Abstract
Temperature is a major environmental factor affecting the growth, development, and productivity of Sargassum fusiforme. We aimed to assess the metabolic processes and regulatory mechanisms in S. fusiforme during a 7-day high-temperature (27 °C and 32 °C) experiment. Changes in chlorophyll content and electrolyte leakage after high-temperature treatment were investigated. Metabolic changes in the leaves of S. fusiforme were analysed using gas chromatography–mass spectrometry. High temperatures suppressed chlorophyll content and increased electrolyte leakage. Further, a strong modulation of various metabolisms was observed: organic acids, amino acids, sugars or sugar alcohols, esters, and amines. These metabolisms were significantly enriched in ten pathways under the 27 °C treatment: aminoacyl-tRNA biosynthesis; glycine, serine, and threonine metabolism; alanine, aspartate, and glutamate metabolism; valine, leucine, and isoleucine biosynthesis; cyanoamino acid metabolism; cysteine and methionine metabolism; arginine and proline metabolism; tyrosine metabolism; citrate cycle (TCA cycle); and glucosinolate biosynthesis. The various metabolisms significantly enriched seven pathways under the 32 °C treatment, namely, alanine, aspartate, and glutamate metabolism; aminoacyl-tRNA biosynthesis; phenylalanine metabolism; tyrosine metabolism; arginine and proline metabolism; nitrogen metabolism; and isoquinoline alkaloid biosynthesis. These changes in metabolic pathways may contribute to the tolerance and adaptability of S. fusiforme to high-temperature stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sargassum fusiforme is a fleshy, juicy, and flavoured marine vegetable with high nutritional and medicinal value (Schepetkin and Quinn 2006; Pugh and Pasco 2011). It is also highly recommended as a healthy longevity food, and is a warm temperate subtropical seaweed that mainly grows in the Northwest Pacific. It is found in the east of China (from the south of the Liaodong Peninsula to the Leizhou Peninsula in Guangdong), Japan (south of Hokkaido, from state to Kyushu), and the coast of Korea (Zhang et al. 2002). In China, where the cultivation area was 2.6% (2482 ha) of the entire coastal area for commercial cultivation of seaweeds, the total production reached 32,000 tonness per year (fresh weight) (Pang et al. 2008). S. fusiforme is largely produced in high quality in the Dongtou islands of Zhejiang costal area (Zeng 2000).
Temperature is a major environmental factor that affects growth, development, and productivity of S. fusiforme. Under natural conditions, S. fusiforme is cultured from September to mid-April or early May of the following year, and the growth temperature range of S. fusiforme is wide, from 5 to 25 °C (Sun et al. 2009). However, in the early seeding period (September to October in south China), S. fusiforme farms often suffer from consistently high temperatures or the well-known “temperature rebound” (a temperature increase followed by a dramatic drop). In mid-April or early May, the seawater temperature is often higher than the optimal temperature. Constant high temperatures can negatively affect S. fusiforme by inhibiting growth, development, and sexual reproduction (Zou and Gao 2005), thus, leading to a substantial reduction in yield. In recent years, high temperatures have markedly affected S. fusiforme cultures and their yields. To adapt to global climate changes and maintain high yields of S. fusiforme, several heat-resistant strains should be selected and extensively cultured in Southern China. Therefore, a deep understanding of the high-temperature resistance mechanisms of S. fusiforme would establish a theoretical foundation for further studies.
In many higher plants, especially in model plants, the response mechanisms to high-temperature stress have been thoroughly studied, and a variety of mechanisms have been summarised. These include maintenance of membrane stability, feedback inhibition of photosynthesis, scavenging of reactive oxygen species (ROS), calcium-dependent protein kinase (CDPK) cascade, antioxidants production, induction of mitogen-activated protein kinase (MAPK), and accumulation and regulation of compatible solutes (Mittler et al. 2012; Hasanuzzaman et al. 2013; Driedonks et al. 2015). These stress responses can be studied by measuring changes in cellular metabolites. A comprehensive study on metabolisms marked by changes in metabolites will help to determine the key factors that regulate metabolism and increase the understanding of how plants respond to various stresses (Zhou et al. 2017). Using metabolomics, the effects of stress on metabolites abundance have been examined in microalgae such as Chlamydomonas reinhardtii (Bolling and Fiehn 2005; Valledor et al. 2013), Ettlia oleoabundans (Matich et al. 2016), Pseudochoricystis ellipsoidea (Ito et al. 2013), Chlorella pyrenoidosa (Guo 2016), Chlorella sorokiniana (Lu 2012), and in marine microalgae such as Paralia sulcata (Yu et al. 2015) and S. fusiforme (Lin 2014). However, there are relatively few studies on the mechanism of high-temperature resistance of algae, especially in S. fusiforme.
This study aims to increase the understanding of the molecular mechanisms of heat stress of S. fusiforme and reveal the global metabolic reaction of S. fusiforme under heat stress. We observed and analysed the physiological changes of leaves under different high-temperature stress. At the same time, we used a gas chromatography–mass spectrometry (GC–MS) method to compare the abundances of different metabolites under normal and heat stress conditions. Metabolomics is another newly developed omics after genomics, transcriptomics and proteomics. The study of metabolomics has also become one of the important components of systems biology. The research results of metabolomics can reveal the changes of the genome, transcriptome and proteome after being affected by genetic or environmental factors, and it is closer to the phenotype of cells or organisms, and is thus becoming more and more widely used (Fiehn 2002). The data obtained in this study will provide important bioinformatic resources for studying the reaction mechanism of S. fusiforme under high-temperature stress.
Materials and Methods
Plant Material and Temperature Treatment
The S. fusiforme used in this study was cultivated by Fisheries Science and the Technology Research Institute of Dongtou District of Wenzhou City in the Zhejiang Province of China. We sampled S. fusiforme at maturity in early May. Uniform samples (morphology and plant height) were screened, impurities were removed, and then the seedlings were clamped by 15 cm of 100 polyethylene ropes; each rope contained seven seedlings before treatments. S. fusiforme seedlings were cultured for 24 h in natural seawater and treated at different temperatures (22 °C as control; 27 °C and 32 °C as heat stress treatments) for 7 days. Then, the leaves near the apical side of the sporophyte of the samples were collected at 0, 1, 3, 5, and 7 days during the treatment period; metabolite analysis was performed only on the 7th-day samples. Four replicas were used in each treatment. Seawater was replaced every 24 h, and the environmental factors during the experiment are presented in Table 1.
Physiological Measurements
The chlorophyll content was measured using a method described by De et al. (1994) with minor modifications. A total of 0.2 g of leaf tissue was incubated in 2 mL of 90% acetone, and quickly ground to shreds. The ground tissue was poured into a centrifuge tube, the mortar was washed two to three times and then the solution was filled to a final volume of 10 mL.
A centrifuge tube containing the extraction solution was centrifuged at 4 °C, 10,000 rpm for 10 min, and the supernatant was absorbed. The resulting solution was measured on a spectrophotometer (Thermo Fisher Model No. GENESYS 10S UV–VIS) at 630 nm, 647 nm, 664 nm, and 750 nm. Acetone (90%) was used as a control (Control, C) and the measurements were repeated three times per sample. The chlorophyll content was calculated on a fresh weight basis using the equations described by Jeffrey and Humphery (1975).
Membrane stability was estimated as electrolyte leakage (EL) using the methods described by Blum and Ebercon (1981). About 0.2 g of fresh leaf was placed in a tube with 15 mL deionised water and then placed on an orbital shaker for 15 h. An initial conductance reading was taken using a conductivity meter (YSI Incorporated, Yellow Springs, OH). Tubes containing the solution were autoclaved at 120 °C for 20 min to kill all leaf tissue. Tubes were placed back on the shaker for an additional 15 h and a final conductance reading was taken. Electrolyte leakage was calculated as a percentage of initial conductance to final conductance, to determine relative damage.
Metabolite Analysis
Sample Preparation
A total of 60 mg of an accurately weighed sample was transferred to a 1.5 mL Eppendorf tube. Two small steel balls were added to the tube. Then, 360 μL of cold methanol and 40 μL of 2-chloro-l-phenylalanine (0.3 mg/mL) dissolved in methanol (internal standard) were added to each sample and placed at − 80 °C for 2 min; samples were ground at 60 Hz in a lapping machine for 2 min. The mixtures were ultrasonicated at room temperature for 30 min. Then, 200 μL of chloroform was added to the samples, the mixtures were vortexed, and 400 μL of water was added. Samples were ultrasonicated at room temperature for 30 min and then centrifuged at 12,000 rpm, 4 °C for 10 min. Two hundred microliters of supernatant in a glass vial was dried in a freeze concentration centrifugal dryer, and 80 μL of methoxylamine hydrochloride pyridine (15 mg/mL) was subsequently added in a glass vial. The resultant mixture was vortexed vigorously for 2 min and incubated in a concussion incubator at 37 °C for 90 min. Eighty microliters of N,O-Bis(trimethylsilyl) trifluoro acetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) and 20 μL n-hexane was added into the mixture, which was vortexed vigorously for 2 min and then derivatised at 70 °C for 60 min. The samples were placed at room temperature for 30 min before GC–MS analysis.
GC Conditions
The derivatised samples were analysed on an Agilent 7890A gas chromatography system coupled to an Agilent 5975C MSD system (Agilent, USA). An HP-5MS fused-silica capillary column (30 m × 0.25 mm × 0.25 μm, Agilent J & W Scientific, Folsom, CA, USA) was utilised to separate the derivatives. Helium (> 99.999%) was used as the carrier gas at a constant flow rate of 6.0 mL/min through the column. The injector temperature was maintained at 280 °C. The injection volume was 1 μL by splitless mode. The initial temperature was 60 °C, ramped to 125 °C at a rate of 8 °C/min, to 190 °C at a rate of 10 °C/min, to 210 °C at a rate of 4 °C/min, to 310 °C at a rate of 20 °C/min, and finally held at 310 °C for 8.5 min.
MS Method
The temperature of the MS quadrupole and ion source (electron impact) was set to 150 °C and 230 °C, respectively. The collision energy was 70 eV. Mass data were acquired in a full-scan mode (m/z 50–600), and continuous sample analysis was carried out in random order to avoid the effect caused by the fluctuation of instrument signals.
Data Analysis
The acquired MS data from the GC–MS were analysed by ChromaTOF software (v 4.34, LECO, St Joseph, MI), the CSV file was obtained with 3D data sets including sample information, peak names, and peak intensities. After internal standards, any known pseudo positive peaks, such as peaks caused by noise or column bleed, were removed from the data set, and the peaks from the same metabolite were carried out and combined. Peak area normalisation method was used to normalise the response intensity of sample mass peaks, and a normalised data matrix was obtained. The integrated data matrix was imported into the SIMCA-P+14.0 software package (Umetrics, Umeå, Sweden), where the unsupervised principal component analysis (PCA) was used to observe the overall distribution of samples and the stability of the whole analysis process; then, the supervised (orthogonal) partial least squares analysis [(O) PLS-DA] was used to distinguish overall differences in metabolic profiles between groups and to find different metabolites in different groups. In (O) PLS-DA analysis, a variable important in projection (VIP > 1, p < 0.05) was considered as differential variables. To prevent the model from overfitting, the quality of the model was investigated by seven-cycle interactive validation and a 200-response sequencing test.
Bioinformatics Analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database that integrates genomic, chemical, and functional information. Differential metabolite information was mapped to the KEGG database, and an enriched KEGG pathway was obtained. The different metabolite items were listed, and different types of statistical graphs were made. The main contents of the analysis of KEGG metabolic pathways include metabolic pathway enrichment table, metabolic pathway enrichment column, metabolic pathway significance analysis, and metabolic pathway map. The p value of metabolites was enriched in this pathway, the Holm method correction calculation and false discovery rate (FDR) correction calculation were carried out based on p value, and p value or FDR value was selected according to the actual needs to determine credibility. The KEGG enrichment results can be visualised by a bar diagram, the ten pathways with the smallest p value were mapped, and the ordinate was the negative logarithm of the E base as the bottom of the p value; the greater the value of − log (p value), the higher wad the significance of the pathway.
Results
Changes in Physiological Indices of Sargassum fusiforme Induced by Heat Stress
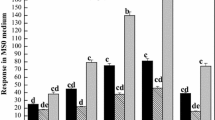
Chlorophyll a and Chlorophyll c content declined in all treatments during heat stress (Fig. 1a, b). By 7 days of heat stress, chlorophyll a content declined by 29% in the control plants, whereas that for 27 °C and 32 °C treatments declined by 37% and 42%, respectively. Plants treated with 27 °C exhibited significantly higher chlorophyll a content than the control plants at 1 day of heat stress; plants treated with 32 °C exhibited significantly higher chlorophyll a content than the control plants at 1, 3, and 5 days of heat stress. Chlorophyll c content declined by 41% in the control plants, and that for the 27 °C and 32 °C treatments declined by 52% and 79%, respectively. Plants treated with the 27 °C and 32 °C treatments exhibited significantly higher chlorophyll c content than the control plants at 1 and 3 days of heat stress.
Effect of heat stress on the chlorophyll content of Sargassum fusiforme leaf. The horizontal axis shows the days of high-temperature treatment (0, 1, 3, 5, and 7 days). Each value represents the mean ± standard deviation (SD) of three replicates. Changes to chlorophyll a content (a) and chlorophyll c content (b) during the 7th day of 27 °C or 32 °C treatments (22 °C as a control) are shown. Vertical bars represent the least significant differences between different treatments at p = 0.05. Different letters represent significant differences
EL increased in all high-temperature treatments, indicating a loss of membrane stability (Fig. 2). By 7 days of heat stress, EL increased to 40.8% and 50.2% in the 27 °C and 32 °C treatments, respectively; whereas there was little change in the control group (22 °C) from 7 days (20.6%) to 0 days (20.4%). Overall, plants treated with the 27 °C and 32 °C treatments significantly increased EL during the 7 days of heat stress.
Effect of heat stress on electrolyte leakage in Sargassum fusiforme leaf. The horizontal axis shows the days of high-temperature treatment (0, 1, 3, 5, and 7 days). Each value represents the mean ± standard deviation (SD) of three replicates. Vertical bars represent the least significance difference values between different treatments at p = 0.05. Different letters represent significant differences
Effects of High Temperature on Metabolites of Sargassum fusiforme
The results of metabolic profiling of plants under heat stress were presented. A total of 31 metabolites were significantly changed in the leaves of S. fusiforme exposed to 27 °C heat stress compared with control; this treatment caused an increase in 5 metabolites and a decrease in 26 metabolites, 9 of which were organic acids, 8 were amino acids, 4 were sugars or sugar alcohols, and 10 were other metabolites (Table 2). When plants were treated with 32 °C heat stress, 80 metabolites were significantly changed (22 metabolites increased and 58 metabolites decreased), 20 of which were organic acids, 20 were amino acids, 10 were sugars or sugar alcohols, 4 were esters, 5 were amines, and 21 were other metabolites ( Table 3). Heat stress treatment (27 °C and 32 °C) caused a decrease in 14 metabolites (phenylacetaldehyde, 2-ketobutyric acid, p-benzoquinone, dibenzofuran, 2-hydroxypyridine, 5-methoxytryptamine, aspartic acid, lyxonic acid, 1,4-lactone, alpha-d-glucosamine 1-phosphate, tyramine, glucose-6-phosphate, cytidine-monophosphate degrprod, dioctyl phthalate, and trehalose), and an increase in tryptophan.
Metabolic Pathways are Affected After Different Heat Stress Temperatures
The identified metabolites were taken as the background set to conduct an enrichment analysis of KEGG pathways, and the abundant pathways in the differential expression metabolite group were determined using a Fisher exact test. After analysing the set of 31 differentially expressed metabolites under the 27 °C treatment and comparing them with the control group (22 °C treatment), 34 associated KEGG signalling/metabolic pathways were extracted (Table 4), among which ten pathways were significantly enriched and obtained by KEGG pathway enrichment analysis (Figs. 3, 5). These ten enriched pathways were: 1 aminoacyl-tRNA biosynthesis, 2 glycine, serine, and threonine metabolism, 3 alanine, aspartate, and glutamate metabolism, 4 valine, leucine, and isoleucine biosynthesis, 5 cyanoamino acid metabolism, 6 cysteine and methionine metabolism, 7 arginine and proline metabolism, 8 tyrosine metabolism, 9 citrate cycle (TCA cycle), and 10 glucosinolate biosynthesis. Analysing the set of 80 differentially expressed metabolites under the 32 °C treatment (compared with the control group), we extracted 38 associated KEGG signalling/metabolic pathways, among which 7 of the significantly enriched pathways were obtained by the KEGG pathway enrichment analysis, and the first 10 metabolic pathways were present in an enrichment map of metabolic pathways of S. fusiform (Figs. 4, 5). These included alanine, aspartate, and glutamate metabolism, aminoacyl-tRNA biosynthesis, phenylalanine metabolism, tyrosine metabolism, arginine and proline metabolism, nitrogen metabolism, isoquinoline alkaloid biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis, and carbon fixation in photosynthetic organisms. After different temperature treatments (27 °C and 32 °C), 29 metabolic pathways remained constant and, of the first 10 metabolic pathways, 4 were the same.
Enrichment map of the metabolic pathways of Sargassum fusiform after the 27 °C treatment. The selected signalling pathways are shown in the abscissa, and the negative logarithms of the p values at the base of E are shown in the ordinate. The greater the value of − log (p value), the more significant was the pathway
Enrichment map of the metabolic pathways of Sargassum fusiform after the 32 °C treatment. The selected signal pathways are shown in the abscissa, and the negative logarithms of the p values at the base of E are shown in the ordinate. The greater the value of − log (p value), the more significant was the pathway
Overall, seven of the ten distinct metabolic pathways enriched after 27 °C treatment were related to amino acid metabolism, and some amino acid metabolisms were promoted by heat stress, such as valine, leucine and isoleucine biosynthesis metabolic pathways (Supplementary Fig. 1). In this pathway, l-valine and l-isoleucine contents were all increased. The other three distinct metabolic pathways were aminoacyl-tRNA biosynthesis (Supplementary Fig. 2), TCA cycle (Supplementary Fig. 3), and glucosinolate biosynthesis (Supplementary Fig. 4). In the aminoacyl-tRNA biosynthesis pathway, l-glutamate, l-aspartate, and l-serine were all down-regulated, which resulted in the down-regulation of l-glutamyl-tRNA, l-aspartyl-tRNA, and l-selenocysteinyl-tRNA, whereas up-regulation of l-valine, l-isoleucine, and l-tryptophan resulted in the up-regulation of l-valyl-tRNA, l-valyl-tRNA, and l-isoleucyl-tRNA. In the TCA cycle pathway, citrate and fumarate contents were all decreased. In the glucosinolate biosynthesis pathway, l-valine, l-isoleucine, and l-tryptophan contents were all increased.
After the 32 °C treatment, five of the seven distinct metabolic pathways were related to amino acid metabolism, and four of them were the same distinct metabolic pathways observed under the 27 °C treatment. These are aminoacyl-tRNA biosynthesis; alanine, aspartate, and glutamate metabolism; arginine and proline metabolism; and tyrosine metabolic pathways in alanine, aspartate, and glutamate metabolic pathway (Supplementary Fig. 5). l-asparagine, l-glutamate, and l-glutamine increased in the arginine and proline metabolic pathway (Supplementary Fig. 6), and l-glutamine, proline, and 5-aminopentanoate accumulated. The different amino acid metabolic pathways compared in the 27 °C treatment were from the phenylalanine metabolic pathway (Supplementary Fig. 7). In the phenylalanine metabolic pathway, phenylacetaldehyde and fumarate were down-regulated, and l-tyrosine, l-phenylalanine, hippurate, trans-4-hydroxycinnamate, and 3-hydroxyphenylacetate were up-regulated. The remaining two metabolisms of the seven distinct metabolic pathways were nitrogen metabolism (Supplementary Fig. 8) and isoquinoline alkaloid biosynthesis (Supplementary Fig. 9) metabolic pathways. l-Glutamine and l-glutamate were up-regulated in the nitrogen metabolic pathway; l-tyrosine and 4-coumarate were up-regulated, and tyramine was down-regulated in the isoquinoline alkaloid biosynthesis metabolic pathway.
Discussion
Effects of Heat Stress on Physiological Indexes of Sargassum fusiform
Chlorophyll reflects the photosynthetic capacity of leaves. The relative water content of plant leaves can partly reflect plant stress resistance. Unchanged membrane function is the key to maintaining photosynthesis and respiration (Lu and Uuo 2003; Xu et al. 2004). Leaf relative EL can indicate the damage degree of the leaf membrane structure, and it can be used as one of the evaluation indicators of plant heat tolerance levels (Zhao et al. 2013). Under high-temperature treatments, the EL of leaf increased. It can be seen that continuous high temperatures caused damage to the membrane structure of S. fusiform leaves, thus increasing EL. Continued high temperatures caused chlorophyll deformation and destruction of thylakoid lamellar structure, resulting in a decrease in chlorophyll content. In this study, under high-temperature stress, chlorophyll a and chlorophyll c content in the leaves of S. fusiforme decreased significantly (the higher the temperature, the more obvious the effect), which was consistent with the result of Fei et al. (2018). The degree of damage caused by high-temperature stress to S. fusiforme may be closely related to the different accumulation of metabolites, which will be discussed in detail below.
Effects of Heat Stress on Metabolites and Metabolic Pathways of Sargassum fusiform
Metabolites play an important role in plant growth and development. In addition to primary metabolism, these metabolites can also act on plants in terms of signal transduction to aid in defence mechanisms or plant tolerance. In this study, we studied survival or tolerance mechanisms against heat stress, and the metabolite changes of S. fusiform under different high-temperature stress conditions.
Amino Acid Metabolism Under Different High Temperatures
Amino acids play an important role in plant growth and metabolism. Amino acids are not only able to synthesise proteins, but also the precursors of many plant responses to abiotic stress-related metabolites (Less and Galili, 2008). In this study, the contents of valine and isoleucine increased significantly after the 27 °C treatment, and the metabolic pathways (valine, leucine, and isoleucine biosynthesis) were also significantly affected. Valine may act as a primary metabolite and produce more secondary metabolites to protect plant tissues from high-temperature stress (Zhao et al. 2015). The increase of valine content after stress has been reported under many adverse conditions (Kaplan et al. 2004; Oliver et al. 2011). Isoleucine can enter the TCA cycle and contribute to the redox potential to help maintain respiratory rate (Kirma et al. 2012), and it also plays a role in the jasmonate signal (Staswick and Tiraki 2004). Increased isoleucine may also increase heat resistance by helping to maintain signalling pathways or TCA cycle activity. However, in our study, the TCA cycle was inhibited, and fumarate and citrate were decreased in the TCA cycle.
Abiotic stress usually leads to protein degradation within the cell, resulting in the accumulation of ammonium (Gilbert et al. 1998). It has been reported that different forms or subtypes of glutamate are often accumulated as stress-responsive substances in plants under different stress environments (Semel et al. 2007). In this study, the glutamate content increased, and the glutamate metabolic pathway was significantly enhanced after the 32 °C treatment. Glutamic acid is a precursor of many other amino acids (Beale et al. 1975). Additionally, glutamic acid plays an important role in nitrogen metabolism and chlorophyll biosynthesis (Forde and Lea 2007). The increase in glutamate can improve heat resistance and nitrogen binding to other cellular molecules; it also indicates that the maintenance and accumulation of glutamic acid contributes to better adaptation and tolerance of S. fusiform to high-temperature stress. Under abiotic stresses, elevated levels of amides, especially asparagine and glutamine, can reabsorb the released free amino acids to reduce the toxic effect of ammonia salts on plants (Good and Zaplachinski 1994; Díaz et al. 2005). On the other hand, asparagine is synthesised by glutamine, and both metabolites work by regulating the storage and transport of nitrogen. Consistently, high accumulation of asparagine was related to stress treatment of wheat leaves (Lea et al. 2007). In this study, both asparagine and glutamine were significantly increased after the 32 °C treatment, results consistent with the research reported by Diaz et al. (2005). Obviously, asparagine is highly accumulated in plants as proline levels increase. The regulation of asparagine in these plants may suggest a biological mechanism by which plants maintain osmotic function (Lea et al. 2007).
Phenylalanine is a precursor of secondary metabolites such as plant antitoxins, alkaloids, lignin, and flavonoids. Increasing phenylalanine levels can increase the synthesis of secondary metabolites, which play an important role in regulating plant heat tolerance. Yamakawa and Hakata (2010) found that the accumulation of phenylalanine in rice grains increased under high temperatures. In this study, phenylalanine content increased significantly, and the phenylalanine metabolic pathway was promoted after the 32 °C treatment. Accumulation of phenylalanine is beneficial for increased production of heat-related secondary metabolites, thus making S. fusiform more heat resistant.
A sulphur-containing amino acid, S-carboxymethyl cinnamic acid, was detected in this study, and its content increased significantly after the 32 °C treatment. Cysteine is not only a basic amino acid for protein synthesis, but it can also be used to synthesise glutathione from glycine and glutamate. Glutathione is an important active substance in plants against oxidative damage. Some defensive organic compounds (such as metallothionein) and active centre sulphur elements in coenzymes are directly or indirectly derived from cysteine (Wahid and Close 2007). Consistently high levels of cysteine in S. fusiform may be related to the synthesis of glutathione for the enhancement of antioxidant activity.
Proline is a non-protein and stress-related amino acid (Liu et al. 2013). Proline plays many roles in the plant defence system as an energy bank, subcellular structure stabiliser, osmotic regulator, free radical scavenger, and stress signal molecule (Nanjo et al. 1999). In this study, proline content and the proline metabolic pathway were significantly increased after the 32 °C treatment, which are consistent with the results of Zhao and Li (2001).
In our study, accumulation of some other amino acids such as hippuric acid, tryptophan, 3-cyanoalanine, oxoproline, and tyrosine was detected, however, there are few studies on the relationship between these amino acids and high-temperature tolerance in plants, which require further study.
Organic Acid Metabolism Under Different High Temperatures
In this study, nine organic acids were significantly altered under the 27 °C treatment; they were 2-ketobutyric acid, lactic acid, fumaric acid, aconitic acid, galactonic acid, citric acid, alpha-d-glucosamine-1-phosphate, creatine, and glucose-6-phosphate. However, the contents of these organic acids all decreased significantly. Under the 32 °C treatment, 20 organic acids changed significantly. Out of these, five organic acids were significantly increased; they were gallic acid, 1-malic acid, c, 5-aminovaleric acid, and 4-hydroxycinnamic acid. As an intermediate product of the TCA cycle, malic acid accumulation may reflect high mitochondrial activity, thus facilitating the formation of more reductants or providing a carbon skeleton for amino acid biosynthesis (Vasquez et al. 2008). Moreover, 1-malic acid content was increased after 7 days in the 32 °C treatment, but the TCA metabolic pathway was not changed significantly. Gallic acid is a type of secondary metabolite in plants. It exists in gallnut, tea, gall, and other plants as a free acid or as an ester, and it acts as a free radical scavenger (Matito et al. 2003). However, little is known about the relationship between gallic acid, 3-hydroxyphenylacetic acid, 5-aminovaleric acid, 4-hydroxycinnamic acid, and plant heat stress.
Sugar and Sugar Alcohol Metabolism Under Different High Temperatures
Sugars play an important role in plant growth and adaptation to stress. Sugars not only provide energy for direct synthesis of other compounds, but also stabilise cell membranes (Hoekstra et al. 1991, 2001), regulate gene expression (Koch 1996), and participate in the signal system of carbohydrate sensing Zeng (2000). Furthermore, it has been reported that carbohydrates may be signal molecules of metabolic regulation (Gibson 2005). After high-temperature stress, fructose (Rizhsky et al. 2004), glucose, and sucrose (Kaplan et al. 2004) content in Arabidopsis thaliana and the sucrose content in barley (Mac and Duffus 1988) and rice Yamakawa and Hakata 2010) increased significantly. In this study, sedoheptulose content increased significantly after 7 days of the 32 °C treatment. Because of the significant correlation between the accumulation of sugars and heat tolerance in plants (Wahid and Close 2007), the increase in this sugar may help to enhance heat tolerance of S. fusiform.
Sugar alcohols are hydrated carbohydrates. Through water-like hydroxyl groups, they can form hydration layers around macromolecules to protect them (Schobert 1977). Sugar alcohols are not only closely involved in carbon transport and storage, cryoprotection, boron transport, and energy transfer, but they are also involved in osmotic regulation and inhibition of ROS (Brown and Hu 1996). In this study, dithioerythritol, 2-butyne-1,4-diol, and ribitol contents changed significantly after 7 days of the 27 °C treatment, and 1,5-anhydroglucitol, glycerol, 2-butyne-1,4-diol, phytol, threitol, 1-hexadecanol, d-arabitol, and mannitol contents changed significantly after 7 days of the 32 °C treatment. When treated with high temperatures, d-arabitol was increased, and other sugar alcohols were decreased. It was speculated that d-arabitol might be a sugar alcohol that resists high-temperature stress in S. fusiform.
Conclusion
Our study indicated that heat stress suppressed chlorophyll content and increased EL. A total of 31 metabolites were significantly changed in the leaves of S. fusiforme exposed to 27 °C heat stress compared with control, and 80 metabolites were significantly changed when plants were treated with 32 °C heat stress. Further, a strong modulation of various metabolisms was observed: organic acids, amino acids, sugars or sugar alcohols, esters, and amines. These metabolisms were significantly enriched in ten pathways under the 27 °C treatment: aminoacyl-tRNA biosynthesis; glycine, serine, and threonine metabolism; alanine, aspartate, and glutamate metabolism; valine, leucine, and isoleucine biosynthesis; cyanoamino acid metabolism; cysteine and methionine metabolism; arginine and proline metabolism; tyrosine metabolism; citrate cycle (TCA cycle); and glucosinolate biosynthesis. The various metabolisms significantly enriched seven pathways under the 32 °C treatment, namely, alanine, aspartate, and glutamate metabolism; aminoacyl-tRNA biosynthesis; phenylalanine metabolism; tyrosine metabolism; arginine and proline metabolism; nitrogen metabolism; and isoquinoline alkaloid biosynthesis. These changes in metabolic pathways may contribute to the tolerance and adaptability of S. fusiforme to high-temperature stress. This information will be useful to identify metabolites that may be incorporated into chemical products for alleviating heat stress and provide knowledge for the breeding of S. fusiforme thermotolerance cultivars.
Abbreviations
- CDPK:
-
Calcium-dependent protein kinase
- EL:
-
Electrolyte leakage
- GC–MS:
-
Gas chromatography–mass spectrometry
- MAPK:
-
Mitogen-activated protein kinase
- (O) PLS-DA:
-
(Orthogonal) partial least squares analysis
- PCA:
-
Principal component analysis
- ROS:
-
Reactive oxygen species
- VIP:
-
Variable important in projection
References
Beale SI, Gough SP, Granick S (1975) Biosynthesis of alpha-aminolevulinic acid from the intact carbon skeleton of glutamic acid in greening barley. Proc Natl Acad Sci USA 72:2719–2723
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Brown PH, Hu H (1996) Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol-rich species. AnnBot-London 77:497–506
Bollingc F (2005) Metabolite profiling of Chlamydomonas reinhardtii under nutrient deprivation. Plant Physiol 139:1995–2005
Dekli JL, Diana C, Campos RJ, Arnoux A, Pellegrini L (1994) Toxicity of methyl mercury and mercury (II) chloride to a brown algae Cyctoseira barbata (Fucales) under laboratory culture conditions detoxify role of calcium. Bot Mar 37:367–379
Díaz P, Borsani O, Marquez A (2005) Nitrogen metabolism in relation to drought stress responses in cultivated and model Lotus species. Lotus Newslr 35:83–92
Driedonks N, Xu J, Peters JL, Park S, Sun JZ (2015) The Effect temperature on the growth of Sargassum fusiforme, Journal of Zhejiang ORieu I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front Plant Sci 6:999
Forde BG, Lea PJ (2007) Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot 58:2339–2358
Fiehn O (2002) Metabolomics-the link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Fei ZX, Liu LL, Hu D, Hou N (2018) Effects of high temperature stress on physiological–biochemical of Rhododendronhybridum. Northernhorticulture 08:102–105
Good AG, Zaplachinski ST (1994) The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol Plantarum 90:9–14
Gilbert GA, Gadush MY, Wilson C (1998) Amino acid accumulation in sink and source tissues of Coleus blumei Benth during salinity stress. J Exp Bot 49:107–114
Gibson SI (2005) Control of plant development and gene expression by sugar signalling. Curr Opin Plant Biol 8:93–102
Guo J (2016) The study of influence of four kinds of PPCPs on the cultivation of microalgae. In: M.D. thesis. Tongji University, Shanghai
Hoekstra FA, Crowe JH, Crowe LM (1991) membranes in intact pollen of Typha latifolia L., as measured with Fourier transform infrared spectroscopy. Plant Physiol 97:1073–1079
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6:431–438
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Ito T, Tanaka M, Shinkawa H (2013) Metabolic and morphological changes of an oil accumulating trebouxiophycean alga in nitrogen-deficient conditions. Metabolomics 9:178–187
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants and algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Koch K (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Biol 47:509–540
Kaplan F, Kopka J, Haskell DW (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136:4159–4164
Kirma M, Araujo WL, Femie AR, Galili G (2012) The multifaceted role of aspartate-family amino acids in plant metabolism. J Exp Bot 63:4995–5001
Lu SY, Uuo ZF (2003) Physiological responses of turlgrass to abiotic stresses. Acta Prataculturae Sin 12:7–13
Lea PJ, Sodek L, Parry MA, Shewry PR, Halford NG (2007) Asparagine in plants. Ann Appl Biol 150:1–26
Less H, Galili G (2008) Principal transcriptional programs regulating plant aminoacid metabolism in response to abiotic stresses. Plant Physiol 147:316–330
Lu ZH (2012) Trans-omics Study of the Cellular Metabolism of Chlorella sorokiniana Cultivation. In: M.D. thesis. Tianjin University, Tianjin
Liu AR, Zhang YB, Zhong ZH (2013) Effect of salt stress- on the growth and osmotica accumulation of Coleus blumei. Acta Prataculturae Sin 22:211–218
Lin LD (2014) The adsorption and pysiological responses to heavy metal copper of Hizikia fusiformis. In: M.D. thesis. Northest Forestry University, Harbin
Mac LL, Duffus C (1988) Reduced starch content and sucrose synthase activity in developing endosperm of barley plants grown at elevated temperatures. Funct Plant Biol 15:367–375
Matito C, Mastorakou F, Centelles JJ (2003) Antiproliferative effect of antioxidant polyphenols from grape in murine Hepa-1c1c7. Eur J Nutr 42:43–49
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Matich EK, Butryn DM, Ghafari M (2016) Mass spectrometry-based metabolomics of value-added biochemicals from Ettlia oleoabundans. Algal Res 19:146–154
Nanjo T, Kobayashi M, Yoshiba Y (1999) Biological functions of prolinein morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J 18:185–193
Oliver MJ, Cuo I, Alexander DC (2011) A sister group contrast untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus staypfianus. Plant Cell 23:1231–1248
Pang SJ, Shan TF, Zhang ZH, Sun JZ (2008) Cultivation of the intertidal brown alga Hizikia fusiformis (Harvey) Okamura: mass production of zygote-derivedseedlings under commercial cultivation conditions, a case study experience. Aquac Res 39:1408–1415
Pugh N, Pasco DS (2011) Characterization of human monocyte activation by a water soluble preparation of Aphanizomenon flosaquae. Phytomedicine 8:445–453
Rizhsky L, Liang H, Shuman J (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Schobert B (1977) Is there an osmotic regulatory mechanism in algae and higher plants. J Theor Biol 68:17–26
Staswick PE, Tiraki L (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127
Schepetkin IA, Quinn MT (2006) Botanical polysaccharides:macrophage immunomodulation and therapeutic potential. Int Immunopharmacol 6:317–333
Semel Y, Schauer N, Roessner U (2007) Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype. Metabolomics 3:289–295
Sun YY, Sun QH, Sun JZ (2009) The effect temperature on the growth of Sargassum fusiforme. J Zhejiang Ocean Univ (Natural Sci) 28:342–347
Vasquez RC, Mane SP, Ulanov AV (2008) Physiological and molecular adaptations to drought in Andean potato genotypes. J Exp Bot 59:2109–2123
Valledor L, Furuhashi T, Hanak AM (2013) Systemic cold stress adaptation of Chlamydomonas reinhardtii. Mol Cell Proteomics 12:2032–2047
Wahid A, Close T (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Xu S, Li JL, Zhao DH (2004) Research advances in physiological ecological and biochemical characteristics of Festuca arundinacea. Acta Pratacul turae Sinica 13:8–64
Yamakawa H, Hakata M (2010) Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol 51:795–809
Yu Q, Wang Q, Han W, Zhang Q, Wu HF, Zhao JM (2015) Comparison of metabolome extraction strategies from Paralia sulate. Marine Sci Bull 34:703–709
Zeng CH (2000) Chinese seaweed. Science Press, Bejing, pp 150–151
Zhao X, Li YL (2001) Variation of several physiological indices of five cool season turfgrasses under high temperature stress. Acta Prataculturae Sin 10:85–91
Zhang Z, Liu JG, Liu JD (2002) Study review of Hizikia fusiformis. Mar Fish Res 23:67–74
Zou DH, Gao KS (2005) Photosynthetic characteristics of the economic brown seaweed Hizikia fusiforme (Sargassaceae phaeophyta) With special reference. J Appl Psychol 17:255–259
Zhao LL, Chen C, Ma L (2013) Physiological response and heat tolerance evaluation o1 Lotus corniculutus under high temperature stress. Pratacult Sci 30:2018–2023
Zhou LY, Li YY, Wang WN, Zhong S (2017) Research Progress in the metabolomics for plants response to temperature stress. J Shanxi Agric Sci 45:317–320
Zhao ZJ, Hu LX, Hu T, Fu JM (2015) Differential metabolic responses of two tall fescue genotypes to heat stress. Acta Pratacult Sin 24:58–68
Acknowledgements
This work was supported by the Science and Technology Plan Projects in Dongtou District of Wenzhou City (N2016Y20B), Key Fishing and Agricultural Science and Technology Project in Dongtou District, Wenzhou, (No. N2018Y03A), and the Fundamental Research funds in Heilongjiang Provincial Universities (special specialty of plant food processing technology) (YSTSXK201877). We would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LL, LL. Performed the experiments: LL. Analysed the data: LL, LL. Contributed reagents/materials/analysis tools: LL, LL.
Corresponding author
Ethics declarations
Conflict of Interests
The author(s) declare no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., Lin, L. Effect of Heat Stress on Sargassum fusiforme Leaf Metabolome. J. Plant Biol. 63, 229–241 (2020). https://doi.org/10.1007/s12374-020-09247-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-020-09247-5