Abstract
Sugarcane is an important crop for tropical countries and to accurately inventory its greenhouse gas (GHG) emissions baseline measurements are needed. In Colombia, sugarcane is one of the most important crops in terms of cultivated area and, paradoxically, scientific information reporting GHG emissions based on field measurements is almost nonexistent. The objective of this work was to quantify the direct emissions of carbon dioxide (CO2) and nitrous oxide (N2O) in the sugarcane-soil system of the Cauca river valley, Colombia. For this purpose, a field experiment was established in a typic haplustert soil cropped with sugarcane. The effects of nitrogen (N) fertilization and sampling site on its GHG emissions were tested using the closed static chamber method over a period of 211 days. The main cumulative emissions were 765.14 ± 34.1 g CO2–C m−2 and 125.4 ± 22.6 mg N2O–N m−2. Overall, GHG emissions were modified by N fertilization, the sampling site, and their interaction. Nitrogen fertilization with urea increased mean and cumulative CO2 and N2O emissions, especially at the row sampling site. This paper highlights the importance of considering these factors when the quantification of GHGs or a reduction of their associated uncertainties are required. This work reportss the first GHG emissions data for a typical sugarcane agroecosystem in Colombia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum spp.) is one the most cultivated plant species in the world, with approximately 26 million hectares harvested in 2019 (FAO, 2021). This crop is responsible for 25% of the world’s production of bioethanol and its production is expected to increase (Thorburn et al. 2011; OECD-FAO 2021). The average yield of this crop ranges between 70 and 90 Mg ha−1, and high doses of nitrogen (N) fertilizer (between 150 and 200 kg N ha−1) are needed to achieve these yields (Thorburn et al. 2011). However, N fertilizers are a major source of N2O emissions, especially when the N dose exceeds crop demand (Chalco Vera et al. 2022). For these reasons, concerns about the environmental impacts associated with sugarcane production make the study of greenhouse gas (GHG) emissions necessary, especially for sugarcane-producing countries with expansion potential.
In Colombia, the agriculture sector accounted for 26% of the total GHG emissions in 2012, contributing 12, 48 and 88% of the total emission of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O), respectively (Pulido et al. 2016). In this country, sugarcane plays an important role in the economy, occupying approximately 500,000 hectares, with about 50% of the cultivated area concentrated in the Cauca River valley region (MinAgricultura 2021). This likely makes sugarcane a major source of anthropogenic GHG emissions in Colombia. Paradoxically, scientific information reporting GHG emissions based on infield measurements is almost non-existent for Colombia. In fact, most of the national GHG emissions estimaties are based on Intergovernmental Panel on Climate Change (IPCC) default emission factors. Thus, field-scale studies to quantify GHG emissions from agroecosystems are still required. Indeed, obtaining specific information on GHG emissions for sugarcane in Colombia (as in other countries) will be useful for reducing uncertainties, identifying regional hotspots, and developing enhanced strategies to mitigate those emissions. In addition, because sugarcane is an important feedstock for bioenergy production in Colombia, field measurements of GHG emissions from this crop are essential for assessing the cost of carbon and GHG emissions from biofuel production and the fossil fuel replacement (Lisboa et al. 2011).
In general, it is well known that GHG emissions (mainly N2O) are characterized by high spatio-temporal fluctuations (Hénault et al. 2012; Butterbach-Bahl et al. 2013) and, in sugarcane, it was found that some soil conditions could generate trade-offs among the GHGs (Chalco Vera et al. 2020). In addition, agricultural management practices associated with N or carbon (C) inputs are important factors influencing GHG emissions (Panosso et al. 2009; Vargas et al. 2014; Chalco et al. 2017; Pitombo et al. 2017; Dattamudi et al. 2019). Thus, efforts to quantify GHG emissions need to address the challenge of having to capture this spatial variability. Accordingly, in-field GHG measurements are needed to determine the effect of representative management practices in variable soil conditions for the sugarcane cropping system of Colombia. The objectives of this study was to quantify the direct emissions of CO2 and N2O from a typical vertisol grown with fertilized and unfertilized sugarcane under two soil sampling conditions in the Cauca River valley in Colombia.
Materials and Methods
Description of the Study Area
The experiment was carried out in a commercial sugarcane field located in the central region of the Cauca River valley (Fig. 1), Colombia (− 76.283825 N; 3.67235 W). Traditionally, this area has been cropped with a monoculture of sugarcane for more than 90 years. The measurements were made in a field planted with the CC01-1940 variety, the dominant variety in the region. The productive cycle normally consists of an annual harvest for a period of five years, with mean yield of 110 Mg ha−1. Nitrogen fertilization is generally performed with urea at rates of 100 to 200 kg N ha−1 and irrigation is applied through surface canals when required. Finally, the harvest is completely mechanized without burning.
The climate is characterized by a daily mean temperature of 24.6 °C and daily mean minimum and maximum temperatures of 19.0 and 30.2 °C, respectively. The mean annual rainfall is about 1000 mm concentrated from March to May (IDEAM, 2020). The soil was classified as a Typic Haplustert, the predominant soil in the Cauca river valley (Carbonell and Osorio 2010). It was characterized by having a pH of 8.4; 2.6% of organic matter; 46.3 ppm of available phosphorus; and 0.4, 54.8, 8.6 and 0.2 cmol ( +) Kg−1 of potassium, calcium, magnesium, and sodium, respectively. Also, this soil has an isohyperthermal regime (mean annual soil temperature ˃ 22 °C), a silty clay texture dominated by the mineral montmorillonite and a high cation exchange capacity (64 cmol ( +) Kg−1) (Geoportal IGAC, 2020).
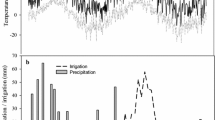
During the experimental period (211 days), the air temperature averaged 23.9 ± 1.0 °C, and the mean minimum and maximum temperatures were 19.2 ± 1.3 and 31.0 ± 1.1 °C, respectively. The total rainfall was 645.0 mm and the relative humidity averaged 78.4 ± 5.6% (Fig. 2). Evapotranspiration averaged 5.1 ± 1.3 mm d−1, solar radiation averaged 460.4 ± 94.3 Cal cm−2, and atmospheric pressure averaged 904.5 ± 1.4 hPa (IDEAM, 2020).
Field Experiment
To capture the natural heterogeneity of soil conditions and determine representative GHG emissions, soil apparent electrical conductivity (ECa) data were used to guide the placement of chambers in the plots (Johnson et al. 2005). A profiler EMP-400 (Geophysical Survey Systems Inc.) soil profiler equipped with an electromagnetic induction sensor and a global positioning system (GPS) was used to measure and georeference ECa data. This profiler sensed soil ECa for the first 50 cm of soil depth. Soil was sensed every 3.5 m apart in an area of 4.8 ha. To map this information, soil ECa data were interpolated using Kriging and a Gaussian model (Nugget = 32 Ms m−1, Sill = 747.9 mS m−1, Range = 188 m and r2 = 0.974) using Qgis ® software (ver. 3.10.5; QGIS.org 2020). After this, the experiment was lay out over an area that included soil ECa values between 25 and 108 mS m−1 (Fig. 1) and was arranged in split-plot factorial design from a cross-combination of N fertilization (control and urea) and sampling sites (row and inter-row) as factors of treatment. Thus, sampling chambers with a maximum distance of 20 m were considered experimental units (Table 1).
Nitrogen fertilization was carried out twice, incorporating solid urea (92 kg N ha−1) in the band row at a depth of 5–10 cm. Additionally, phosphorus (P), potassium (K), sulfur (S) and zinc (Zn) were applied to supply the crop requirements according to Viveros Valens (2018). To reproduce possible application effects in the control treatment, the fertilization machinery was used without fertilizer. Management practices summarized in (Table 2).
Gas Sampling
Greenhouse gases were sampled using the closed static chamber method (Norman et al. 1997; de Klein et al., 2012). Each chamber consisted of two attachable PVC-cylinders (base and lid) 0.1 m in height with an inside diameter of 0.25 m. The base was buried 0.05 m into the ground. The lid had two rubber stoppers: one to insert a digital thermometer and record air temperature, and the other to draw the air sample using 20 mL polypropylene syringes. Immediately after coupling cylinders, four samples were extracted (0, 20, 40 and 60 min) from each chamber and they were stored individually in 5.9 ml evacuated vials (Exetainer, Labco ®).
Field gas sampling was performed between 8 and 11 AM to reduce environmental variations. Sampling began after the second harvest of the crop and continued a weekly for the first four months and, thereafter, every 30 days for the remainder of the growing season (211 day in total). In addition, when N fertilization was scheduled, gas sampling was performed one day before and the three days following application of the N fertilizer, totaling 20 sampling dates. The same sampling frequency was used in the unfertilized plots. Mobility restrictions established by the Colombian government to mitigate the spread of the SARS-Covid 2 virus impeded sampling from day 97 to day 168 of the evaluation. The concentration (ppm) of each GHG was determined by mean of gas chromatography using a Shimadzu ® GC-201 (Shimadzu Corp., Japan) equipped with a flame ionization detector for CO2 and an electron capture detector for N2O (GC-2014, 2020).
Calculation of GHG Emissions
The GHG emissions were calculated using a restricted quadratic regression as explained by Venterea et al. (2020). To screen data the minimum detectable flux (MDF) was calculated for CO2 and N2O following Parkin et al. (2012). The daily mean emissions were estimated based on the method and constants proposed by Parkin and Kaspar (2003) for CO2 and Parkin and Kaspar (2006) for N2O. To calculate the cumulative emission of each of the GHG, the daily mean emissions were projected according to the day of evolution (daily emission fluxes), then the projected points were linearly interpolated and the area under the curve was calculated. This method was applied including the interrupted sampling period.
Statistical Analysis
In order to test differences among treatment effects over time, a linear mixed model was adjusted following akaike criteria (Chalco Vera et al. 2017) considering factors of treatments (fertilizer and sampling site) as fixed effects and time as a random effect. The type III hypothesis test was used to control for possible directional, non-directional, and combined errors during hypothesis testing (Shaffer 2002), and the LSD Fisher test with at 0.05 level was used to compare adjusted means (Westermann et al. 2021). In addtion, ANOVAs at 0.05 level were used to determine significant treatment effectson cumulative GHG emissions.
Results
CO 2 Emissions
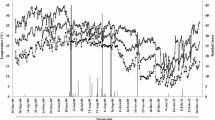
Although the CO2 emissions had a gap of 71 days due to the interruption of sampling (Fig. 3A,C), the daily mean CO2 emissions differed significantly for the fertilization factor (p<0.01). The interaction between fertilization and the sampling site also generated a significant effect (p < 0.05). The highest CO2 emissions were observed at 45, 50, 80 and 211 days of evaluation, and the lowest CO2 emissions were recorded during the first four days of evaluation (0–3 days) (Fig. 3A, C). The time (random effect) only explained 20,5% of the random variance of the model. Overall, urea generated a higher daily mean CO2 emission than the control (3.55 ± 0.3 vs. 2.84 ± 0.35 g CO2–C m−2 d−1, respectively). The ranking of the daily mean CO2 emissions for the interaction fertilization-sampling site (from highest to lowest) was: 3.6 ± 0.34; 3.5 ± 0.31; 3.3 ± 0.41 and 2.3 ± 0.41 g CO2–C m−2 d−1 for Urea*Inter-row, Urea*Row, Control*Row and Control*Inter-row, respectively. The mean cumulative CO2 emission during the evaluation period (211 days) was 765.14 ± 34.1 g CO2–C m−2. Fertilization factor had a significant effect on cumulative CO2 emissions (p < 0.05) (Fig. 3B), but the sampling site of the chambers or the fertilization-by-sampling site interaction did not have a significant effect (p > 0.05) (Fig. 3D). The urea generated higher cumulative CO2 emissions than the control (810.2 ± 38.1 vs. 640.4 ± 89.7 g CO2–C m−2, respectively).
Dynamics of emissions and cumulative emissions of CO2, considering the fertilization factor (A and B, respectively) and sampling site factor (C and D, respectively) for the evaluated growing season of sugarcane in the Cauca River valley, Colombia. Black (continuous) and blue (dotted) vertical lines indicate irrigation and nitrogen fertilization applications, respectively. Bars represent standard errors
N 2 O Emissions
The N2O emissions had a gap similar to that of the CO2 flux (Fig. 4A, C). However, the daily mean N2O emissions had no significant differences between fertilizer treatments, sampling site, or their interaction (p > 0.05). In fact, there was important variability in the N2O emissions: approximately 385 and 363% for fertilized and unfertilized treatments, respectively. The daily mean N2O emission of urea and control was 1.52 ± 0.77 and 0.99 ± 0.05 mg N m−2 d−1, respectively. The dinamic of N2O emissions showed two emission peaks: at 14 days after the first fertilizer application and two days after the second fertilizer application (Day 55) (Fig. 4A, C). The time (random effect) only explains 30.1% of the random variance of the model. The mean cumulative N2O emission during the evaluation period (211 days) was 125.4 ± 22.6 mg N2O–N m−2. The factors fertilization, sampling site, and their interaction had no significant effect (p > 0.05) (Fig. 4B, D). Although there was no significant effect (p = 0.32), urea tended to produce higher cumulative N2O emissions than the control (140.7 ± 27.5 and 68.2 ± 22.7 mg N2O–N m−2, respectively).
Dynamics of emissions and cumulative emissions of N2O, considering the fertilization factor (A and B, respectively) and sampling site factor (C and D, respectively) for the evaluated growing season of sugarcane in the Cauca River valley, Colombia. Black (continuous) and blue (dotted) vertical lines indicate N fertilization and irrigation applications, respectively. Bars represent standard errors
Discussion
Despite there is valuable information regarding GHG emissions in sugarcane, this manuscript reports the first GHG emission data obtained in the field for a typical sugarcane agroecosystem in the Cauca River valley, Colombia. It is important to note that in this work the uncertainty associated with the interruption of measurements (see Material and Methods section) was partially addressed through linear interpolation of data. Although this method has some shortcomings (Levy et al. 2017), the overall uncertainty generated on the effects of N fertilization or chamber location could be considered negligible, since the interruption began 40 days after the last N fertilization, outside the critical period caused by N application (Smith and Dobbie 2001; Reeves and Wang 2015; Ferrari Machado et al. 2019).
Our results showed significant CO2 and N2O emission rates during the crop cycle. In fact, CO2 emissions ranged between about 1.98 ± 0.25 and 243.9 ± 15.87 g CO2–C m−2 d−1 over the entire experimental period. These emissions are close to those informed by others studies for sugarcane under tropical climate (La Scala et al. 2006; Silva-Olaya et al. 2013; Farhate et al. 2019). However, they were higher than those reported by Vasconcelos et al. (2018) for a Typic Acrudox, this difference could be associated with the high content of organic matter in our conditions, which could promote more decomposition and CO2 production. In the same way, N2O emissions found in this study (between about 0.09 and 33.9 mg N2O–N m−2 d−1) were similar to those reported by Bolfarini et al. (2018) for an oxisol in Sorocaba, Brazil (0.6–22.8 mg N2O–N m−2 d−1); but lower than those found in Piracicaba, Brazil (≤ 0.7 mg N2O–N m−2 d−1) by Vasconcelos et al. (2018).
Concerning the effect of N fertilization on GHG emissions, we found that the peaks in emissions of N2O and CO2 following urea application events were expected and in agreement with the literature (Dattamudi et al. 2016; Tamale, van Straaten, et al., 2021; Vasconcelos et al. 2022). For our conditions, N fertilization increased CO2 emissions by 24.6% with respect to unfertilized treatment. We attribute the significant difference to the reaction and extra CO2 release of the carbonyl group of urea in soil (De Klein et al. 2006). In addition, the supply of N most likely enhanced microbial activity since it could support the required amino acid synthesis (Tian et al. 2015; R. Liu et al. 2017), which ultimately resulted in higher CO2 emissions. Our results agree with those of Dattamudi et al. (2019) who found higher CO2 emissions in sugarcane when it was fertilized with N; but contrast with those found for a subtropical sugarcane agroecosystem (Chalco Vera and Acreche 2018). In this last case, the soil had a much lower pH (5.9) than our condition (8.4), which probably decreased CO2 emissions due to a reduction in urea hydrolisis (Cabrera et al. 1991).
With respect to N2O emissions, this study quantified the influence of N fertilization for the first time for sugarcane soils in Colombia, where, until now, no previous data was available. Nitrogen fertilization tended to increase (106%) cumulative N2O emissions in relation with unfertilized treatment. This could be attributed to an increase in soil inorganic N availability which enhanced nitrification and denitrification processes (Denmead et al. 2010; Signor et al. 2013; da Silva et al. 2014; Neto et al. 2016; W. J. Wang et al. 2016). However, our research did not address soil variables to understand the mechanisms that control GHG emissions in the soil. Therefore, this will be mandatory to better explain the effects of sugarcane management practices on GHG emissions. Moreover, we highlight the need to determine and validate the variables behind the soil ECa that explain its influence on GHG emissions.
On the other hand, our results showed that the location of the chambers had a significant effect on N2O emissions. The importance of chamber location in the crop has also been demonstrated by Allen et al. (2010) and Westermann et al. (2021), and it is key for monitoring N2O emission peaks generated by fertilizer application (Williams et al. 1999; Tamale et al. 2021a, b). Thus, this study suggests that the allocation of chambers in the inter-row site could not be avoided to reduce the cost of intensive gas sampling when the specific objective of testing N fertilization strategies to mitigate N2O emissions is addressed.
Conclusions
Greenhouse gas emissions from the sugarcane soil system in the Cauca River valley were closely modified by N fertilization, the sampling site and their interaction. This work demonstrates the importance of considering these factors when representative GHG quantification is targeted. Nitrogen fertilization was an important management practice that increased N2O and CO2 emissions, which until now had not been quantified in Colombia. This shows the necessity to address new studies assessing mitigating management practices focused on N fertilization alternatives. This paper contributes to our understanding of the dynamics of CO2 and N2O emissions from sugarcane soils in Colombia. However, more experiments should be performed to analyze in more detail the effects of soil conditions on GHG emissions.
References
Allen, D.E., G. Kingston, H. Rennenberg, R.C. Dalal, and S. Schmidt. 2010. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agriculture, Ecosystems and Environment 136 (3–4): 209–217. https://doi.org/10.1016/j.agee.2009.11.002.
Bolfarini, C.B., S. Filoso, L.M. Pitombo, H. Cantarella, R. Rossetto, L.A. Martinelli, and J. B do Carmo. 2018. Impacts of sugarcane agriculture expansion over low-intensity cattle ranch pasture in Brazil on greenhouse gases. Journal of Environmental Management 206: 980–988. https://doi.org/10.1016/j.jenvman.2017.11.085.
Butterbach-Bahl, K., E.M. Baggs, M. Dannenmann, R. Kiese, and S. Zechmeister-Boltenstern. 2013. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society b: Biological Sciences 368 (1621): 20130122. https://doi.org/10.1098/rstb.2013.0122.
Cabrera, M.L., D.E. Kissel, and B.R. Bock. 1991. Urea hydrolysis in soil: effects of urea concentration and soil pH. Soil Biology and Biochemistry 23 (12): 1121–1124. https://doi.org/10.1016/0038-0717(91)90023-D.
Carbonell, J.G., and C.A. Osorio. 2010. Characterization of different areas with maximum potential productivity planted with sugarcane in the cauca river valley (colombia). International Symposium on Voronoi Diagrams in Science and Engineering 2010: 266–272. https://doi.org/10.1109/ISVD.2010.37.
Chalco Vera, J., A. Valeiro, G. Posse, and M.M. Acreche. 2017. To burn or not to burn: the question of straw burning and nitrogen fertilization effect on nitrous oxide emissions in sugarcane. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2017.02.172.
Chalco Vera, J., and M.M. Acreche. 2018. Towards a baseline for reducing the carbon budget in sugarcane: three years of carbon dioxide and methane emissions quantification. Agriculture, Ecosystems and Environment 267: 156–164. https://doi.org/10.1016/j.agee.2018.08.022.
Chalco Vera, J., R.N. Curti, and M.M. Acreche. 2020. Integrating critical values of soil drivers for mitigating GHGs: an assessment in a sugarcane cropping system. Science of the Total Environment 704: 135420. https://doi.org/10.1016/j.scitotenv.2019.135420.
Chalco Vera, J., R. Portocarrero, G. Piñeiro, and M.M. Acreche. 2022. Increases in nitrogen use efficiency decrease nitrous oxide emissions but can penalize yield in sugarcane. Nutrient Cycling in Agroecosystems 122 (1): 41–57. https://doi.org/10.1007/s10705-021-10180-3.
da Silva, D., A.C.R. da Lessa, S.A.C. de Sant’Anna, R.M. Boddey, S. Urquiaga, and B.J.R. Alves. 2014. Nitrous oxide emission and ammonia volatilization induced by vinasse and N fertilizer application in a sugarcane crop at Rio de Janeiro. Brazil. Nutrient Cycling in Agroecosystems 98 (1): 41–55. https://doi.org/10.1007/s10705-013-9594-5.
Dattamudi, S., J.J. Wang, S.K. Dodla, A. Arceneaux, and H.P. Viator. 2016. Effect of nitrogen fertilization and residue management practices on ammonia emissions from subtropical sugarcane production. Atmospheric Environment 139: 122–130. https://doi.org/10.1016/j.atmosenv.2016.05.035.
Dattamudi, S., J.J. Wang, S.K. Dodla, H.P. Viator, R. DeLaune, A. Hiscox, M. Darapuneni, C. Jeong, and P. Colyer. 2019. Greenhouse gas emissions as influenced by nitrogen fertilization and harvest residue management in sugarcane production. Agrosystems, Geosciences and Environment 2 (1): 190014. https://doi.org/10.2134/age2019.03.0014.
Denmead, O.T., B.C.T. Macdonald, G. Bryant, T. Naylor, S. Wilson, D.W.T. Griffith, W.J. Wang, B. Salter, I. White, and P.W. Moody. 2010. Emissions of methane and nitrous oxide from Australian sugarcane soils. Agricultural and Forest Meteorology 150 (6): 748–756. https://doi.org/10.1016/j.agrformet.2009.06.018.
de Klein, C., Harvey, M., Clough, T., Rochette, P., Kelliher, F., Grace, P., Venterea, V., Alfaro, M, and Chadwic, D. 2012. Nitrous oxide chamber methodology guidelines. Global Research Alliance on Agricultural Greenhouse Gase.
GC-2014. (2020). https://www.shimadzu.com/an/products/gas-chromatography/gas-chromatograph/gc-2014/index.html.
Geoportal IGAC. (2020). https://www.igac.gov.co/.
IDEAM. (2020). Geoportal. http://dhime.ideam.gov.co/atencionciudadano/.
FAO. (2021). FAOStat. https://www.fao.org/faostat/es/#data/QCL
Westermann, M., R. Brackin, N. Robinson, M. Salazar Cajas, S. Buckley, T. Bailey, M. Redding, J. Kochanek, J. Hill, S. Guillou, J.C.M. Freitas, W. Wang, C. Pratt, R. Fujinuma, and S. Schmidt. 2021. Organic wastes amended with sorbents reduce N2O emissions from sugarcane cropping. Environments. https://doi.org/10.3390/environments8080078.
Farhate, C.V.V., Z.M. de Souza, N. La Scala Jr, A.C.M. de Sousa, A.P.G. Santos, and J.L.N. Carvalho. 2019. Soil tillage and cover crop on soil CO2 emissions from sugarcane fields. Soil Use and Management 35 (2): 273–282. https://doi.org/10.1111/sum.12479.
Ferrari Machado, P.V., C. Wagner-Riddle, R. MacTavish, P.R. Voroney, and T.W. Bruulsema. 2019. Diurnal variation and sampling frequency effects on nitrous oxide emissions following nitrogen fertilization and spring-thaw events. Soil Science Society of America Journal 83 (3): 743–750. https://doi.org/10.2136/sssaj2018.10.0365.
Hénault, C., A. Grossel, B. Mary, M. Roussel, and J. Léonard. 2012. Nitrous oxide emission by agricultural soils: a review of spatial and temporal variability for mitigation. Pedosphere 22 (4): 426–433. https://doi.org/10.1016/S1002-0160(12)60029-0.
Johnson, C.K., K.M. Eskridge, and D.L. Corwin. 2005. Apparent soil electrical conductivity: applications for designing and evaluating field-scale experiments. Computers and Electronics in Agriculture 46 (1): 181–202. https://doi.org/10.1016/j.compag.2004.12.001.
De Klein, C., Novoa, R., Ogle, S., Smith, K., Rochette, P., Wirth, T., McConkey, B., Mosier, A. and Rypdal. K. 2006. Chapter 11 N2O emissions from managed soils, and CO2 emissions from lime and urea application. In: 2006 IPCC Guidelines for National Greenhouse Gas Inventaries: Vol. 4: Agriculture, Forestery and Other Land Use.
La Scala, N., D. Bolonhezi, and G.T. Pereira. 2006. Short-term soil CO2 emission after conventional and reduced tillage of a no-till sugar cane area in southern Brazil. Soil and Tillage Research 91 (1): 244–248. https://doi.org/10.1016/j.still.2005.11.012.
Levy, P.E., N. Cowan, M. van Oijen, D. Famulari, J. Drewer, and U. Skiba. 2017. Estimation of cumulative fluxes of nitrous oxide: uncertainty in temporal upscaling and emission factors. European Journal of Soil Science 68 (4): 400–411. https://doi.org/10.1111/ejss.12432.
Lisboa, C.C., K. Butterbach-Bahl, M. Mauder, and R. Kiese. 2011. Bioethanol production from sugarcane and emissions of greenhouse gases - known and unknowns: GHG emissions and bioethanol from sugarcane. GCB Bioenergy 3 (4): 277–292. https://doi.org/10.1111/j.1757-1707.2011.01095.x.
Liu, R., H.L. Hayden, H. Hu, J. He, H. Suter, and D. Chen. 2017. Effects of the nitrification inhibitor acetylene on nitrous oxide emissions and ammonia-oxidizing microorganisms of different agricultural soils under laboratory incubation conditions. Applied Soil Ecology 119: 80–90. https://doi.org/10.1016/j.apsoil.2017.05.034.
MinAgricultura. (2021). Evaluaciones Agropecuarias—EVA y Anuario Estadístico del Sector Agropecuario. Agronet. https://www.agronet.gov.co/estadistica/paginas/home.aspx?cod=59.
Neto, M.S., M.V. Galdos, B.J. Feigl, C.E.P. Cerri, and C.C. Cerri. 2016. Direct N2O emission factors for synthetic N-fertilizer and organic residues applied on sugarcane for bioethanol production in Central-Southern Brazil. GCB Bioenergy 8 (2): 269–280. https://doi.org/10.1111/gcbb.12251.
Norman, J.M., C.J. Kucharik, S.T. Gower, D.D. Baldocchi, P.M. Crill, M. Rayment, K. Savage, and R.G. Striegl. 1997. A comparison of six methods for measuring soil-surface carbon dioxide fluxes. Journal of Geophysical Research: Atmospheres 102 (D24): 28771–28777. https://doi.org/10.1029/97JD01440.
OECD-FAO. 2021. OECD-FAO agricultural outlook 2021–2030. OECD. https://doi.org/10.1787/19428846-en.
Panosso, A.R., J. Marques, G.T. Pereira, and N. La Scala. 2009. Spatial and temporal variability of soil CO2 emission in a sugarcane area under green and slash-and-burn managements. Soil and Tillage Research 105 (2): 275–282. https://doi.org/10.1016/j.still.2009.09.008.
Parkin, T.B., and T.C. Kaspar. 2003. Temperature controls on diurnal carbon dioxide flux. Soil Science Society of America Journal 67 (6): 1763–1772. https://doi.org/10.2136/sssaj2003.1763.
Parkin, T.B., and T.C. Kaspar. 2006. Nitrous oxide emissions from corn-soybean systems in the midwest. Journal of Environmental Quality 35 (4): 1496–1506. https://doi.org/10.2134/jeq2005.0183.
Parkin, T.B., R.T. Venterea, and S.K. Hargreaves. 2012. Calculating the detection limits of chamber-based soil greenhouse gas flux measurements. Journal of Environmental Quality 41 (3): 705–715. https://doi.org/10.2134/jeq2011.0394.
Pitombo, L.M., H. Cantarella, A.P.C. Packer, N.P. Ramos, and J. B. do Carmo. 2017. Straw preservation reduced total N2O emissions from a sugarcane field. Soil Use and Management 33 (4): 583–594. https://doi.org/10.1111/sum.12384.
Pulido, A. D., Turriago, J. D., Jiménez, R., Torres, C. F., Rojas, A., Chaparro, N., Ortiz, E. Y., Granados, S., Rodríguez, J., Berrío, V., Figueroa, I. C., Bohórquez, Á. V., Rojas, S. and López, J. A. 2016. Inventario nacional y departamental de Gases Efecto Invernadero–Colombia (Tercera Comunicación Nacional de Cambio Climático). IDEAM, PNUD, MADS, DNP, CANCILLERÍA.
QGIS.org. 2020. QGIS Geographic Information System (3.10.5) [Computer software]. QGIS Association.
Reeves, S., and W. Wang. 2015. Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system. Science of the Total Environment 530–531: 219–226. https://doi.org/10.1016/j.scitotenv.2015.05.117.
Shaffer, J.P. 2002. Multiplicity, directional (Type III) errors, and the Null Hypothesis. Psychological Methods 7 (3): 356–369. https://doi.org/10.1037/1082-989X.7.3.356.
Signor, D., C.E.P. Cerri, and R. Conant. 2013. N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environmental Research Letters 8 (1): 015013. https://doi.org/10.1088/1748-9326/8/1/015013.
Silva-Olaya, A.M., C.E.P. Cerri, N. La Scala Jr, C.T.S. Dias, and C.C. Cerri. 2013. Carbon dioxide emissions under different soil tillage systems in mechanically harvested sugarcane. Environmental Research Letters 8 (1): 015014. https://doi.org/10.1088/1748-9326/8/1/015014.
Smith, K.A., and K.E. Dobbie. 2001. The impact of sampling frequency and sampling times on chamber-based measurements of N2O emissions from fertilized soils. Global Change Biology 7 (8): 933–945. https://doi.org/10.1046/j.1354-1013.2001.00450.x.
Tamale, J., R. Hüppi, M. Griepentrog, L.F. Turyagyenda, M. Barthel, S. Doetterl, P. Fiener, and O. van Straaten. 2021a. Nutrient limitations regulate soil greenhouse gas fluxes from tropical forests: evidence from an ecosystem-scale nutrient manipulation experiment in uganda. The Soil 7 (2): 433–451. https://doi.org/10.5194/soil-7-433-2021.
Tamale, J., O. van Straaten, R. Hüppi, L.F. Turyagyenda, P. Fiener, and S. Doetterl. 2021b. Soil Greenhouse Gas Fluxes following Conversion of Tropical Forests to Fertilizer-Based Sugarcane Systems in Northwestern Uganda. Agriculture, Ecosystems & Environment. https://doi.org/10.2139/ssrn.3967094.
Thorburn, P.J., J.S. Biggs, A.J. Webster, and I.M. Biggs. 2011. An improved way to determine nitrogen fertiliser requirements of sugarcane crops to meet global environmental challenges. Plant and Soil 339 (1): 51–67. https://doi.org/10.1007/s11104-010-0406-2.
Tian, Z., J.J. Wang, S. Liu, Z. Zhang, S.K. Dodla, and G. Myers. 2015. Application effects of coated urea and urease and nitrification inhibitors on ammonia and greenhouse gas emissions from a subtropical cotton field of the Mississippi delta region. Science of the Total Environment 533: 329–338. https://doi.org/10.1016/j.scitotenv.2015.06.147.
Vargas, V.P., H. Cantarella, A.A. Martins, J.R. Soares, and de C. A. Andrade. 2014. Sugarcane crop residue increases N2O and CO2 emissions under high soil moisture conditions. Sugar Tech 6: 174–179.
Vasconcelos, A.L.S., M.R. Cherubin, B.J. Feigl, C.E.P. Cerri, M.R. Gmach, and M. Siqueira-Neto. 2018. Greenhouse gas emission responses to sugarcane straw removal. Biomass and Bioenergy 113: 15–21. https://doi.org/10.1016/j.biombioe.2018.03.002.
Vasconcelos, A.L.S., M.R. Cherubin, C.E.P. Cerri, B.J. Feigl, A.F. Borja Reis, and M. Siqueira-Neto. 2022. Sugarcane residue and N-fertilization effects on soil GHG emissions in south-central. Brazil. Biomass and Bioenergy 158: 106342. https://doi.org/10.1016/j.biombioe.2022.106342.
Venterea, R.T., S.O. Petersen, C.A.M. de Klein, A.R. Pedersen, A.D.L. Noble, R.M. Rees, J.D. Gamble, and T.B. Parkin. 2020. Global Research Alliance N2O chamber methodology guidelines: flux calculations. Journal of Environmental Quality 49 (5): 1141–1155. https://doi.org/10.1002/jeq2.20118.
Viveros Valens, C. A. 2018. Características agronómicas y de productividad de la variedad Cenicaña Colombia (CC) 01–1940. Centro de Investigación de la Caña de Azúcar de Colombia.
Wang, W.J., S.H. Reeves, B. Salter, P.W. Moody, and R.C. Dalal. 2016. Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia. Agriculture, Ecosystems and Environment 216: 137–146. https://doi.org/10.1016/j.agee.2015.09.035.
Williams, D.. Ll.., P. Ineson, and P.A. Coward. 1999. Temporal variations in nitrous oxide fluxes from urine-affected grassland. Soil Biology and Biochemistry 31 (5): 779–788. https://doi.org/10.1016/S0038-0717(98)00186-2.
Acknowledgements
The authors would like to thank the Universidad de los Llanos (Unillanos), The Alliance of Bioversity International and CIAT, the Centro de Investigación de la Caña de Azúcar de Colombia (CENICAÑA), the Pontificia Universidad Javeriana de Cali. This work has been funded by the Program "ÓMICAS: In-silico Multiscale Optimization of Sustainable Agricultural Crops (Infrastructure and validation in Rice and Sugarcane)", sponsored within the Colombia Scientific Ecosystem, formed by the World Bank, Ministry of Science, Technology, and Innovation (Minciencias), Icetex, Ministry of Education and Ministry of Industry and Tourism, Project ID: FP44842-217-2018.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Valencia-Molina, M.C., Chalco Vera, J., Loaiza, S. et al. Carbon Dioxide and Nitrous Oxide Emissions from a Typical Sugarcane Soil in the Cauca River Valley, Colombia. Sugar Tech 26, 171–179 (2024). https://doi.org/10.1007/s12355-023-01328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-023-01328-2