Abstract
Background
Angiogenesis has increasingly been a target for imaging and treatment over the last decade. The integrin αvβ3 is highly expressed in cells during angiogenesis and are therefore a promising target for imaging. In this study, we aimed to investigate the PET tracer [68Ga]Ga-RGD as a marker of angiogenesis following MI and its ability to predict cardiac functional parameters.
Methods
First, the real-time interaction between [68Ga]Ga-RGD and integrin αvβ3 was investigated using surface plasmon resonance (SPR). Second, an animal study was performed to investigate the [68Ga]Ga-RGD uptake in the infarcted area after one and four weeks following MI in a rat model (MI = 68, sham surgery = 36). Finally, the specificity of the [68Ga]Ga-RGD tracer was evaluated ex vivo using histology, autoradiography, gamma counting and flow cytometry.
Results
SPR showed that [68Ga]Ga-RGD has a high affinity for integrin αvβ3, forming a strong and stable binding. PET/CT showed a significantly higher uptake of [68Ga]Ga-RGD in the infarcted area compared to sham one week (p < 0.001) and four weeks (p < 0.001) after MI. The uptake of [68Ga]Ga-RGD after one week correlated to end diastolic volume (r = 0.74, p < 0.001) and ejection fraction (r = − 0.71, p < 0.001) after four weeks.
Conclusion
This study demonstrates that [68Ga]Ga-RGD has a high affinity for integrin αvβ3, which enables the evaluation of angiogenesis and remodeling. The [68Ga]Ga-RGD uptake after one week indicates that [68Ga]Ga-RGD may be used as an early predictor of cardiac functional parameters and possible development of heart failure after MI. These encouraging data supports the clinical translation and future use in MI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse cardiac remodeling after myocardial infarction (MI) is a process of structural and functional changes which could lead to heart failure. Remodeling of the myocardium occurs as a response to inadequate repair after an MI.1,2,3 The repair of the myocardium after MI consists of three phases: the acute, the proliferation and scar maturation phase. During the proliferation phase, endothelial cells proliferate and infiltrate the infarcted area, leading to the formation of a dense microvascular network, which supplies oxygen and nutrients to the infarcted area.4,5 This process is known as angiogenesis and is essential to repair after MI.6 The third phase is the maturation phase, in which most of the myofibroblasts transition to a phenotype that promotes scar maturation.3,7 Angiogenesis peaks seven days after MI and slowly decreases over the next 14 to 28 days.8 Since angiogenesis is vital to repair, the targeting and stimulation of angiogenesis has been of clinical interest for many years.
To develop and optimize treatment that promotes angiogenesis, it is crucial to establish a non-invasive method for the real-time monitoring of angiogenesis. Positron emission tomography (PET) is a modality with high sensitivity and acceptable resolution that enables continuous in vivo monitoring of angiogenesis in human subjects. The integrin αvβ3 has been extensively studied and the highest expression of this integrin has been found in activated endothelial cells undergoing angiogenesis.9,10,11 The integrin αvβ3 is expressed at low levels in normal healthy tissue like intestinal, vascular and smooth muscle cells.12 Other cell types with expression of integrin αvβ3 is bone resorbing osteoclasts, activated macrophages, angiogenic endothelial cells and migrating smooth muscle cells.13 The tripeptide motif Arg-Gly-Asp (RGD) is a specific ligand to αvβ3 and has been the major peptide used in the molecular imaging of αvβ3, since it is recognized by the α-subunit of the integrin14,15 on the endothelial cell. Several different RGD-based PET tracers have previously been used for imaging integrin, primarily in animal studies. The tracers differ in characteristics like linkers, chelators, radionuclides.16,17,18.
The aim of this study was to investigate the emerging PET radiotracer [68Ga]Ga-NODAGA-E[(cRGDyK)]2 ([68Ga]Ga-RGD), as a marker of angiogenesis and its potential use in predicting outcome following MI. This was done by (a) evaluating the binding between αvβ3 and [68Ga]Ga-RGD using surface plasmon resonance (SPR), (b) in vivo animal experiment imaging angiogenesis in a myocardial infarction rodent model using [68Ga]Ga-RGD PET/CT and (c) ex vivo evaluation to confirm infarction (histology), cell distribution (flow cytometry) and verify tracer accumulation using autoradiography and gamma counting.
Methods
Study design
Binding kinetics
The assessment of real-time biomolecular interaction was performed using a Biacore X100 (Biacore, Uppsala, Sweden), with the determination of binding kinetics of [68Ga]Ga-RGD and integrin αvβ3. To evaluate the assay development and results, vitronectin and fibronectin was used as comparison.
Ethical statement
The Danish Animal Experiments Inspectorate approved experimental protocols (Permit No. 2016-15-0201-00920). All animal procedures performed are in accordance with the guidelines in Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Animal experiment
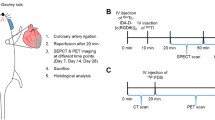
104 animals were included and underwent open chest surgery. The rats were randomly assigned to either sham or LAD ligation to induce an experimental myocardial infarction in a 1:3 ratio. One week after operation, the surviving rats (n = 88) were PET/CT scanned with 2-deoxy-2-[18F]fluoro-D-glucose (2-[18F]FDG) and the following day [68Ga]Ga-RGD. After the [68Ga]Ga-RGD scan 52 of the rats were euthanized and the excised heart used for flow cytometry or autoradiography and histology. Four weeks after the operation, a follow-up scan of 2-[18F]FDG and [68Ga]Ga-RGD were performed (n = 36). Following [68Ga]Ga-RGD PET/CT scan the animals were euthanized, and myocardial tissue used for either flow cytometry or histology and autoradiography (flowchart depicted in Figure 1).
Workflow of the study. At first, the rats underwent either sham or permanent LAD-occlusion. The rats were PET/CT scanned after 1 week and follow-up scanned 4 weeks after operation. LAD, left anterior descending coronary artery; [68Ga]Ga-RGD, [68Ga]Ga-NODAGA-E[(cRGDyK)]2; PET, positron emission tomography; CT, computed tomography
See supplementary for full description of methods.
Results
Integrin αvβ3, SPR experiments by single cycle kinetics
The binding between integrin αvβ3 and [68Ga]Ga-RGD showed a strong and stable interaction in the presence of Mg2+ and an even slower dissociation rate constant in the presence of Mg2+ and Mn2+ (Figure 2).
Integrin αvβ3 is the natural receptor for vitronectin, and SPR showed likewise a strong interaction with vitronectin in the presence of Mg2+ with no change in the presence of Mg2+ and Mn2+ and thereby validate the SPR analyses of integrin αvβ3 and [68Ga]Ga-RGD.
Fibronectin showed steady state interaction with integrin αvβ3. (supplementary figure S1 and table S1).
The glycoprotein CD4 + was used as a control ligand. This would demonstrate if [68Ga]Ga-RGD binds unspecific in the present of a protein on the CM5 chip. There was no interaction between CD4 + and [68Ga]Ga-RGD, indicating that [68Ga]Ga-RGD does not have unspecific binding in the presence of a protein.
Animal experiments
Myocardial infarction extent
In 1 examination out of the total 124 (0.8%), 2-[18F]FDG-PET were of poor quality and excluded from the dataset(1 week: sham = 1). In the MI group, PET showed a lack of 2-[18F]FDG uptake in the anterolateral wall of the left ventricle corresponding to the infarcted area. As expected, the extent of the defect was higher in the MI group, compared to sham after one week (p < 0.001). After four weeks, the extent of the defect in the MI group was still larger than sham (p < 0.001). (Table 1).
In vivo [68Ga]Ga-RGD PET-uptake
PET-uptake measurements of [68Ga]Ga-RGD in %ID/g are shown in Table 1. The intraclass correlation coefficient for %ID/g of the anterior wall (0.84; 95% CI 0.75–0.9) and posterior wall (0.86; CI 95% 0.79–0,91) measurements were good.
One week after total chronic occlusion of the LAD, the MI group showed a significantly higher uptake of [68Ga]Ga-RGD by in vivo PET, compared to the sham group in the anterior wall of the left ventricle (LV) (Supplementary figure S4). After four weeks, the MI group still showed a significantly higher uptake of the [68Ga]Ga-RGD in the anterior wall, compared to sham animals. In the non-infarcted posterior LV wall, there was no difference in uptake of [68Ga]Ga-RGD after one and four weeks, when comparing the MI group with the sham group (Figure 3).
Representative PET/CT images of the same rat one and four weeks after surgery. The 2-[18F]FDG -PET shows a significant lack of tracer uptake in the anterolateral parts of the myocardium at one week and four weeks post-surgery. The [68Ga]Ga-RGD PET shows that the uptake corresponds to the area with a lack of tracer uptake on the 2-[18F]FDG-PET. SA, short axis view; VLA, vertical axis view; HLA, horizontal axis view. Other abbreviations as in previous figures
EDV and EF measured from gated 2-[18F]FDG-PET and correlation to [68Ga]Ga-RGD
ECG-gated 2-[18F]FDG-PET/CT was used to analyze EDV, ESV and EF. The MI groups had a significantly higher EDV after one week compared to the sham group (p = 0.015). After four weeks the EDV in the MI group had increases and was significantly higher than the sham, indicating a progression towards a heart failure phenotype in the MI group (Table 1). The MI group had a significantly lower EF, compared to sham after one week. After four weeks, the difference in EF was still evident. There was no difference in EF from one week to four-week scan between the groups (Table 1). There was a positive correlation between mean [68Ga]Ga-RGD uptake (%ID/g) in the anterior LV wall after one week and the four weeks end diastolic volume (r = 0.74, p < 0.001). A negative correlation was observed between max [68Ga]Ga-RGD uptake (%ID/g) at one week and EF at four weeks (r = -0.71, p < 0.001) (Figure 4).
[68Ga]Ga-RGD uptake in the anterior wall of the LV after one week (A) and four weeks (B). The uptake is higher after one week, but there is no difference between the MI groups between week one and week four. There is a positive correlation between EDV at four weeks and uptake of [68Ga]Ga-RGD after one week (C). There is a negative correlation between EF at four weeks and uptake of [68Ga]Ga-RGD after one week (D). EDV, end diastolic volume; EF, ejection fraction; FU, follow-up. Other abbreviations as in previous figures
In multiple regression analysis with EDV after four weeks, only %ID/g is significant in predicting EDV after four weeks (%ID/g p = 0.005, LVEF acute p = 0.305, FDG extent acute p = 0.127).
Uptake of [68Ga]Ga-RGD following myocardial infarction correlates with αv 1 expression on endothelial cells.
Cellular distribution of αvβ3 was evaluated in whole rat hearts by flow cytometry one and four weeks after MI. One week after MI there was an increase in the amount of immune cells and endothelial cells compared to the sham group (p = 0.0006 and 0.018, respectively) which four weeks after MI returned to the cellular composition to the levels of sham treated animals (p > 0.05 for all cell types; Figure 5A). The overall amount of cells positive for αvβ3 across cell types increased drastically 1 week after MI (p < 0.0001) and returned to sham levels 4 weeks after MI (p > 0.05; Figure 5B). Besides the changes in cellular composition of the heart and the overall increase in αv and β3 one week after MI, myocytes increased their expression of αv and β3 (p = 0.0016 and < 0.0001, respectively) while only β3 was increased on endothelial cells and immune cells (p < 0.0001 and = 0.0026, respectively). The increased expression of β3 returned to sham levels in all cell types four weeks after MI (p > 0.05) while αv had a statistically insignificant trend towards being slightly increased across cell types (Figure 5C).
Flow cytometry evaluation of heart after MI. Cellular composition of hearts (A). Positivity of αv and β3 of all cells in hearts (B). αv and β3 expression on endothelial cells (C), myocytes, and immune cells. Correlation of [68Ga]Ga-RGD uptake to αv on endothelial cells, myocytes and immune cells (D). n = 12 one week after MI, n = 12 four weeks after MI and n = 10 for sham. Statistical differences were determined using mixed-effect analyses with Dunnetts correction for multiple comparisons to sham in A–C and pearsons correlation in D. MI, myocardial infarction; %ID/g, % injected dose per gram tissue. Other abbreviations as in previous figures
Correlating the investigated cellular findings to the mean %ID/g [68Ga]Ga-RGD uptake revealed the best correlation for αv expression on endothelial cells (r = 0.52, P = 0.002) which was less apparent on immune cells and myocytes (Figure 5D) as well as other possible correlations (Supplementary Figure S2).
Histological analysis of the heart
The presence of an infarcted area in the myocardium was confirmed by histological evaluation of the heart. The infarcted area on HE stains showed regions with a lack of myocytes and many cells with atypical nuclei (Figure 6). This was more profound and transmural at four weeks compared to after one week. MT stains showed collagen accumulation (fibrosis) in the infarcted area, represented as blue staining. This was more delineated at four weeks compared to after one week. When measuring fibrosis density (percentage of total area) on MT-stained cross-sections of the heart (Figure 7 A,B), there was a significantly higher degree of fibrosis in the MI group, compared to sham after one week and after four weeks (sham: 1.1 ± 1.3; one week: 11.7 ± 10; four weeks: 9.1 ± 7.0, p = 0.047, Figure 7 D).
Masson’s trichrome staining shows a clear area of fibrosis compared to healthy tissue (A, B). The immunohistochemically staining for integrin β3 shows an accumulation in the vessel wall (C). The quantification of fibrotic tissue on MT shows a significant difference between sham and the MI groups (D). The extent of fibrosis is unchanged between week 1 and 4 in the MI group, indicating a stable and consistent ligation of the LAD
Immunohistochemically staining for the integrin β3 chain showed a stronger positivity for β3 in infarcted tissue compared to healthy tissue (Figure 7 C, red arrow), with the β3 being primarily present in the wall of the vessel.
[68Ga]Ga-RGD uptake after ischemia measured by autoradiography and gamma counting of the heart ex vivo
For autoradiography analysis, the sham group, showed no focal [68Ga]Ga-RGD accumulation in the myocardium, only homogeneous background activity in the myocardium. In the MI group, an increased focal [68Ga]Ga-RGD uptake was observed, corresponding to the infarcted area. This was evident both one week and four weeks after MI. Gamma counting showed a significantly higher %ID/g of [68Ga]Ga-RGD in the MI group, compared to the sham group. After one week, the mean %ID/g were 0.12 vs 0.068 (p < 0.001) and after four weeks 0.11 vs 0.063 %ID/g (p < 0.001). There was a significant correlation between gamma counting and autoradiography (r = 0.62, p < 0.001).
Discussion
The major finding of this study is that [68Ga]Ga-RGD PET-uptake in the infarcted area correlates with cardiac functional parameters at a later time point. More specifically, we found that the uptake of [68Ga]Ga-RGD in the infarcted area after one week correlated to the EDV and EF measured after four weeks. The [68Ga]Ga-RGD tracer used was recently developed by our group,19,20,21,22 and demonstrated in the present study, a stronger binding to αvβ3 than that of its natural ligand vitronectin. We also demonstrated that after a MI, endothelial cells express a higher level of αvβ3 after one week, declining after four weeks. The focal uptake of [68Ga]Ga-RGD on autoradiography was present in the infarcted area and not in healthy myocardium, correlating to measured [68Ga]Ga-RGD counts in the respective slices of the heart. In vivo imaging of angiogenesis using [68Ga]Ga-RGD with PET/CT showed a higher uptake of [68Ga]Ga-RGD in the infarcted area compared to sham after both one and four weeks.
Our study is not the first to image angiogenesis based on integrin αvβ3 targeted PET tracer in a rat model of acute MI. However, in this study, we present in vivo and ex vivo data that support that binding between integrin αvβ3 and [68Ga]Ga-RGD is better than previously for other RGD targeting PET tracers, e.g., 64Cu-NOTA-PEG4-cRGD, a cyclic RGD-peptide radiolabelled with 64Cu for PET imaging.16 This radiolabelled version has a different chelator (NOTA-PEG4), a different isotope (64Cu) and used a cyclic RGD-peptide rather than the dimeric RGD-peptide radiolabelled version used in our study (chelator: NODAGA, isotope: 68Ga). We previously showed that the dimeric [68Ga]Ga-NODAGA-E[(cRGDyK)]2 has a higher affinity towards integrin αvβ3 than the monomer.23 SPR analysis of 64Cu-NOTA-PEG4-cRGD and integrin αvβ3 showed the same KD as we present,16 but the dissociation rate is slower between our [68Ga]Ga-RGD and integrin αvβ3 than 64Cu-NOTA-PEG4-cRGD and integrin αvβ3. This could indicate a more stable complex between [68Ga]Ga-RGD and integrin αvβ3 than 64Cu-NOTA-PEG4-cRGD and integrin αvβ3.
SPR has previously been used to assess the binding between integrin αvβ3 and RGD.24 This study found that the binding between integrin αvβ3 and vitronectin was improved with the presence of Mn2+. Our SPR analysis of vitronectin and integrin αvβ3 showed the same level of affinity, which confirms the accuracy of our setup. We found that binding of [68Ga]Ga-RGD to integrin αvβ3 was 1000-fold stronger than vitronectin in the presence of Mn2+, suggesting that [68Ga]Ga-RGD binds specifically and with a stable complex to integrin αvβ3.
Flow cytometry analysis of the heart showed that the level of αvβ3 positive cells were significantly higher after one week in the MI group compared to the sham in the myocytes/myofibroblasts, immune cells, and endothelial cells. The literature describes αvβ3 to be involved with different actions of the different cell types, all which relates to myocardial wound healing, which is activation and migration of endothelial cells, infiltration and anti-inflammatory actions for immune cells,25 resistance to apoptosis for myocytes26 and matrix remodeling for myofibroblasts.27 The flow cytometry analysis supports the many different actions of αvβ3, since the levels of positive αvβ3 cells were present in myocytes, immune cells and endothelial cells. However, the best correlation was between RGD uptake and endothelial cells, which indicates that even though the integrin is present in other cell types relating to myocardial wound healing, the signal detected by RGD PET is mostly derived from angiogenesis.
The percentages of αvβ3 positive immune cells and endothelial cells both declined from one to four weeks after MI, indicating that the immunomodulation and formation of new blood vessels had declined. The decline in αvβ3 positive cells by flow cytometry was in our study paralleled by a decline in in vivo uptake of [68Ga]Ga-RGD.
In vivo PET imaging showed a significant increase in [68Ga]Ga-RGD uptake in the infarcted myocardium, compared to non-infarcted myocardium. This was evident both at one and four weeks after MI which correlated best with αv expression on endothelial cells, indicating that [68Ga]Ga-RGD is a promising PET tracer in detecting angiogenesis and remodeling. The first scan was performed after one week, to ensure that the initial inflammatory response following MI did not affect the [68Ga]Ga-RGD uptake. The follow-up scan was done after four weeks, to investigate the potential shift from angiogenesis to remodeling. Several other studies have shown angiogenesis imaged with other RGD PET tracers17,18,28,29,30 and three clinical trial has been performed.31,32,33 However, there are some inconsistency towards the conclusions of these studies. Sherif et al. performed a preclinical trial with permanent ligation of the LAD artery and F-galatco-RGD 1 week after MI. In this study a low uptake of RGD was associated with lower EF and higher EDV. Jenkins et al. showed that in 21 patient with ST-elevation myocardial infarction, high uptake of F-fluciclatide RGD was predicting regions of recovery.
It is established that left ventricular dilation is correlated with subsequent heart failure independently of risk factors and EF in humans.34 Our findings of a correlation between EDV at four weeks follow-up with [68Ga]Ga-RGD uptake one week after MI, indicate that [68Ga]Ga-RGD could be used as a non-invasive method to early identify patients at risk of developing dilated cardiomyopathy following a MI. The clinical implications of such a tool may be paramount. While it seems documented in our study that [68Ga]Ga-RGD is increased in the presence of MI with high sensitivity and specificity, further studies are needed to confirm the correlation between early uptake of [68Ga]Ga-RGD and subsequent adverse remodeling.
One of the great advantages of the [68Ga]Ga-RGD tracer used in the present study is the use of a generator produced radionuclide, which circumvents the need of an on-site cyclotron. The production of [68Ga]Ga-NODAGA-E[(cRGDyK)]2 is fast, simple, with a high yield, and the PET tracer is stable. Other RGD PET tracers, such as those labeled with 18F, need a cyclotron close by.
Study limitations
This study investigated permanent ligation of the LAD as a MI model. To assess the broader applicability of [68Ga]Ga-RGD studies in reperfusion models with transient occlusion of the LAD should be conducted in the future. A reperfusion model would be a more clinically relevant and translate better to investigate acute myocardial infarction in humans.
This study was not designed to investigate heart failure following MI. To establish if [68Ga]Ga-RGD uptake correlates with heart failure, the animals needed to be observed for a longer period of time.
In this study the functional cardiac parameters were EDV, ESV and EF. To assess the cardiac function in greater detail, magnetic resonance imaging or a perfusion tracer such as 13N-NH3 could be used to evaluate myocardial blood flow and myocardial flow reserve. However, 13N-NH3 requires an on-site cyclotron, making it difficult to examine in a rodent model.35
Conclusion
This study demonstrates that [68Ga]Ga-RGD has a high affinity for integrin αvβ3 which enables the evaluation of angiogenesis following an MI, using PET/CT. The in vivo RGD uptake after one week correlated to ejection fraction and end diastolic volume after four weeks, indicating that [68Ga]Ga-RGD may be used as an early predictor of cardiac functional parameters and possible development of heart failure after an MI. These encouraging data support a clinical translation.
New knowledge gained:
The PET tracer [68Ga]Ga-NODAGA-E[(cRGDyK)]2 forms a stable and strong binding to the integrin αvβ3 αvβ3, which enable the detection of angiogenesis. The angiogenic response after one week correlated to predictors of early onset of heart failure phenotype.
Abbreviations
- AHA:
-
American Heart Association
- EDV:
-
End diastolic volume
- EF:
-
Ejection fraction
- ESV:
-
End systolic volume
- HE:
-
Hematoxylin & Eosin
- KD:
-
Dissociation constant (M)
- koff :
-
Dissociation rate constant (s−1)
- kon :
-
Association rate constant (M−1 s−1)
- LAD:
-
Left anterior descending coronary artery
- MI:
-
Myocardial infarction
- MT:
-
Masson’s Trichrome
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- SPR:
-
Surface plasmon resonance
- %ID/g:
-
Percentage injected dose per gram
- 2-[18F]FDG:
-
2-Deoxy-2-[18F]fluoro-D-glucose
- [68Ga]Ga-RGD:
-
[68 Ga]Ga-NODAGA-E[(cRGDyK)]2
References
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262‐75.
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316‐25.
Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Vol. 365, Cell and Tissue Research. New York: Springer; 2016. p. 563‐81.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255‐65.
Tonnesen MG, Feng X, Clark RAF. Angiogenesis in wound healing. J Invest Dermatol Symposium Proc 2000;5:40‐6.
van der Laan AM, Piek JJ, van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol 2009;6:515‐23.
Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal 2006;8:1907‐39.
Sun M, Opavsky MA, Stewart DJ, Rabinovitch M, Dawood F, Wen WH, et al. Temporal response and localization of integrins β1 and β3 in the heart after myocardial infarction: Regulation by cytokines. Circulation 2003;107:1046‐52.
Eliceiri BP, Cheresh DA. The role of αv integrins during angiogenesis: Insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227‐30.
Verjans J, Wolters S, Laufer W, Schellings M, Lax M, Lovhaug D, et al. Early molecular imaging of interstitial changes in patients after myocardial infarction: Comparison with delayed contrast-enhanced magnetic resonance imaging. J Nucl Cardiol 2010;17:1065‐72.
Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 2004;113:1684‐91.
Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis. 2002;61:96‐9.
Eliceiri BP, Cheresh DA. Molecular medicine the role of av integrins during angiogenesis. Mol Med 1998;4:741‐50.
Smith JW, Cheresh DA. Integrin (alpha v beta 3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990;265:2168‐72.
Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell 1989;58:945‐53.
Hedhli J, Czerwinski A, Schuelke M, Płoska A, Sowinski P, La HL, et al. Synthesis, chemical characterization and multiscale biological evaluation of a dimeric-cRGD peptide for targeted imaging of α V β 3 integrin activity. Sci Rep 2017;7:3185.
Laitinen I, Notni J, Pohle K, Rudelius M, Farrell E, Nekolla SG, et al. Comparison of cyclic RGD peptides for αvβ3 integrin detection in a rat model of myocardial infarction. EJNMMI Res 2013;3:1‐9.
Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, et al. Assessment of v 3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res 2008;78:395‐403.
Rasmussen T, Follin B, Kastrup J, Brandt-Larsen M, Madsen J, Emil Christensen T, et al. Angiogenesis PET tracer uptake (68Ga-NODAGA-E[(cRGDyK)]2) in induced myocardial infarction in minipigs. Diagnostics 2016;6:26.
Clemmensen A, Hansen AE, Holst P, Schøier C, Bisgaard S, Johannesen HH, et al. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 PET and hyperpolarized [1-13C] pyruvate MRSI (hyperPET) in canine cancer patients: simultaneous imaging of angiogenesis and the Warburg effect. Eur J Nucl Med Mol Imaging 2021;48:395‐405.
Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg 2012;72:428‐36.
Rasmussen T, Follin B, Kastrup J, Brandt-Larsen M, Madsen J, Emil Christensen T, et al. Angiogenesis PET tracer uptake (68Ga-NODAGA-E[(cRGDyK)]2) in induced myocardial infarction and stromal cell treatment in minipigs. Diagnostics 2018;8:33.
Oxboel J, Brandt-Larsen M, Schjoeth-Eskesen C, Myschetzky R, El-Ali HH, Madsen J, et al. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl Med Biol 2014;41:259‐67.
Takagi J, Petre BM, Walz T, Springer TA. Global conformational earrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 2002;110:599‐611.
Ward PA. Inflammation and α vβ 3 integrin. Vol. 185, American Journal of Respiratory and Critical Care Medicine. American Thoracic Society; 2012. p. 5–6.
Wei L, Zhou Q, Tian H, Su Y, Fu GH, Sun T. Integrin β3 promotes cardiomyocyte proliferation and attenuates hypoxia-induced apoptosis via regulating the PTEN/akt/mTOR and ERK1/2 pathways. Int J Biol Sci 2020;16:644‐54.
Graf K, Neuss M, Stawowy P, Hsueh WA, Fleck E, Law RE. Angiotensin II and α(v)β3 integrin expression in rat neonatal cardiac fibroblasts. Hypertension 2000;35:978‐84.
Lee MS, Park HS, Lee BC, Jung JH, Yoo JS, Kim SE. Identification of angiogenesis rich-viable myocardium using RGD dimer based SPECT after myocardial infarction. Sci Rep 2016;10:6.
Eo JS, Paeng JC, Lee S, Lee YS, Jeong JM, Kang KW, et al. Angiogenesis imaging in myocardial infarction using 68Ga-NOTA-RGD PET. Coron Artery Dis 2013;24:303‐11.
Sherif HM, Saraste A, Nekolla SG, Weidl E, Reder S, Tapfer A, et al. Molecular imaging of early α vβ 3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med 2012;53:318‐23.
Makowski MR, Rischpler C, Ebersberger U, Keithahn A, Kasel M, Hoffmann E, et al. Multiparametric PET and MRI of myocardial damage after myocardial infarction: correlation of integrin αvβ3 expression and myocardial blood flow. Eur J Nucl Med Mol Imaging. 2020;48:1070‐80.
Sun Y, Zeng Y, Zhu Y, Feng F, Xu W, Wu C, et al. Application of 68Ga-PRGD2 PET/CT for ? v ? 3-integrin imaging of myocardial infarction and stroke. Theranostics 2014;4:778‐86.
Jenkins WSA, Vesey AT, Stirrat C, Connell M, Lucatelli C, Neale A, et al. Cardiac α V β 3 integrin expression following acute myocardial infarction in humans. Heart 2017;103:607‐15.
Yeboah J, Bluemke DA, Hundley WG, Rodriguez CJ, Lima JAC, Herrington DM. Left ventricular dilation and incident congestive heart failure in asymptomatic adults without cardiovascular disease: Multi-Ethnic Study of Atherosclerosis (MESA). J Card Fail 2014;20:905‐11.
Ghotbi AA, Clemmensen A, Kyhl K, Follin B, Hasbak P, Engstrøm T, et al. Rubidium-82 PET imaging is feasible in a rat myocardial infarction model. J Nucl Cardiol 2019;26:798‐809.
Funding
Open access funding provided by Royal Danish Library. The authors received funding from the European Union’s Horizon 2020 research and innovation program under grant agreements no. 670261 (ERC Advanced Grant) and 668532 (Click-It), the Lundbeck Foundation, the Novo Nordisk Foundation, the Innovation Fund Denmark, the Danish Cancer Society, Arvid Nilsson Foundation, the Neye Foundation, the Research Foundation of Rigshospitalet, the Danish National Research Foundation (grant 126), the Research Council of the Capital Region of Denmark, the Danish Health Authority, the John and Birthe Meyer Foundation and Research Council for Independent Research.
Author information
Authors and Affiliations
Contributions
SB participated in conception of the study as well as collection of the data, analysis, drafting the manuscript and final approval of the manuscript. JKJ, CEG, BF, JSM and AC contributed with data collection, analysis and revision of the manuscript. LRP, EC and CC contributed to data analysis and revision of the manuscript. TB, PH, RSR and AK contributed with conception and design of the study as well critical revision of the manuscript and final approval.
Corresponding author
Ethics declarations
Disclosure
A.K. is an inventor on patents covering the PET tracer used (EP3706809A1 and US16/762,873). No other potential conflicts of interest relevant to this article exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Rob deKemp, PhD.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bentsen, S., Jensen, J.K., Christensen, E. et al. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 angiogenesis PET following myocardial infarction in an experimental rat model predicts cardiac functional parameters and development of heart failure. J. Nucl. Cardiol. 30, 2073–2084 (2023). https://doi.org/10.1007/s12350-023-03265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-023-03265-9