Abstract
Background
Radionuclide ventriculography (RNVG) can be used to quantify mechanical dyssynchrony and may be a valuable adjunct in the assessment of heart failure with reduced ejection fraction (HFrEF). The study aims to investigate the effect of beta-blockers on mechanical dyssynchrony using novel RNVG phase parameters.
Methods
A retrospective study was carried out in a group of 98 patients with HFrEF. LVEF and dyssynchrony were assessed pre and post beta-blockade. Dyssynchrony was assessed using synchrony, entropy, phase standard deviation, approximate entropy, and sample entropy from planar RNVG phase images. Subgroups split by ischemic etiology were also investigated.
Results
An improvement in dyssynchrony and LVEF was measured six months post beta-blockade for both ischemic and non-ischemic groups.
Conclusions
A significant improvement in dyssynchrony and LVEF was measured post beta-blockade using novel measures of dyssynchrony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure affects approximately 1%-2% of the adult population in developed countries, increasing to over 10% for those > 70 years.1,2,3,4 Various treatments are available which aim to improve symptoms, morbidity, and mortality. However, identifying the underlying cause is crucial to determine the most appropriate treatment. This research focuses on non-valvular heart failure with reduced ejection fraction (HFrEF). In HFrEF, LVEF is known to be a good predictor of outcome, and is included in the decision criteria for many HFrEF treatments.5
There is some interest in measures of left ventricular dyssynchrony for patients with HFrEF. For example, there have been many studies published investigating imaging parameters as predictors to cardiac resynchronization therapy response, with varying degrees of success as summarized in the review by Hawkins et al.6 Despite promising results in single-center studies,7,8 the results have not been reproduced in larger multi-center trials,9 leaving many unanswered questions in this area.
Beta-blockade therapy is well established and currently recommended by the ESC guidelines as first-line treatment for patients in symptomatic HFrEF.5 Several large-scale clinical trials have demonstrated the benefits of beta-adrenoceptor blockers for heart failure patients, with a reduction in morbidity and mortality.10,11 It is known that post beta-blockade therapy, patients show significantly improved LVEF,12 but the effect of beta-blockade on cardiac dyssynchrony has not been widely investigated. There are several published studies that investigate the use of echo-derived dyssynchrony parameters, such as septal to lateral wall delay, for heart failure patients.13,14,15 However, the authors are not aware of any published studies investigating the effect of beta-blockade on dyssynchrony measured from radionuclide ventriculography (RNVG) imaging.
Dyssynchrony measures from RNVG phase analysis
RNVG phase parameters offer an alternative index for the quantification of ventricular dyssynchrony and may be a valuable adjunct in the assessment of patients with heart failure. Various measures to quantify dyssynchrony, including synchrony, entropy, approximate entropy (ApEn), and standard deviation of the phase histogram (phase SD), can be used to provide additional information from planar RNVG images with high reproducibility.16,17,18,19,20,21,22,23 Sample entropy (SampEn)24 is novel for this application and has not been previously investigated.
This work aims to investigate the effect of beta-blocker therapy on dyssynchrony, as assessed by planar RNVG phase images, for symptomatic HFrEF. Subgroups split by ischemic etiology will also be compared to determine if there is a difference in response.
Method
Study outline
A retrospective study was carried out for 98 heart failure patients who attended the department in 2005-2006. The inclusion criteria are defined in Table 1. All patients who were included had evidence of left ventricular systolic dysfunction, NYHA class II-IV, and were stabilized on standard HF treatment. Patients who had recent intervention, including CABG, PCI, CRT, and RV pacing, were excluded to ensure that any change in function would be secondary to beta-blocker and not intervention related. Those with atrial fibrillation or severe valve disease were excluded as these conditions can make the assessment of LV systolic function less reliable. None of the patients who were included had any intervention between the baseline and follow-up RNVG. No other heart failure medications were changed during this period.
All of the patients included had a planar RNVG and Thallium-201 myocardial perfusion (MPI) scan pre titration of beta-blocker and a repeat RNVG six months post beta-blocker. Patients were initially given 1.25 mg of Bisoprolol, with the dose increasing stepwise to 2.5 mg, 5 mg, 7.5 mg, and 10 mg at intervals of two weeks. Before each step increase, patients underwent clinical review. Each patient continued on their maximum tolerated dose of Bisoprolol. Patients who did not tolerate the prescribed beta-blockers and those who did not attend the second RNVG scan were excluded from this study. Of the 12 patients who were excluded, 8 patients did not tolerate beta-blocker, 3 patients did not attend for the second RNVG for unknown reasons, and 1 patient died before the second RNVG. After the exclusion criteria were applied, there were 86 patients remaining.
The patients were grouped depending on whether or not they had heart failure of ischemic etiology. Of this patient cohort, 54 were ischemic, and 32 were non-ischemic. A patient was categorized in the ischemic heart failure group if at least one of the following criteria was met:
-
(i)
A stenosis of more than 50% in at least one of the three major coronary arteries as assessed by coronary angiogram (where available)
-
(ii)
Previous MI or PCI
-
(iii)
Positive Thallium-201 MPI (defined by two experienced reporters).
Data acquisition and processing
All patients underwent planar RNVG imaging before and six months after beta-blockade. In-vivo labeling was performed using intravenous administration of pyrophosphate 20 minutes prior to injection of technetium-99m pertechnetate. The administered dose for each scan was 600 MBq (16.2 mCi). The gamma camera was positioned to achieve the best septal separation between the left and right ventricles. Imaging was acquired on an Optima gamma camera (GE Healthcare, Waukesha, WI) using list mode acquisition and processed using MAPS Link Medical 10000 software. The raw data were reconstructed into a 24 frame 64 × 64 matrix with the exclusion of heartbeats 10% greater than the mean inter-beat (R–R) interval. All data were reviewed to check heart rate, gating, image quality, and ensure adequate septal separation. The acquisition angle was recorded to ensure the same angle was used for the repeat scan.
The pre and post therapy images were anonymized and randomized before analysis. LVEF was calculated from the gated images by an experienced operator and reviewed by a second operator. To assess intra-operator variability each of the anonymized images were analyzed twice by the same operator, without reference to their first ROI. A manual single region of interest method was used following the standard clinical protocol at the time of the study. The single region of interest technique systematically underestimates the ejection fraction, but has good reproducibility. The locally established normal range for LVEF by this method is > 40% and the inter-observer variability is 3.1%.25
Phase analysis
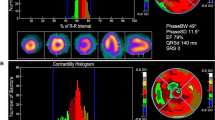
Phase images were created using a first-order harmonic fit of the time–activity curve for each pixel, representing the timing of ventricular contraction relative to the R wave of the ECG.26,27 The phase angle defines the point in the time–activity curve where the Fourier function reaches its peak, representing the onset of contraction. An example of a phase and amplitude image with the associated time–activity curve and phase histogram for a patient with normal ventricular contraction is shown in Figure 1. The R–R duration is measured in seconds but can be converted to degrees, where 360° represents the length of one cardiac cycle. Any areas of dyssynchronous contraction will appear as delays in the phase images and phase histogram.
In-house software was used to calculate synchrony, entropy, ApEn, SampEn, and phase SD from the RNVG phase images both pre and post beta-blockade. To calculate synchrony, each pixel within the ROI was defined by a vector where the length was defined as the amplitude (maximum change in counts), and the direction was defined as the phase angle. Synchrony is then defined as the vector sum of the pixel values divided by the scalar sum. A ventricle with completely synchronous contraction would have a synchrony value of 1 and a completely asynchronous contraction would have a synchrony value of 0. Entropy, as derived from Shannon information theory,28 was used as a measure of randomness in the phase histogram.16 A higher value of entropy indicates a more random contraction.
ApEn and SampEn were calculated using Eqs. 1 and 2, by considering the pixel values within the region of interest on the phase image as a data series. Both ApEn and SampEn calculate the probability that a series of pixels of length m from the phase image remains similar within a tolerance r at the next sequence in the data series. SampEn is a modification of ApEn described by Richman and Moorman,24 but unlike ApEn, SampEn displays relative consistency regardless of sequence length and tolerance values used, and it is independent of data length. ApEn is defined as
where N is the length of data, m is the sequence length, and r is the tolerance. \(C{_{i^{m}}}(r)\) is the conditional probability that when a sequence of pixels is within the tolerance, then the next sequence will also be within tolerance. SampEn is defined as
where \(B_i^m(r)\) is the number of sequences of length m that are within tolerance r, excluding self matches, and \(B^m(r)\) is the probability that two sequences of length m are similar.
The values of input parameters m and r significantly affect the results so they must be optimized for the application. Based on the previous optimization work, ApEn was calculated using sequence length m = 2 and tolerance r = 7 and SampEn was calculated using sequence length m = 2 and tolerance r = 423. To calculate ApEn and SampEn, each group of m = 2 pixels within the region of interest in the phase image was compared to every other group of m = 2 pixels within the region of interest. If they were similar within the defined tolerance r, it was counted as a match. This was carried out for every group of ‘m’ pixels then repeated with groups of ‘\(m+1\).’ Unlike synchrony and entropy, ApEn and SampEn take into account the similarity of adjacent pixel values. A higher value of ApEn and SampEn would indicate a more dyssynchronous contraction. Correlation with LVEF and intra-operator variability was assessed for these novel dyssynchrony parameters.
Statistical analysis
All data analysis and statistics were performed in R 3.6.3.29,30 Shapiro–Wilk’s test was used to check the normality for each parameter, and significance testing was performed, using the t-test or Wilcoxon rank-sum test, depending on the outcome of the univariate test of normality. For paired data, a paired two-sample t test or Wilcoxon signed-rank test was used. The chi-squared test was used to test the significance of categorical parameters. The correlation between the parameters was tested using Pearson’s correlation. A P value of < .05 was considered significant for all tests.
Results
At baseline, there was no significant difference in sex, NYHA class, presence of hypertension, diabetes or ICD between the ischemic and non-ischemic groups. However, there was a significant difference in age (P < .001) and baseline heart rate (P = .02) when comparing the ischemic (age = 69 ± 9, HR = 82 ± 14) and non-ischemic (age = 54 ± 16, HR = 88 ± 16) patients. The correlation coefficients between LVEF and each dyssynchrony parameter were calculated to be .538 (P < .001) for synchrony, − .780 (P < .001) for entropy, − .338 (P = .001) for ApEn and − .675 for SampEn (P < .001) and − .602 (P < .001) for phase SD.
Intra-operator variability results are shown in Table 2. All of the parameters tested had excellent intra-operator variability with correlation coefficients ranging from .991 to .997. The original data were not available to test inter-operator variability.
Comparison was made pre and post beta-blockade as summarized in Table 3. There was a significant improvement in all of the dyssynchrony parameters and LVEF measured post beta-blockade. The only parameter that did not significantly improve after beta-blockade was ApEn for the non-ischemic group. There was no significant difference in dyssynchrony between the ischemic and non-ischemic groups at baseline (P > .05). There was a weak relationship (correlation = .31, P = .004) between change in heart rate and change in LVEF post beta-blockade.
Discussion
The results suggest that beta-blockers improve dyssynchrony for HFrEF of both ischemic and non-ischemic etiology. As expected LVEF also improves post beta-blockade. The results are consistent with the studies by Kaya et al and Takemoto et al.15,31 Both studies used septal to lateral delay as measured by echo to assess dyssynchrony and did not include any patients with ischemic heart failure. Kaya et al. found that beta-blockade improved LV synchrony and LVEF for heart failure patients with idiopathic dilated cardiomyopathy and LV dyssynchrony. Taketmoto et al. also found that patients with a QRS <120ms and sinus rhythm experienced an improvement in both LVEF and dyssynchrony after beta-blocker therapy. The mechanism for this improvement is not fully understood. Improved dyssynchrony has been linked with improved survival, as shown in CRT studies. For example, a sub-study of the EchoCRT trial found that persistent or worsening dyssynchrony six months post CRT was associated with worse clinical outcomes, in particular, heart failure hospitalizations.32
While the effect of dyssynchrony and beta-blockers on survival would be of clinical interest, this cohort is too small to provide any meaningful results. Treatment for these patients would have varied, and some went on to have PCI, CABG, or heart transplant after the study. Previous attempts to create models to predict mortality for heart failure patients have had only moderate accuracy, and those trying to predict a combined endpoint of hospitalization or death had even poorer results.33,34
There are currently no studies using newer echo dyssynchrony parameters, such as global longitudinal strain, to investigate the effect of beta-blockers on cardiac dyssynchrony. This study is the only work to date assessing dyssynchrony for heart failure patients using RNVG phase parameters. Further work to investigate the inter-operator variability should also be carried out, but good intra and inter-operator variabilities have been previously demonstrated for synchrony, entropy, and phase standard deviation.16,18,21,35 Heart failure treatment may benefit from further investigation of mechanical dyssynchrony in larger trials.
Limitations
The results are from a single-center study, limited by a small patient sample with no control group. This study also assumes patient compliance with taking prescribed drugs. The authors also acknowledge the complexity in defining heart failure etiology. Some of the patients in the non-ischemic group may have mild ischemia.
Conclusion
An improvement in dyssynchrony and LVEF was measured six months post beta-blockade for both ischemic and non-ischemic heart failure patients using novel phase parameters. A larger study with data from multiple centers would be desirable to confirm the results of this study.
New Knowledge Gained
A significant improvement in dyssynchrony after beta-blockade therapy has been demonstrated for heart failure patients using novel measures of dyssynchrony from RNVG phase images.
Abbreviations
- LVEF:
-
Left ventricular ejection fraction
- RNVG:
-
Radionuclide ventriculography
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with reduced ejection fraction
- MI:
-
Myocardial Infarction
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass graft
- COPD:
-
Chronic obstructive pulmonary disease
- PFTs:
-
Pulmonary function tests
- ICD:
-
Implantable cardioverter defibrillator
- HR:
-
Heart rate
- ApEn:
-
Approximate entropy
- SampEn:
-
Sample entropy
- SD:
-
Standard deviation
- NYHA:
-
New York Heart Association
- ESC:
-
European Society of Cardiology
References
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93(9):1137-46.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194-202.
Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: The rotterdam study. Eur Heart J 2004;25(18):1614-19
Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, et al. Prevalence of chronic heart failure in southwestern europe: the EPICA study. Eur J Heart Fail 2002;4(4):531-9
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016;37(27):2129-200.
Hawkins NM, Petrie MC, MacDonald MR, Hogg KJ, McMurray JJ. Selecting patients for cardiac resynchronization therapy: Electrical or mechanical dyssynchrony? Eur Heart J 2006;27(11):1270-81.
Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, Van Erven L, Boersma E, et al. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol 2003;92(10):1238-40.
Yu C, Fung W, Lin H, Zhang Q, Sanderson JE, Lau C. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003;91(6):684-8.
Chung ES, Leon AR, Tavazzi L, Sun J, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to crt (PROSPECT) trial. Echocardiography 2008;2608:2616.
Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel J. Clinical effects of \(\beta \)-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 1998;98(12):1184-91.
Brophy JM, Joseph L, Rouleau JL. \(\beta \)-blockers in congestive heart failure: A Bayesian meta-analysis. Ann Internal Med 2001;134(7):550-60.
CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure the cardiac insufficiency bisoprolol study (CIBIS). Circulation 1994;90:1765-73.
Bader H, Garrigue S, Lafitte S, Reuter S, Jaïs P, Haïssaguerre M, et al. Intra-left ventricular electromechanical asynchrony: a new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 2004;43(2):248-56.
Cho G, Song J, Park W, Han S, Choi S, Doo Y, et al. Mechanical dyssynchrony assessed by tissue doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol 2005;46(12):2237-43.
Takemoto Y, Hozumi T, Sugioka K, Takagi Y, Matsumura Y, Yoshiyama M, et al. Beta-blocker therapy induces ventricular resynchronization in dilated cardiomyopathy with narrow QRS complex. J Am Coll Cardiol 49(7):2007;778-83.
O’Connell JW, Schreck C, Moles M, Badwar N, DeMarco T, Olgin J, et al. A unique method by which to quantitate synchrony with equilibrium radionuclide angiography. J Nucl Cardiol 2005;12(4):441-50.
Cullen J, Saleem A, Swindell R, Burt P, Moore C. Measurement of cardiac synchrony using approximate entropy applied to nuclear medicine scans. Biomed. Signal Process. Control 2010;5(1):32-6.
Wassenaar R, O’Connor D, Dij B, Ruddy R, Birnie D. Optimisation and validation of radionuclide angiography phase analysis parameters for quantification of mechanical dyssynchrony. J Nucl Cardiol 2009;16:895-903.
Johnson CJ, Roberts JD, James JH, Hoffmayer KS, Badhwar N, Ku IA, et al. Comparison of radionuclide angiographic synchrony analysis to echocardiography and magnetic resonance imaging for the diagnosis of arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2015;12(6):1268-75.
Badhwar N, James J, Hoffmayer K, O’Connell J, Green D, De Marco T, et al. Utility of equilibrium radionuclide angiogram-derived measures of dyssynchrony to predict outcomes in heart failure patients undergoing cardiac resynchronization therapy. J Nucl Med 57:1880-6.
Vallejo E, Jiménez L, Rodríguez G, Roffe F, Bialostozky D. Evaluation of ventricular synchrony with equilibrium radionuclide angiography: assessment of variability and accuracy. Arch Med Res 2010;41(2):83-91.
Jones KA, Small AD, Ray S, Hamilton DJ, Robinson J, Goodfield NER. Radionuclide ventriculography phase analysis for risk stratification of patients undergoing cardiotoxic cancer therapy. J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02277-z
Jones KA, Paterson CA, Hamilton DJ, Small AD, Martin W, Robinson J, et al. Optimising approximate entropy for assessing cardiac dyssynchrony with radionuclide ventriculography. Biomed Signal Process Control 2021;68:102703.
Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000;278(6):H2039-49.
McGowan J, Martin W, Burgess M, McCurrach G, Ray S, McDonagh T, et al. Validation of an echocardiographic wall motion index in heart failure due to ischaemic heart disease. Eur J Heart Fail 2001;3(6):731-7.
Botvinick EH, Frais MB, Shosa DW, O’Connell MA, Pacheco-Alvarez JA, Scheinman M, et al. An accurate means of detecting and characterizing abnormal patterns of ventricular activation by phase image analysis. Am J Cardiol 1982;50:289-98.
Frais M, Botvinick EH, Shosa DW, O’Connell WJ, Scheinman MM, Hattner RS, et al. Phase image characterization of ventricular contraction in left and right bundle branch block. Am J Cardiol 1982;50:95-105.
Shannon CE. A mathematical theory of communication. Bell Syst Tech J 1948;27:379-423.
Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer; 2016.
Cheng J. ggpval: Annotate statistical tests for ’ggplot2’. R package version 0.2.3, 2019.
Kaya MG, Sarli B, Akpek M, Kaya EG, Yarlioglues M, Topsakal R, et al. Evaluation of beta-blockers on left ventricular dyssynchrony and reverse remodeling in idiopathic dilated cardiomyopathy: A randomized trial of carvedilol and metoprolol. Cardiol J 2014;21(4):434-41.
Gorcsan J III, Sogaard P, Bax JJ, Singh JP, Abraham WT, Borer JS, et al. Association of persistent or worsened echocardiographic dyssynchrony with unfavourable clinical outcomes in heart failure patients with narrow QRS width: a subgroup analysis of the EchoCRT trial. Eur Heart J 2016;37(1):49-59.
Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2(5):429-36.
Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, et al. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail 2014;2(5):440-6.
Marcassa C, Campini R, Verna E, Ceriani L, Giannuzzi P. Assessment of cardiac asynchrony by radionuclide phase analysis: Correlation with ventricular function in patients with narrow or prolonged QRS interval. Eur J Heart Fail 2007;9(5):484-90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, K.A., Paterson, C.A., Ray, S. et al. Beta-blockers and mechanical dyssynchrony in heart failure assessed by radionuclide ventriculography. J. Nucl. Cardiol. 30, 193–200 (2023). https://doi.org/10.1007/s12350-022-03142-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-03142-x