Abstract
Ischemic heart disease remains one of the leading causes of death and disability worldwide. However, most patients referred for a noninvasive computed tomography coronary angiogram (CTA) or invasive coronary angiogram for the investigation of angina do not have obstructive coronary artery disease (CAD). Approximately two in five referred patients have coronary microvascular disease (CMD) as a primary diagnosis and, in addition, CMD also associates with CAD and myocardial disease (dual pathology). CMD underpins excess morbidity, impaired quality of life, significant health resource utilization, and adverse cardiovascular events. However, CMD often passes undiagnosed and the onward management of these patients is uncertain and heterogeneous. International standardized diagnostic criteria allow for the accurate diagnosis of CMD, ensuring an often overlooked patient population can be diagnosed and stratified for targeted medical therapy. Key to this is assessing coronary microvascular function—including coronary flow reserve, coronary microvascular resistance, and coronary microvascular spasm. This can be done by invasive methods (intracoronary temperature-pressure wire, intracoronary Doppler flow-pressure wire, intracoronary provocation testing) and non-invasive methods [positron emission tomography (PET), cardiac magnetic resonance imaging (CMR), transthoracic Doppler echocardiography (TTDE), cardiac computed tomography (CT)]. Coronary CTA is insensitive for CMD. Functional coronary angiography represents the combination of CAD imaging and invasive diagnostic procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease (IHD) remains one of the leading causes of death and disability worldwide.1,2 Angina is the most common symptom of IHD, and the investigation and management of angina is often focused on the detection and treatment of flow-limiting epicardial coronary artery disease (CAD).3 However, most patients referred for an invasive coronary angiogram for the investigation of stable anginal symptoms do not have obstructive CAD.4 Multiple studies have shown that a large proportion of these patients have coronary microvascular dysfunction (CMD).5,6,7 However, this condition is rarely diagnosed and the onward management of these patients is uncertain and heterogenous. CMD is not benign. It is associated with increased risk of major adverse cardiovascular events,8,9 persistent anginal symptoms,10 impaired quality of life,11 and considerable health resource utilization due to recurrent hospitalizations and repeat invasive angiograms.8 Recent studies have highlighted the importance of establishing an accurate diagnosis and have demonstrated that stratified medical therapy leads to a marked and sustained improvement in angina symptoms and quality of life.5,12
Definition
Camici and Crea defined Type 1 CMD as coronary microvascular dysfunction in the absence of obstructive CAD and myocardial diseases (Table 1).6 Type 2 CMD associates with cardiomyopathy, Type 3 CMD occurs in the presence of obstructive CAD, and Type 4 CMD is iatrogenic. Information gaps on the definition of microvascular angina and vasospastic angina stimulated the Coronary Vasomotion Disorders International Study Group (COVADIS), to set out standardized diagnostic criteria for the for the diagnosis of these conditions.13 The criteria for diagnosis required: (1) Symptoms of myocardial ischemia; (2) Absence of obstructive CAD; (3) Objective evidence of myocardial ischemia; (4) Evidence of impaired coronary microvascular function (Table 2). They define “Definite MVA” as the presence of all four criteria. However, “Suspected MVA” is diagnosed if criteria (1) and (2) are present, in addition to either (3) or (4). Therefore, a cornerstone of making the diagnosis of CMD is the use of diagnostic tests to specifically assess coronary microvascular function.
Assessment of Coronary Microvascular Function
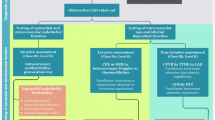
Options for CMD evaluation include invasive and non-invasive techniques, each with strengths and limitations. Contemporary guidelines recommend that the test choice should be guided by local availability and expertise.14 A detailed description of each technique used to diagnose CMD will be covered in other manuscripts in this themed issue. These techniques are summarized below along with a proposed algorithm for the investigation of patients with suspected CMD (Figure 1).
Invasive Techniques
The combination of coronary angiography with adjunctive tests of coronary vascular function is described as ‘functional coronary angiography’. A key attribute of invasive assessment of coronary microvascular function is the ability to test both endothelium-dependent and -independent pathways, which is not yet possible with non-invasive methods. Novel approaches, such as the interventional diagnostic procedure (IDP),15 combine direct measurements of microvascular function using guidewire sensors with pharmacological coronary reactivity testing, such as with acetylcholine (ACh).
The 2019 ESC CCS guidelines16 and the 2021 ACC/AHA Guideline for the Evaluation and Diagnosis of Chest pain14 have both given a IIa recommendation (‘should be considered’) to the use of guidewire-based measurement of coronary flow reserve (CFR) and/or measurements of microvascular resistance in patients with non-obstructive CAD and persistent chest pain symptoms. Similarly, intracoronary ACh testing can be used to assess for endothelium-dependent coronary microvascular spasm, supported by a IIa recommendation in ACC/AHA guidelines,14 and IIb in ESC guidelines.16 These trial recommendations are underpinned by the results of the coronary microvascular angina (CorMicA) randomized, controlled trial of stratified medical therapy.5,12
Coronary Flow Reserve
CFR reflects the vasodilatory capacity of the coronary microcirculation. It is assessed by calculating the ratio of maximal hyperemic blood flow (usually induced by the intravenous infusion of a vasodilator such as adenosine), to resting blood flow. CFR gives a measure of flow through both the epicardial coronary arteries and the coronary microcirculation. However, in the absence of obstructive epicardial disease, CFR can be used as a marker of CMD.
CFR can be measured by a thermodilution technique using a pressure-temperature sensor guidewire, which estimates CFR indirectly using a saline bolus, by dividing resting mean transit time by hyperemic mean transit time. CFR can also be measured using a Doppler flow wire represented by flow velocity divided by resting flow velocity. In the absence of obstructive CAD, a CFR > 2.5 is considered normal, and a CFR < 2.0 is abnormal.6,13,17 An impaired CFR underpins a functional vasomotor disorder, i.e., functional microvascular angina.
Measures of Microvascular Resistance
CMD may also be caused by an increase microvascular resistance, which underpins a structural vasomotor disorder, i.e., structural microvascular angina. Microvascular resistance can be measured in one or more coronary artery territories using a guidewire sensor and coronary thermodilution,18 including by use of intravenous adenosine18 or continuous intracoronary infusion of saline pressure and flow.19 This can be measured by combining pressure and flow measurements. Unlike CFR, this is independent of epicardial vascular function and is specific to the microcirculation. An additional benefit is that measures of microvascular resistance are independent of resting coronary flow.
The index of microvascular resistance (IMR) is measured using a manual saline bolus thermodilution technique and is calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time.18 An IMR > 25 is abnormal, and consistent with a diagnosis of CMD.20 Alternatively, hyperemic myocardial velocity resistance (HMR) can be measured using a Doppler-based technique. This is calculated by dividing intracoronary pressure by hyperemic flow velocity. Studies have suggested a HMR >2.5 mmHg·cm−1·s−1 is consistent with CMD,21 however, there is less consensus around this cut-off and further validation is required.
The coronary resistive reserve ratio (RRR, derived using saline bolus thermodilution)22 and relatedly, myocardial resistance reserve (MRR, derived using continuous thermodilution),23 reflect microvascular resistance. MRR is derived from CFR divided by fractional flow reserve (FFR) corrected for driving pressure.23
TIMI Frame Count
TIMI frame count provides a semi-quantitative method of assessing microvascular resistance. In the absence of obstructive CAD, a corrected TIMI frame count > 27 (images acquired at 30 frame·s−1)24 suggests MVA due to impaired resting flow (coronary slow-flow phenomenon).13 While this technique is low cost and easy to perform, it is less sensitive than other invasive techniques.
Pharmacological Intracoronary Reactivity Testing
Coronary vasomotor tone is a function of endothelium-mediated vasorelaxation and constriction mediated by the vascular smooth muscle cells (VSMC). The vasomotor effects of vasoactive substances is a function of endothelial and non-endothelial responses. Vasoactive substances that mainly exert effects on the endothelium include ACh, ergonovine, and substance P.24 However, their effects are dose-dependent. ACh is the most widely used in clinical practice and can be used to assess endothelial function (low dose, < 1 µg dose of ACh), microvascular spasm (1-50 µg dose of ACh), (which causes MVA), as well as epicardial vasospasm (which causes vasospastic angina) (> 50-150 µg dose of ACh). The pharmacological protocols vary somewhat between practitioners but the general objectives are the same. This is achieved by briefly infusing ACh at increasing doses and assessing response. In healthy individuals, ACh stimulates nitric oxide release from the endothelium, leading to vasodilation of vascular smooth muscle. However, in patients with MVA it causes coronary microvascular spasm, defined as reproduction of symptoms, ischemic ECG shift, but no angiographic evidence of epicardial spasm.13
Non-invasive Techniques
There are multiple non-invasive methods for assessing for CMD, and advances in the accuracy and availability of these has now been reflected in contemporary guidelines. The 2021 ACC/AHA Guideline for the Evaluation and Diagnosis of Chest Pain14 gives stress PET or stress CMR with MBFR a IIa recommendation, and stress echocardiography with MBVR a IIb recommendation, for the diagnosis of CMD in patients with stable chest pain and suspected INOCA.
PET
Myocardial perfusion imaging using stress/rest PET is the most well established and validated non-invasive method for the global detection of CMD. As a result, it is currently the most widely used non-invasive method and is recognized as the gold standard reference.25 PET uses radiotracers (e.g., 82Rb, 13N-ammonia, 15O-H2O) and dynamic first-pass vasodilator stress and then rest imaging to quantify absolute myocardial blood flow (MBF) at stress (maximal hyperemia) and rest in mL·g−1·min−1. Post-processing software gives accurate measurements for both regional and global stress and rest MBF.26,27,28
These measurements then allow for the calculation of the myocardial perfusion reserve (MPR), defined as the ratio of stress MBF to rest MBF. An MPR < 2.0, in the absence of obstructive CAD, is widely accepted as the diagnostic threshold for CMD. MPR has been shown to be an independent predictor of major cardiovascular events.29 In addition, use of MPR improves risk stratification in patients with suspected CMD.30
MBF and MPR measured by PET have been validated against invasive measurements in several studies, and have been shown to be accurate and reproducible.31 An advantage of PET over other diagnostic methods is that it allows for global assessment of coronary microvascular function in all coronary territories. This is particularly important as it is recognized that CMD often has a heterogeneous distribution over the myocardium, and diagnosis may be missed if only one coronary territory is assessed (e.g., by invasive angiographic assessment of a single coronary artery).32 Furthermore, PET involves relatively low radiation exposure. This can be reduced even further by the use of high-sensitivity 3D PET scanners, while still allowing for accurate MBF quantification.33,34
PET is widely available in many healthcare systems, notably in North America and some parts of Europe. Elsewhere, as is also the case with CMR, PET is less available in part due to costs and logistics. Further, interpretation of myocardial perfusion scans (PET or CMR) for CMD are facilitated by coronary angiography to clarify the presence, distribution, and severity of CAD before CMD can be diagnosed. PET imaging offers slightly lower spatial resolution than CMR (typical spatial resolution for PET = 5-7 mm, for CMR = 1.5 × 1.5 × 10 mm3).35
CMR
Dynamic first-pass imaging of MBF using cardiovascular magnetic resonance (CMR) is well established for the evaluation of suspected obstructive CAD. However, more recently stress perfusion CMR has increasingly been used to assess for CMD.
Historically, this technique involved semi-quantitative, visual interpretation of stress/rest CMR perfusion scans. Dynamic first-pass vasodilator stress imaging is followed by rest perfusion imaging to calculate a semi-quantitative MPR index (MPRI), which correlated with CFR.36 This qualitative approach had some diagnostic and prognostic value for patients with CMD in the setting of non-obstructive CAD.36 However, this method had inherent limitations in diagnostic accuracy, mainly due to variations in MPRI related to resting flow.
Recently, there have been significant advances and the development of fully automatic, pixel-wise quantitative mapping of myocardial perfusion by CMR (Figures 2 and 3). This method developed by Kellman and colleagues automatically generates pixel-encoded maps of MBF (mL·min−1·g−1 tissue) which are acquired during vasodilator (hyperemic) stress and resting conditions. MPR may therefore be calculated in a similar way to PET.37,38,39 This method has been validated against both invasive measures of coronary function and PET.38,40 Furthermore, it has been shown that MBF and MPR measured using this method are strong independent predictors of adverse cardiovascular outcomes.41
Multi-modality investigations for a 66 year old woman referred to the cardiology clinic with chest pain. A Exercise Treadmill Test was inconclusive with minor upsloping ST depression without symptoms. B Invasive coronary angiogram showed smooth unobstructed coronary arteries. C Stress/rest perfusion CMR at 1.5 T coupled with inline pixel mapping of myocardial blood flow revealed normal myocardial perfusion, with normal stress MBF (Global stress MBF = 3.40 mL·min−1·g−1) and normal MPR (Global MPR = 3.47). The final diagnosis was non-cardiac chest pain (Acknowledgement Dr. P. Kellman and Dr. H Xue, National Institutes of Health)
Multi-modality investigations in a 70 year old woman with recurrent hospitalizations with chest pain and consistently associated with high-sensitivity troponin I concentrations within the normal sex-specific range (< 16 ng·L−1). A The exercise treadmill test was strongly positive for ischemia with widespread horizontal ST depression. B The invasive coronary angiogram disclosed minor atherosclerotic plaque only, with no obstructive CAD. C Stress/rest perfusion CMR imaging coupled with inline pixel mapping of myocardial blood flow revealed a circumferential subendocardial perfusion defect, low stress MBF (Global stress MBF = 1.80 mL·min−1·g−1), and low MPR (Global MPR = 1.67). These findings are diagnostic of CMD. The final diagnosis was microvascular angina (Acknowledgement Dr. P. Kellman and Dr. H Xue, National Institutes of Health)
MBF may be considered at a global level (all segments) or at a segmental level (16- or 32-segments) and prior studies have established a cut-off for global hyperemic blood flow of 2.25 mL·min−1·g−1 tissue.42 Since the microcirculation has a sub-endocardial distribution, microvascular dysfunction may be spatially localized with a ‘sub-segmental’ distribution of hypoperfusion relative to the sub-epicardium. This threshold of 2.25 mL·min−1·g−1 may be applied to sub-endocardial segments.42 Myocardial perfusion ratio (MPR) represents the ratio of hyperemic blood flow achieved during stress testing indexed to resting blood flow. MPR can also be calculated specifically for the subendocardial layer (MPRENDO) (defined as: hyperemic MBPENDO/rest MBFENDO). A MPRENDO of 2.41 has been identified a specific cut-off for microvascular dysfunction.43 The average resting blood flow is approximately 0.6-0.8 mL·min−1·g−1 tissue, however, resting blood flow may vary within the adult population, and this is especially the case in women43,44 and in patients with diabetes45 who commonly have ‘high’ resting blood flow > 1.0 mL·min−1·g−1 tissue. In these patients, MPR may be falsely low. An MPR threshold of 2.2 has been proposed as a cut-off for microvascular dysfunction.43
Other methods using stress CMR to detect CMD are also being developed. Adenosine stress CMR T1-mapping has potential for detection of microvascular dysfunction in patients with type 2 diabetes without obstructive CAD[46 and may, potentially, distinguish epicardial from microvascular coronary disease.47 This method has the added advantage of not requiring gadolinium contrast media, and therefore would be suitable for patient with severe renal impairment, in whom stress perfusion CMR is usually contraindicated.
A major advantage of CMR is that it allows for assessment of cardiac structure, function, and tissue characterization of myocardial scar, inflammation and extracellular volume, while simultaneously assessing for CMD. There is no exposure to ionizing radiation, and all coronary artery territories are simultaneously assessed, as is the case with PET. However, CMR does have some limitations, notably comparatively high costs and ineligible patient groups, e.g., severe renal disease, claustrophobia, implantable devices. And while CMR is generally more accessible than PET, software for quantification of MBF is not universally available.
Cardiac CT
CT coronary angiography is well established for the assessment anatomical assessment of CAD. However, there have been advances in the use of CT for functional assessment as well. Myocardial first-pass dynamic CT allows for semi-quantitative assessment of MBF and MPR.48,49 However, there have been very few studies investigating its ability to detect CMD and it has not been validated. The main advantage of CT would be the ability to combine anatomical and functional imaging in one imaging examination, reducing the need for additional investigations. However, CT involves exposure to ionizing radiation and the risk of contrast-induced nephropathy. Also, iodine-based contrast media risks overestimation of MBF, due to the vasodilatory properties of the contrast.
Echocardiography
Transthoracic Doppler echocardiography (TTDE) of the left anterior descending (LAD) coronary artery can be used to calculate the coronary flow velocity ratio (CFVR). This is calculated by the ratio of peak diastolic flow in the artery at rest and at stress (induced by adenosine, dipyridamole, or regadenoson).50 In the absence of obstructive epicardial CAD, CFVR acts as a measure of coronary microvascular function. There is a lack of consensus on an exact threshold, but CFRV ≤ 2.0-2.5 is thought to be consistent with CMD.13
TTDE is comparatively inexpensive compared with other imaging modalities, is readily available, and involves no radiation exposure. However, limitations include operator dependency, and the need for extensive training. There will be patient related technical issues that are encountered with all forms of transthoracic echo (e.g., poor acoustic windows). Furthermore, TTDE is mainly limited to assessing the LAD territory, and therefore fails to account for heterogeneous microvascular dysfunction. Myocardial contrast echocardiography using intravenous injection of microbubbles to assess MPR has been validated against PET,51 however, it is rarely used in clinical practice.
Single-Photon Emission Computed Tomography
Single-photon emission computed tomography (SPECT) uses gamma rays for measuring MBF and MFR. Until recently, the use of SPECT for detection of CMD was largely limited by the pharmacokinetics of the radiotracers used. However, advances in SPECT with cadmium zinc telluride (CZT) detectors now allows quantification of MBF and MFR, and studies have shown reasonable correlation between PET and CZT SPECT.52
Epidemiology
Prevalence
CMD may occur in the presence or absence of CAD and/or myocardial disease (Table 1). Most patients referred for a noninvasive computed tomography coronary angiogram (CTA) or invasive coronary angiogram for the investigation of angina do not have obstructive CAD.4 Many of these patients may have underlying CMD. However, establishing the prevalence with reliable accuracy and precision has been challenging. Reasons include the lack of specific noninvasive testing, methodological differences between diagnostic methods, variations in the diagnostic threshold used to define CMD, selected populations, and sample size.
One of the largest studies examining the prevalence of CMD in patients with chest pain and non-obstructive CAD used invasive coronary function testing (including ACh reactivity testing) to diagnose CMD in 1,439 patients. They found that more than two-thirds of patients had some form of CMD (1,171/1,439).7 These findings were in keeping with a previous smaller study in which patients with angina and non-obstructive CAD were assessed in the same way. This study found that 59% (120/203) patients had CMD.53
The Women’s Ischemia Syndrome Evaluation (WISE) Study found that 74/189 (39%) of women with chest pain but normal coronary arteries had an abnormal CFR consistent with CMD.54 Similar results have been demonstrated using non-invasive diagnostic methods. In a large study of 1,218 patients with angina but no history of CAD and no visual evidence of CAD on rest/stress PET myocardial perfusion imaging, PET was used to quantify CFR to investigate for the presence of CMD (CFR < 2.0 = abnormal). They found that CMD was highly prevalent with 641/1,218 (53%) of patients having CFR < 2.0, consistent with CMD.55
However, a lower prevalence was noted in the iPOWER study in which 241/963 (26%) with chest pain but no obstructive CAD on angiography, had markedly impaired CFRV consistent with CMD when measured by transthoracic Doppler echo.56 This finding could be related to the use of echocardiography (and related reduced test sensitivity for CMD).
A recent systematic review investigating the prevalence of microvascular angina in patients with stable angina in the absence of obstructive CAD, defined MVA in different settings according to the COVADIS criteria. They found a median prevalence of 43% for suspected MVA using non-invasive ischemia testing, 28% for suspected MVA using specific modalities for MVA, and 30% for definite MVA.57
The Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study is a multicenter cohort study and stratified medicine trial that will prospectively characterize the prevalence of disease endotypes in 250 patients with known or suspected angina and no obstructive coronary arteries,58 as defined by coronary CTA. The nested randomized, controlled trial will assess whether disclosure of the endotypes with linked therapy will improve health and economic outcomes, as compared to standard coronary CTA guided management.
Sex
Most individuals who have anginal symptoms and no obstructive coronary arteries are female.4,8 Despite the lower prevalence of obstructive CAD, women have a similar prevalence of ST elevation myocardial infarction, and have excess mortality compared with men.59 CMD is not a benign condition; it associated with increased morbidity and major adverse cardiovascular events,8,60 and CMD is likely to contribute to the cardiovascular morbidity encountered by women with INOCA.
Meta-analyses have shown that CMD is more prevalent in women,57 and particularly among post-menopausal women.54 Other large studies have shown that while the prevalence of CMD is higher in women than men, the prevalence was generally high in both populations and is associated with adverse outcomes regardless of sex.53,55
Traditional Cardiovascular Risk Factors
Multiple cardiovascular risk factors are associated with increased prevalence of CMD. Several large studies, including the WISE, iPOWER, and CorMicA studies, have shown that hypertension, dyslipidemia, diabetes, and age are all associated with impaired coronary microvascular function.54,56,61 CMD is also more prevalent in inflammatory conditions,62,63,64 and has been shown to be associated with serum biomarkers of inflammation.65,66
Future Developments
CMD is highly prevalent and has significant unmet clinical need. More research and large-scale studies are therefore urgently needed to address this important health condition.
Encouragingly there are multiple current studies of the prevalence, detection, and treatment of CMD.
Multiple non-invasive methods for the detection of CMD are now available, but there is a paucity of evidence that management guided by noninvasive imaging improves clinical outcomes The CorCMR trial (NCT04805814) will assess the clinical utility of quantitative stress perfusion CMR for the diagnosis of CMD and determine whether stratified medical therapy guided by the results of the stress perfusion CMR improves symptoms, well-being, cardiovascular risk and health and economic outcomes.
At present there are no evidence-based, disease-modifying therapies for the treatment of CMD. Some studies have suggested that intensive medical therapy including statins and angiotensin converting enzyme inhibitors (ACE-I) or receptor blockers (ARB) may be beneficial. The Women’s Ischemia Trial to Reduce Events In Non-Obstructive CAD (WARRIOR) trial (NCT03417388) is a multicenter, prospective, randomized, blinded outcome evaluation, to assess the impact of intensive medical therapy (high dose statin + ACEi/ARB) versus usual care on major adverse cardiovascular events in women with INOCA.67
There is also much anticipation for the Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial (NCT04097314). Zibotentan is a potent, oral, selective inhibitor of the endothelin A receptor. Dysregulation of the endothelin system is implicated in the development of CMD, and therefore zibotentan has potential as a disease-modifying therapy for CMD. The PRIZE trial will assess the efficacy and safety of zibotentan as a novel treatment for CMD.68
Conclusion
CMD is a highly prevalent and important health problem, however, it often goes unrecognized by clinicians. Accurate diagnosis and stratified medical therapy are crucial to improve symptoms, quality of life, and long-term outcomes for patients with CMD. International standardized criteria provide a structured approach for the diagnosis of this condition, and the use of both invasive and non-invasive diagnostic methods are essential for the accurate diagnosis of CMD. However, ongoing research is required to continue to develop diagnostic methods and novel disease-modifying therapies.
Abbreviations
- CAD:
-
Coronary artery disease
- CFR:
-
Coronary flow reserve
- CMD:
-
Coronary microvascular disease
- CMR:
-
Cardiovascular magnetic resonance
- COVADIS:
-
COVADIS: Coronary Vasomotion Disorders International Study Group
- IHDT:
-
Ischemic heart disease
- IMR:
-
Index of microcirculatory resistance
- MBF:
-
Myocardial blood flow
- MPR:
-
Myocardial perfusion reserve
- PET:
-
Positron emission tomography
References
Khan MA, et al. Global epidemiology of ischemic heart disease: Results from the Global Burden of Disease Study. Cureus 2020;12:e9349.
GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859‐922.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C. ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019. https://doi.org/10.1093/eurheartj/ehz425.
Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886‐95.
Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: CorMicA Trial. J Am Coll Cardiol 2018;72:2841‐55.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830‐40.
Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445‐53.
Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734‐44.
Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840‐9. https://doi.org/10.1093/eurheartj/ehx721.
Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: Highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol 2013;102:571‐81.
Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2016;2:117‐24.
Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv 2020;13:33‐45.
Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16‐20.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;144:e368‐454. https://doi.org/10.1161/CIR.0000000000001029.
Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: Why, how, and when. JACC Cardiovasc Interv 2020;13:1847‐64.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407‐77.
Pijls NHJ. Coronary thermodilution to assess flow reserve: Validation in humans. Circulation 2002;105:2482‐6.
Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, et al. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129‐32.
Verhoeff BJ, van de Hoef TP, Spaan JA, Piek JJ, Siebes M. Minimal effect of collateral flow on coronary microvascular resistance in the presence of intermediate and noncritical coronary stenoses. Am J Physiol Heart Circ Physiol 2012;303:H422‐8.
Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: The index of microcirculatory resistance. Circ Cardiovasc Interv 2017. https://doi.org/10.1161/circinterventions.117.005361.
Williams RP, de Waard GA, De Silva K, Lumley M, Asrress K, Arri S, et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol 2018;121:1‐8.
Layland J, Carrick D, McEntegart M, Ahmed N, Payne A, McClure J, et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2013;6:231‐6.
De Bruyne B, Pijls NHJ, Gallinoro E, Candreva A, Fournier S, Keulards DCJ, et al. Microvascular resistance reserve for assessment of coronary microvascular function: JACC Technology Corner. J Am Coll Cardiol 2021;78:1541‐9.
Radico F, Cicchitti V, Zimarino M, De Caterina R. Angina pectoris and myocardial ischemia in the absence of obstructive coronary artery disease: Practical considerations for diagnostic tests. J Am Coll Cardiol Interv 2014;7:453‐63.
Slomka PJ, Berman DS, Alexanderson-Rosas E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging 2014;2:343‐58.
Kaufmann AP, Camici PG. Myocardial blood flow measurement by PET: Technical aspects and clinical applications. J Nucl Med 2005;46:75‐88.
Nagamachi S, Czernin J, Kim AS, Sun KT, Böttcher M, Phelps ME, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med 1996;37:1626‐31.
Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med 1999;40:1848‐56.
Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325‐36.
Murthy VL, Bateman TM, Beanlands RS, et al. Clinical quantification of myocardial blood flow using PET: Joint position paper of the SNMMI cardiovascular council and the ASNC. J Nucl Cardiol 2018;25:269‐97.
Feher A, Sinusas AJ. Quantitative Assessment of Coronary Microvascular Function. Circ Cardiovasc Imaging 2017;10:1‐21.
Marroquin OC, Holubkov R, Edmundowicz D, Rickens C, Pohost G, Buchthal S, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: Implications for clinical practice. Am Heart J 2003;145:628‐35.
Schafers KP, Spinks TJ, Camici PG, Bloomfield PM, Rhodes CG, Law MP, et al. Absolute quantification of myocardial blood flow with H(2)(15)O and 3-dimensional PET: An experimental validation. J Nucl Med 2002;43:1031‐40.
Schepis T, Gaemperli O, Treyer V, Valenta I, Burger C, Koepfli P, et al. Absolute quantification of myocardial blood flow with 13N-ammonia and 3-dimensional PET. J Nucl Med 2007;48:1783‐9.
Schindler TH, Dilsizian V. Coronary microvascular dysfunction: Clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging 2020;13:140‐55.
Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015. https://doi.org/10.1161/CIRCIMAGING.114.002481.
Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: Optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 2017;19:43.
Engblom H, Xue H, Akil S, Carlsson M, Hindorf C, Oddstig J, et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reson 2017;19:78.
Hsu L-Y, Jacobs M, Benovoy M, Ta AD, Conn HM, Winkler S, et al. Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2018;11:697‐707.
Knott KD, Camaioni C, Ramasamy A, Augusto JA, Bhuva AN, Xue H, et al. Quantitative myocardial perfusion in coronary artery disease: A perfusion mapping study. J Magn Reson Imaging 2019. https://doi.org/10.1002/jmri.26668.
Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N, et al. The prognostic significance of quantitative myocardial perfusion: An artificial intelligence-based approach using perfusion mapping. Circulation 2020;141:1282‐91.
Kotecha T, Chacko L, Chehab O, O’Reilly N, Martinez-Naharro A, Lazari J, et al. Assessment of multivessel coronary artery disease using cardiovascular magnetic resonance pixelwise quantitative perfusion mapping. JACC Cardiovasc Imaging 2020;13:2546‐57.
Rahman H, Scannell CM, Demir OM, Ryan M, McConkey H, Ellis H, et al. High-resolution cardiac magnetic resonance imaging techniques for the identification of coronary microvascular dysfunction. JACC Cardiovasc Imaging 2021;14:978‐86.
Suppogu N, Wei J, Nelson MD, Cook-Wiens G, Cheng S, Shufelt CL, et al. Resting coronary velocity and myocardial performance in women with impaired coronary flow reserve: Results from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study. Int J Cardiol 2020;309:19‐22.
Sørensen MH, Bojer AS, Pontoppidan JRN, Broadbent DA, Plein S, Madsen PL, et al. Reduced myocardial perfusion reserve in type 2 diabetes is caused by increased perfusion at rest and decreased maximal perfusion during stress. Diabetes Care 2020;43:1285‐92.
Levelt E, Piechnik SK, Liu A, Wijesurendra RS, Mahmod M, Ariga R, et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J Cardiovasc Magn Reson 2017;19:81.
Liu A, Wijesurendra RS, Liu JM, Greiser A, Jerosch-Herold M, Forfar JC, et al. Gadolinium-free cardiac MR stress T1-mapping to distinguish epicardial from microvascular coronary disease. J Am Coll Cardiol 2018;71:957‐68.
Branch KR, Haley RD, Bittencourt MS, Patel AR, Hulten E, Blankstein R. Myocardial computed tomography perfusion. Cardiovasc Diagn Ther 2017;7:452‐62.
Ho K-T, Ong H-Y, Tan G, Yong Q-W. Dynamic CT myocardial perfusion measurements of resting and hyperaemic blood flow in low-risk subjects with 128-slice dual-source CT. Eur Heart J Cardiovasc Imaging 2015;16:300‐6.
Vegsundvåg J, Holte E, Wiseth R, Hegbom K, Hole T. Coronary flow velocity reserve in the three main coronary arteries assessed with transthoracic Doppler: A comparative study with quantitative coronary angiography. J Am Soc Echocardiogr 2011;24:758‐67.
Vogel R, Indermuhle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: Algorithm and validation. J Am Coll Cardiol 2005;45:754‐62.
Giubbini R, Bertoli M, Durmo R, et al. Comparison between N13NH3-PET and 99mTc-Tetrofosmin-CZT SPECT in the evaluation of absolute myocardial blood flow and flow reserve. J Nucl Cardiol 2021;28:1906‐18.
Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc 1998;73:1133‐40.
Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825‐32.
Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518‐27.
Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc 2016;5:e003064.
Aribas E, van Lennep JER, Elias-Smale SE, Piek JJ, Roos M, Ahmadizar F, et al. Prevalence of microvascular angina among patients with stable symptoms in the absence of obstructive coronary artery disease: A systematic review. Cardiovasc Res 2021. https://doi.org/10.1093/cvr/cvab061.
Sidik NP, McEntegart M, Roditi G, Ford TJ, McDermott M, Morrow A, et al. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study. Am Heart J 2020;221:48‐59.
Cenko E, van der Schaar M, Yoon J, Kedev S, Valvukis M, Vasiljevic Z, et al. Sex-specific treatment effects after primary percutaneous intervention: A study on coronary blood flow and delay to hospital presentation. J Am Heart Assoc 2019;8:e011190.
Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754‐63.
Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, et al. Ischemia and no obstructive coronary artery disease: Prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv 2019;12:e008126.
Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27‐33.
Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30:1837‐43.
Weber B, Perez-Chada LM, Divakaran S, Brown JM, Taqueti V, Dorbala S, et al. Coronary microvascular dysfunction in patients with psoriasis. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02166-5.
Schroder J, Mygind ND, Frestad D, Michelsen M, Suhrs HE, Bove KB, et al. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int J Cardiol Heart Vasc 2019;24:100370.
Mommersteeg PMC, Naude PJW, Bagijn W, Widdershoven J, Westerhuis B, Schoemaker RG. Gender differences in associations of depressive symptoms and anxiety with inflammatory markers in patients with non-obstructive coronary artery disease. J Psychosom Res 2019;125:109779.
Handberg EM, Merz CNB, Cooper-Dehoff RM, Wei J, Conlon M, Lo MC, et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J 2021;237:90‐103.
Morrow AJ, Ford TJ, Mangion K, Kotecha T, Rakhit R, Galasko G, et al. Rationale and design of the Medical Research Council’s Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J 2020;229:70‐80.
Disclosures
CB and CB are employed by the University of Glasgow, which holds consultancy and research agreements with companies that have commercial interests in the diagnosis and management of angina, including Abbott Vascular, AstraZeneca, Boehringer Ingelheim, GSK, Heartflow, Menarini Pharmaceuticals, Neovasc, Siemens Healthcare and Valo Health. The authors received no support from any of these organizations for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bradley, C., Berry, C. Definition and epidemiology of coronary microvascular disease. J. Nucl. Cardiol. 29, 1763–1775 (2022). https://doi.org/10.1007/s12350-022-02974-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-02974-x