Abstract
Adenocarcinoma (AC) with neuroendocrine carcinoma (NEC) or enteroblastic (ENT) differentiation rarely develops in Barrett’s esophagus (BE). A 76-year-old man was diagnosed with Barrett’s AC (cT1bN0M0) and underwent thoracoscopic esophagectomy. A type 0-IIc + 0-Is lesion measuring 26 × 21 mm was macroscopically observed on a background of long segment BE (pT1bN0M0). The tumor comprised three different histological types of carcinoma (NEC, AC with ENT differentiation and moderately differentiated AC). NEC showed positivity for synaptophysin, chromogranin A and insulinoma-associated protein 1 with a Ki-67 index of 60.6%. ENT tumors were immunopositive for AFP and sal-like protein 4, and focally immunopositive for human chorionic gonadotrophin. The amounts of NEC, ENT and AC were 40%, 40% and 20%, respectively. p53 expression was positive throughout the tumor. Rb expression was negative at the NEC, but positive at the ENT and AC. CD4 and CD8 densities were lower in the NEC segment than in the AC and ENT segments, and PD-L1 expression was negative throughout the tumor. Early cancer arising in BE with a combination of tubular AC, ENT tumors and NEC is very rare. Our observations might contribute to understanding the carcinogenetic pathways and tumor microenvironment of NEC and ENT tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barrett’s esophagus (BE), which is characterized by the replacement of normal esophageal squamous cell epithelium with columnar metaplasia, affects approximately 1% of the global population and increases the risk of developing esophageal carcinoma (EC) [1]. Most neoplasms arising in BE are tubular adenocarcinoma (AC) [2], while other tissue types, such as neuroendocrine carcinoma (NEC) or adenocarcinoma with enteroblastic (ENT) differentiation, are rare in BE [3,4,5]. These rare tumors reportedly exhibit more aggressive biology than AC [6, 7]. However, due to rarity, their histopathological features and immune profiles have yet to be fully investigated [8]. Herein, we document a very rare case with a tumor arising in BE, which was comprised of tubular AC, AC with ENT differentiation, and NEC.
Case presentation
A 76-year-old man visited our facility for detailed examination of a flat protruding lesion in the esophagogastric junction that had been detected by esophagogastroduodenoscopy during a medical examination. The patient had a history of hypertension and early prostate cancer. He had long experienced symptoms associated with gastroesophageal reflux disease, and had taken proton-pump inhibitors. The blood examination results were normal except for chronic renal dysfunction (eGFR 42.4). Serum levels of several tumor markers were elevated; alpha-fetoprotein (AFP) (19.6 ng/ml, normal range < 10 ng/ml), carbohydrate antigen 19–9 (40 U/ml, normal range < 37 U/ml) and pro-gastrin-releasing peptide (103.6 pg/ml, normal range < 67 pg/ml). Other tumor markers, such as carcinoembryonic antigen (CEA), neuron-specific enolase and anti-p53-antibody, were within their normal ranges.
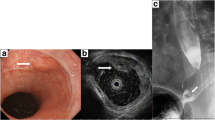
Esophagography revealed a type 0-IIc + 0-Is elevated lesion in the abdominal esophagus, with a size of 20 mm, that showed apparent invasion into the submucosa (Fig. 1a). Esophagogastroduodenoscopy confirmed a flat, erythematous, slightly protruding 20 mm lesion (type 0-IIc + 0-Is) on the posterior wall of the esophagogastric junction (Fig. 1b, c). The proximal end of the lesion did not reach the white normal squamous epithelium. The lesion was observed to be on a background of long segment BE, in which columnar-appearing mucosa was accompanied by palisade vessels. Pathologic evaluation of the biopsy specimen indicated portions of the tubular adenocarcinoma, which was covered with normal stratified squamous epithelium. Neither distant metastasis nor lymph node involvement was seen on thoracoabdominal contrast computed tomography. The patient was diagnosed with Barrett's adenocarcinoma, classified as clinical stage I (T1bN0M0) according to the 8th TNM classification [9].
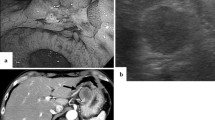
Esophagography and endoscopic findings before surgery, and in the resected specimen. a Esophagography findings. A type 0-IIc + 0-Is elevated lesion was found in the abdominal esophagus. b, c Upper gastrointestinal endoscopy detected a protruding 20 mm lesion (type 0-IIc + 0-Is) on the posterior wall of the esophagogastric junction. The lesion was on a background of long segment Barrett’s esophagus. d A type 0-IIc + 0-Is lesion measuring 26 × 21 mm was macroscopically observed at the squamous-columnar junction. e Lugol’s iodine staining
The patient was treated by thoracoscopic middle to inferior mediastinal lymph node dissection, proximal gastrectomy, distal esophagectomy and reconstruction with jejunal interposition. He was discharged from the hospital 21 days after the surgery, without complications.
Histopathological findings
In the resected specimen, a type 0-IIc + 0-Is lesion measuring 26 × 21 mm was macroscopically observed at the squamous-columnar junction (Fig. 1d). Staining with Lugol’s iodine revealed that part of the flat protruding lesion was covered by squamous epithelium (Fig. 1e). Histological examination of the resected specimens revealed some islands of squamous epithelium, esophageal glands beneath the columnar epithelium, and double-layered muscularis mucosae, suggesting the neoplastic lesion to be within BE. The background mucosa of BE mainly comprised of the cardiac-type mucosa, and intestinal-type mucosa was slightly recognized nearby the oral margin of the tumor [12].
The tumor was comprised of three different histological types of carcinoma with transitional areas (Fig. 2a), showing characteristic immunohistochemical phenotypes described below. Most of the tumor consisted of the two neoplastic cell types. The first component was round carcinoma cells with round or oval nuclei in a nested or sheeted pattern (Fig. 2b). The tumor cells showed positivity for neuroendocrine markers such as synaptophysin (Fig. 3a), chromogranin A (Supplementary Fig. 1a) and insulinoma-associated protein 1 (INSM1) (Supplementary Fig. 1b) with a Ki-67 index of 60.6%, indicating the cells to be NEC. The second consisted of columnar cells with prominent clear cytoplasm growing in tubular, papillary and solid patterns (Fig. 2c). Immunohistochemical evaluation showed that the cells were immunopositive for sal-like protein 4 (SALL4, a fetal gastrointestinal epithelial marker) (Fig. 3b), focally immunopositive for human chorionic gonadotrophin (hCG) (Fig. 3c), largely immunopositive for AFP (Supplementary Fig. 1c), and partially immunopositive for Glypican3 (Supplementary Fig. 1d), suggesting the cells to exhibit ENT differentiation. The rest consisted of moderately differentiated AC (Fig. 2d). Carcinoma involved the submucosa (sm, 5000 μm from the surface of the lesion).
All parts of the tumor were positive for p53 (Fig. 3d). The AC and the NEC component were both positive for CEA (Supplementary Fig. 2a). Most of the component that was positive for CEA was separate from the CEA-negative ENT component area, though several areas showed positivity for both CEA and AFP (Supplementary Fig. 2b-c).
The amounts of AC, NEC and ENT (including focal hepatoid features and areas of indistinct transition) were approximately 20%, 40% and 40%, respectively. Other immunohistochemical results are summarized in Table 1. Placental alkaline phosphatase, p40 (deltaNp63) and HER2 were negative in all parts of the tumor. Vimentin, a marker for carcinosarcoma, was negative. Ki-67 labeling indexes were 45.8%, 60.6% and 62.4% in the AC, NEC and ENT components, respectively. Mismatch repair proteins were positive in all parts of the tumor. Immunohistochemistry showed that CDX-2 was positive, while MUC-6 and MUC-5AC were negative, suggesting the tumor to be intestinal-type. Retinoblastoma gene protein (Rb) was negative in the NEC, but positive in the AC and ENT (Fig. 4). CD4 and CD8 densities were lower in the NEC segment than in the AC and ENT segments, and programmed cell death ligand-1 (PD-L1) expression was negative in all three components (Supplementary Fig. 3a-d).
There was no evidence of either lymphatic or blood vessel invasion. Nodal metastasis was absent. The tumor was pathologically classified as pT1bN0M0, stage I [9].
Discussion
Herein, we have demonstrated a very rare case with an early EC arising in BE. The tumor showed a multidirectional differential potential. In our present case, the tumor was comprised of tubular AC, NEC and AC with ENT differentiation. A recent study documented a case of AC with ENT differentiation arising in the ampulla of Vater [10], but only a few reports have described Barrett’s esophageal adenocarcinoma with ENT and NEC components [5]. Notably, most of the previously documented cases had advanced tumors, such that our present case with an early tumor is quite rare in the literature. Furthermore, we conducted detailed analyses on various immunohistochemical markers and tumor immune microenvironments.
Histologically, BE is characterized by proper esophageal glands or ducts beneath the overlying columnar epithelium, squamous epithelial islets in the columnar epithelium, and double-layered lamina muscularis mucosae [11]. In our present case, CDX-2 was strongly positive while MUC-6 and MUC-5AC were negative, a finding in line with previous reports suggesting most Barrett carcinomas to arise from intestinal-type metaplasia [12].
Esophageal NEC is very rare, accounting for approximately 0.2% of all cases of EC [13]. Neuroendocrine neoplasms are diagnosed by immunostaining, such as specific stains for synaptophysin, chromogranin A and INSM1 [14]. Given the high sensitivity of synaptophysin for NEC [15], we identified the NEC as synaptophysin-positive lesions. Recently, INSM1 has emerged as an additional general neuroendocrine marker because it was shown to have better diagnostic ability than synaptophysin and chromogranin A in gastrointestinal tumors [15, 16].
Adenocarcinoma with ENT differentiation arising in BE is also rare. A primitive intestine-like structure, composed of cuboidal or columnar cells with clear cytoplasm, is reportedly a characteristic feature of enteroblastic lesions [6, 17]. Fetal gastrointestinal epithelial markers such as AFP, Glypican 3, and SALL4 have been widely used to immunohistochemically confirm ENT features [6]. In particular, AFP expression is reportedly detectable in nearly half of gastric tumors with ENT differentiation [6]. Motoyama et al. previously reported combined choriocarcinoma, NEC and AC, and detected the expression of hCG in the choriocarcinoma component [5]. In our present case, hCG was focally expressed in the ENT lesion, though the amount was too small for analysis.
We also evaluated the immune microenvironment for each component. Our present case suggested intratumoral CD4 + and CD8 + T cell infiltrations to be modest in NEC as compared to the other components. This observation agrees with a previous study revealing NEC to show low intratumoral CD3 + T cell infiltration [18]. PD-L1 expression was low in all of three histological tumor types observed in our case. A recent study demonstrated PD-L1 expression to be elevated in a high proportion of NEC samples [8], while another study suggested the opposite results [18]. Tumor microenvironment analysis merits further scrutiny in patients with NEC and AC with ENT differentiation.
The carcinogenetic pathway of NEC is not yet fully understood, but two hypotheses have been proposed. First, the originally present malignant exocrine cell develops into a neuroendocrine tumor. Second, monoclonal multi-potent stem cells differentiate into two components. In our present case, both the NEC and AC components were positive for CEA. These observations, taken together with previous findings [19], suggest malignant cells which had originally been directed towards the development of AC to differentiate into NEC. It is noteworthy that Rb expression was absent only in the NEC component in our case. A recent investigation revealed alterations of Rb to be associated with the development of gastrointestinal NEC [20].
Prior studies have revealed AC with ENT differentiation to develop from AC with an intestinal phenotype [17, 21, 22]. In our present case, AFP-positive cells were also observed at the bases of AC tubules, a finding suggesting the origin of ENT to be AC. Furthermore, CEA was focally positive in the ENT lesion. The lesion might have been comprised of tumor cells that partially differentiated into ENT from AC, thereby expressing both CEA and AFP. It is noteworthy that a recent study documented a case which suggested de novo development of gastric AC with ENT differentiation [23]. Further studies are warranted to elucidate the origin, growth, and morphology of this malignancy.
The optimal treatment strategy remains to be determined in esophageal AC, with both NEC and ENT differentiation, due to the rarity of such malignancies. Esophageal NEC, as well as EC with ENT differentiation, is reportedly associated with poor clinical outcomes and high hepatic metastatic potential [7, 24]. Therefore, close follow-up observation is necessary for our present case.
In conclusion, we have described a very rare case with a combination of NEC, AC with ENT differentiation and tubular AC arising in BE. Our present case might contribute to understanding the carcinogenesis, biology and immune microenvironments of such rare malignancies, as well as helping clinicians to manage these complex cases.
Abbreviations
- BE:
-
Barrett’s esophagus
- EC:
-
Esophageal carcinoma
- NEC:
-
Neuroendocrine carcinoma
- ENT:
-
Enteroblastic
- AFP:
-
Alpha-fetoprotein
- CEA:
-
Carcinoembryonic antigen
- AC:
-
Adenocarcinoma
- INSM1:
-
Insulinoma-associated protein 1
- hCG:
-
Human chorionic gonadotrophin
- SALL4:
-
Sal-like protein 4
- Rb:
-
Retinoblastoma gene protein
- PD-L1:
-
Programmed cell death ligand-1
References
Sharma P. Barrett esophagus: a review. JAMA. 2022;328:663–71.
van Munster SN, Verheij EPD, Nieuwenhuis EA, et al. Extending treatment criteria for Barrett’s neoplasia: results of a nationwide cohort of 138 endoscopic submucosal dissection procedures. Endoscopy. 2022;54:531–41.
Kinoshita T, Ishikawa S, Inaba T, et al. Neuroendocrine carcinoma arising from Barrett’s esophageal adenocarcinoma: a case report. Clin J Gastroenterol. 2020;13:1028–35.
Kawazoe T, Saeki H, Edahiro K, et al. A case of mixed adenoneuroendocrine carcinoma (MANEC) arising in Barrett’s esophagus: literature and review. Surg Case Rep. 2018;4:45.
Motoyama T, Higuchi M, Taguchi J. Combined choriocarcinoma, hepatoid adenocarcinoma, small cell carcinoma and tubular adenocarcinoma in the oesophagus. Virchows Arch. 1995;427:451–4.
Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19:498–507.
Maru DM, Khurana H, Rashid A, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32:1404–11.
Yamashita S, Abe H, Kunita A, et al. Programmed cell death protein 1/programmed death ligand 1 but not HER2 is a potential therapeutic target in gastric neuroendocrine carcinoma. Histopathology. 2021;78:381–91.
Brierley JD, Gospodarowicz MK, Wittekind C, editors. The TNM classification of malignant tumours. 8th ed. Oxford: Wiley Blackwell; 2017.
Mitsuma K, Taniguchi H, Kishi Y, et al. A case of adenocarcinoma with enteroblastic differentiation of the ampulla of Vater. Pathol Int. 2016;66:230–5.
Takubo K, Vieth M, Aida J, et al. Histopathological diagnosis of adenocarcinoma in Barrett’s esophagus. Dig Endosc. 2014;26:322–30.
Aida J, Vieth M, Shepherd NA, et al. Is carcinoma in columnar-lined esophagus always located adjacent to intestinal metaplasia? A histopathologic assessment. Am J Surg Pathol. 2015;39:188–96.
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2010. Esophagus: Off J Jpn Esophageal Soc. 2017;14:189–214.
Board WCTE. WHO classification of tumours. Digestive system tumours: WHO classification of tumours, vol. 1. World Health Organization; 2019.
Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022;33:115–54.
González I, Lu HC, Sninsky J, et al. Insulinoma-associated protein 1 expression in primary and metastatic neuroendocrine neoplasms of the gastrointestinal and pancreaticobiliary tracts. Histopathology. 2019;75:568–77.
Kinjo T, Taniguchi H, Kushima R, et al. Histologic and immunohistochemical analyses of α-fetoprotein–producing cancer of the stomach. Am J Surg Pathol. 2012;36:56–65.
Busse A, Mochmann LH, Spenke C, et al. Immunoprofiling in neuroendocrine neoplasms unveil immunosuppressive microenvironment. Cancers (Basel). 2020;12:3448.
La Rosa S, Vanoli A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. 2014;67:938–48.
Yachida S, Totoki Y, Noë M, et al. Comprehensive genomic profiling of neuroendocrine carcinomas of the gastrointestinal system. Cancer Discov. 2022;12:692–711.
Kumashiro Y, Yao T, Aishima S, et al. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857–63.
Akiyama S, Tamura G, Endoh Y, et al. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer. 2003;106:510–5.
Kimura T, Hikichi T, Nakamura J, et al. Gastric adenocarcinoma with enteroblastic differentiation followed endoscopically: a case report. Clin J Gastroenterol. 2020;13:1074–82.
Wang J, Liu W, Parikh K, et al. Alpha-fetoprotein-producing esophageal adenocarcinoma: a mimicker of hepatocellular carcinoma. Clin J Gastroenterol. 2017;10:7–12.
Acknowledgements
We greatly appreciate Mr. Satoshi Takahashi, Saitama Cancer Center and Ms. Kimie Nomura, Saitama Cancer Center for their technical assistance.
Author information
Authors and Affiliations
Contributions
KS, IN and HK contributed to drafting the manuscript; KS, TF, YK, DO and YK, planning the surgical procedure, performing the operation, and treating the post-operative patients; all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All the procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The institutional ethics committee approved the publication of this case report.
Consent for publication
Oral informed consent was obtained from the patient for publication of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12328_2023_1791_MOESM1_ESM.tiff
Supplementary file1 NEC and ENT markers. The NEC component showed positivity for (a) chromogranin A and (b) insulinoma-associated protein 1 (INSM1). The ENT component was (c) largely immunopositive for AFP and (d) partially immunopositive for Glypican3 (TIFF 7298 KB)
12328_2023_1791_MOESM2_ESM.tiff
Supplementary file2 CEA and AFP expressions. (a) Both the adenocarcinoma and the neuroendocrine component stained positive for CEA. (b-c) Several areas were positive for both CEA and AFP (TIFF 7473 KB)
12328_2023_1791_MOESM3_ESM.tiff
Supplementary file3 CD4, CD8 and PD-L1 status according to the histological type. (a-c) CD4 and (d-f) CD8 densities were lower in the NEC segment than in the AC and ENT segments, and (g-i) all three components were negative for PD-L1 expression (TIFF 6671 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sugawara, K., Fukuda, T., Kishimoto, Y. et al. Combined tubular adenocarcinoma, neuroendocrine carcinoma and adenocarcinoma with enteroblastic differentiation arising in Barrett esophagus. Clin J Gastroenterol 16, 501–507 (2023). https://doi.org/10.1007/s12328-023-01791-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01791-0