Abstract
Introduction
Ralinepag is a potent, titratable, orally administered prostacyclin (IP) receptor agonist to treat pulmonary arterial hypertension. A phase II randomized, double-blind, parallel-group, placebo-controlled, 22-week study of immediate-release (IR) ralinepag safety and efficacy met its primary endpoint, significantly reducing pulmonary vascular resistance (PVR) compared with placebo. This phase II open-label extension (OLE) study assessed long-term safety and tolerability of ralinepag.
Methods
Participants were eligible for the OLE if they completed the parent study or experienced a clinical worsening event while receiving placebo. Those previously receiving IR ralinepag remained on their current dose, and participants formerly administered placebo were titrated to the highest tolerated dose. Participants were transitioned to an extended-release ralinepag formulation toward the end of the OLE. The primary objective evaluated long-term safety and tolerability; secondary endpoints included changes in 6-min walk distance (6MWD), World Health Organization/New York Heart Association functional class, clinical worsening, and hemodynamic measures.
Results
In total, 45/61 participants enrolled in the OLE study, 30 from the IR ralinepag group and 15 from the placebo group. The most common adverse events (AEs) were known prostacyclin-related effects (e.g., headache, 64.4%; diarrhea, 37.8%; jaw pain, 33.3%). There was a notable decline in AEs after reaching and maintaining a stable dose. At month 24 after entering the OLE, 6MWD significantly increased by a mean of 36.3 m (P = 0.004) from OLE baseline, and most participants remained stable in their functional class (84.8%). Post-baseline PVR in 1 or 2 years decreased by a median of 52.2 dyn.s/cm5 and mean pulmonary arterial pressure decreased by a median of 2.0 mmHg (P = 0.05).
Conclusion
Ralinepag produced sustained, durable improvements in 6MWD along with durable reductions in PVR and a manageable AE profile. Most participants continuing treatment with ralinepag maintained functional measures throughout the OLE and those switching from placebo to ralinepag often experienced functional improvements.
Plain Language Summary
Pulmonary arterial hypertension is a rare disease caused by elevated pressure in the blood vessels connecting the heart to the lungs. A previous phase 2 study found that ralinepag significanlty reduced pulmonary vascular resistance (the force or resistance that blood encounters as it flows through the blood vessels in the lungs) compared with placebo. This clinical study of 45 patients investigated whether ralinepag was safe and effective for long-term use to treat people with pulmonary arterial hypertension. All participants received ralinepag twice daily until a new once daily pill was available later in the study. The primary endpoints were long-term safety and tolerability, and secondary endpoints included exercise capacity, impact on daily life (functional class), clinical worsening, and hemodynamic measures (metrics to measure how well the heart is working). The study found that ralinepag had a manageable side effect profile, with a decrease in side effects for patients who continued taking ralinepag over time. Moreover, the study showed that ralinepag improved the ability to exercise, maintained functional measures, and helped to reduce pressure in the blood vessels connecting the heart to the lungs over a 24-month period for participants with pulmonary arterial hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
Pulmonary arterial hypertension (PAH) is a rare, progressive, and often fatal disease, and there is a continuing unmet need for tolerable treatments that improve clinical parameters. | |
This phase II open-label extension study in patients with PAH who completed the blinded phase II parent trial assessed the long-term safety and tolerability of ralinepag and included secondary hemodynamic endpoints. | |
What was learned from the study? | |
Safety was consistent with the parent study, with a decline in adverse events over time for patients continuing on ralinepag treatment. | |
Treatment with ralinepag produced durable improvements in 6-min walk distance and hemodynamic measures, and those continuing treatment from the parent study often maintained functional measures. |
Introduction
Pulmonary arterial hypertension (PAH) is a rare progressive disease characterized by an increase in mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR), with normal pulmonary arterial wedge pressure [1]. PAH remains a severe and often fatal condition, despite the approval of therapies for PAH that have increased the overall median survival rate [2,3,4]. There is a continuing unmet need to develop alternative, less burdensome, and more tolerable treatments that provide improvements in clinical outcomes.
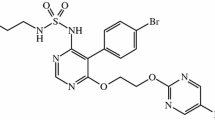
Ralinepag is a potent, titratable, orally administered prostacyclin (IP) receptor agonist being developed to treat PAH. Ralinepag promotes vasodilation and inhibits smooth muscle cell proliferation and platelet aggregation through activation of the IP receptor, which leads to increased levels of cyclic adenosine monophosphate (cAMP) [5, 6]. Ralinepag has been characterized in previous studies and has a predictable and manageable safety profile [7,8,9]. Originally designed as an immediate-release (IR) formulation, current ongoing phase III studies are utilizing an updated extended-release (XR) oral formulation, with a once-daily dosing regimen that mimics the pharmacokinetics of parenteral prostacyclin therapy [10,11,12]. With this formulation, ralinepag can be continually titrated with no maximum dose. Additionally, the prolonged half-life of the XR formulation results in lower peak-to-trough fluctuations, reducing maximum concentration (Cmax) tolerability issues and potential diminished efficacy associated with trough values of IR formulations [7, 10, 13].
A phase II, randomized, double-blind, placebo-controlled, 22-week study of participants with PAH was completed to evaluate the efficacy, safety, and tolerability of IR ralinepag [9]. The study met its primary endpoint, showing a significant reduction (29.8%, P = 0.03) in median PVR after 22 weeks of treatment with ralinepag compared with placebo (− 163.9 dyn.s/cm5 vs 0.7 dyn.s/cm5; P = 0.02). Participants reached an average dose of 259 µg (standard deviation [SD] 168 µg) of the IR formulation over the 22-week study period, with adverse events (AEs) consistent with the known safety profile of prostanoids.
During the open-label extension (OLE) of the phase II study, participants were given the opportunity to transition from the IR to the XR formulation, once it was made available. Here, we report findings from the phase II OLE study to assess the long-term safety and tolerability of ralinepag in participants with PAH who completed the phase II randomized controlled study.
Methods
Study Overview
The phase II OLE study evaluated the long-term safety and tolerability of ralinepag in participants who completed the 22 week, double-blind, parallel-group, placebo-controlled, phase II study unless the participant withdrew from treatment because of an AE or serious AE in the randomized study. All participants from the placebo group who experienced a clinical worsening event were eligible to enroll in the OLE study. All participants in the OLE study received treatment with ralinepag.
The preceding phase II study was a multicenter, randomized, double-blind, parallel-group, placebo-controlled, 22-week study [9]. Full details of the study and entry criteria have been previously published [9]. Briefly, the blinded phase II study included participants on a stable dose of one or two oral, approved, PAH therapies. Participants were titrated up to the maximum tolerated dose (MTD) reached over 9 weeks, followed by a 13-week maintenance period.
Written informed consent was obtained from all study subjects, and protocols were approved by the institutional review board at each participating study site. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Dosing
Participants continuing in the OLE study who were previously in the placebo group transitioned to orally administered ralinepag 10 µg twice-daily dosing (BID) with an individualized 9-week dose titration schedule during which dosages were uptitrated weekly according to each patient’s tolerance until an MTD was determined. This was followed by maintenance treatment. Patients previously on ralinepag continued on their dose from the preceding phase II study. Dose reductions and incremental dose increases were allowed during the maintenance phase at the investigators’ discretion. During the phase II OLE study, the XR oral formulation of ralinepag being utilized in the phase III study became available and participants were given the opportunity to transition from IR to XR ralinepag.
Primary and Secondary Objectives
The primary objective of the phase II OLE study was to evaluate the long-term safety and tolerability of ralinepag. Safety assessments included clinical laboratory assessments, vital signs, physical examination, electrocardiograms, and any AEs. Secondary objectives included changes from baseline OLE in 6MWD, World Health Organization (WHO)/ New York Heart Association (NYHA) functional class (FC), plasma N-terminal pro-brain natriuretic peptide (NT-proBNP), incidence of clinical worsening, and hemodynamic measures obtained by right heart catheterization. Baseline was defined as the last measurement prior to the first dose of ralinepag in the phase II OLE study. As a result of the reduced sample size, changes in 6MWD, WHO/NYHA FC, and NT-proBNP are presented up to month 24. Clinical worsening events included acute or chronic right heart failure, worsening of WHO/NYHA FC or at least a 20% decrease in 6MWD from baseline, addition or change in dose of PAH-specific concomitant medication, hospitalization for worsening PAH, heart–lung or lung transplant, or death or onset of treatment-related AE with fatal outcome occurring at most 14 days after discontinuing treatment. The investigator’s discretion was used to continue the current dose of ralinepag, increase dosage, interrupt treatment, or discontinue therapy if participants met the criteria for clinical worsening. Clinical assessments were performed monthly for the first 3 months for participants who were previously in the placebo group, and then every 3 months thereafter for all participants, until discontinuation or study end. After study completion, participants could elect to transition over to the ongoing phase III OLE study.
Statistical Analysis
All analyses were based on the safety population, which was defined as any participant who received ralinepag at any time during the course of the study. Baseline for all measurements was defined as the last assessment prior to the first dose of ralinepag in the OLE study. Descriptive statistics of continuous variables included the mean and SD, or median and interquartile range (IQR) of observed values, as appropriate. Discrete variables were analyzed using frequency and percentages. The time to clinical worsening was calculated from the treatment start date in the OLE study to the first occurrence of clinical worsening. Any participants who did not experience clinical worsening were censored using the last date of clinical worsening assessment or the last study visit date. No imputation was implemented for other missing data.
Results
Participant Demographics
In total, 45 participants from the 61 recruited into the blinded phase II study elected to enroll in the OLE study (Table 1 and Fig. 1). Thirty participants had previously received ralinepag (RAL to RAL), and 15 were from the placebo group (PBO to RAL). The mean age was 49.7 years, 86.7% were female, 93.3% were white, and the majority of participants were in WHO/NYHA FC II (71.1%) or III (22.2%). There was a significant difference in age between the two groups (RAL to RAL, mean 46.4 years [SD 13.2 years] vs PBO to RAL, mean 56.4 years [SD 10.8 years]; P = 0.01).
While changes in PAH background therapy were not allowed in the parent study, 12 participants (PBO to RAL, 4; RAL to RAL, 8) changed their background therapy medication (added, removed, or changed drugs within a class) after enrolling in the OLE study, which was consistent with standard of care in a global study.
Drug Exposure and Immediate Release to Extended Release
The median study drug exposure was 153.4 weeks overall, with a total exposure of 124.5 participant years (Table 2). The median exposure to IR ralinepag was 103.0 weeks (102.8 for RAL to RAL and 111.9 weeks for the PBO to RAL group), while the median exposure to XR ralinepag was 48.1 weeks (43.1 and 49.4 weeks, respectively). 6WMD and WHO/NYHA FC are reported through month 24; at this time point, 3 of the 45 participants had transitioned to the XR formulation from the IR formulation. The remainder of the study participants transitioned after month 24.
Throughout the OLE study, 26.7% of participants achieved a maximum total daily dose (TDD) of less than 200 µg, 24.4% achieved a maximum TDD of 200–400 µg, 33.3% achieved a maximum TDD of 400–600 µg, and 16% achieved a maximum TDD of at least 600 µg of ralinepag (Fig. 2). Overall, 45 participants reached a median maximum TDD in the OLE study of 350 µg.
Efficacy Endpoints
After continuing into the OLE study, participants initially assigned to PBO or RAL all experienced significant increases in mean 6MWD to month 24 of the OLE (36.3 m [SD 67.0]; P = 0.004; Fig. 3). When OLE baseline and month 24 values were compared, participants in the RAL to RAL group experienced a mean (SD) increase of 33.6 m (68.2 m) (P = 0.04) and those in the PBO to RAL group had a mean (SD) increase of 40.3 m (67.6 m) (P = 0.05). Additionally, when blinded phase II study baseline and month 24 OLE values were compared, participants in the RAL to RAL group experienced a mean (SD) increase of 76.2 m (96.2 m) and those in the PBO to RAL group had a mean (SD) increase of 87.6 m (71.5 m) (Fig. 3).
Most participants remained stable in their WHO/NYHA FC through month 24 of the OLE (28/33; 84.8%), but 2/33 (6.1%) improved and 3/33 (9.1%) deteriorated. Across the two groups, 2/20 (10.0%) participants in the RAL to RAL group and no participants in the PBO to RAL group improved; 16/20 (80.0%) and 12/13 (92.3%) participants maintained their WHO/NYHA FC, and 2/20 (10.0%) and 1/13 (7.7%) participants deteriorated, respectively.
The median plasma NT-proBNP from the OLE baseline to month 24 was largely unchanged for both groups (median change RAL to RAL, − 21.0 pg/mL; PBO to RAL, + 1.0 pg/mL), while the median NT-proBNP change from the baseline of the blinded phase II study to month 24 of the OLE study was − 158.5 pg/mL for RAL to RAL (n = 10) and 37.0 pg/mL for PBO to RAL (n = 5).
After 24 months, 42.9% of participants improved their Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) Lite 2 risk category (RAL to RAL, 50.0%; PBO to RAL, 25.0%) and 42.9% maintained their risk category (RAL to RAL, 40.0%; PBO to RAL, 50.0%; Table 3 and Fig. 4). Additionally, 40.0% of participants had improvements in the number of French non-invasive low-risk criteria at month 24 (RAL to RAL, 40.0%; PBO to RAL, 40.0%) while 46.7% maintained the number of low-risk criteria (RAL to RAL, 50.0%; PBO to RAL, 40.0%).
Overall, 12/45 (26.7%) participants showed signs of clinical worsening (8 [26.7%] RAL to RAL participants and 4 [26.7%] PBO to RAL participants; P = 1.0). The overall median time to the first clinical worsening was 58.0 weeks (35.5 weeks for RAL to RAL participants vs 71.1 weeks for PBO to RAL participants).
In total, follow-up hemodynamic assessments were completed for 31/45 participants in the OLE study with a median (IQR) follow-up time from baseline OLE of 17.9 months (14.5, 25.1 months). A delayed analysis of PVR was performed to observe changes in PVR across the blinded phase II and the OLE studies. As previously reported, PVR decreased for the RAL group in the blinded phase II study, whereas it increased slightly in the PBO group [9]. After enrolling in the OLE, both groups experienced PVR reductions at the OLE follow-up time point (Fig. 4a), with very similar median (IQR) reductions for both groups (RAL to RAL, − 223.9 dyn.s/cm5 [− 342.6, − 88.6 dyn.s/cm5]; PBO to RAL, − 202.6 dyn.s/cm5 [− 283.1, 210.8 dyn.s/cm5]; Fig. 4b).
Safety and Tolerability
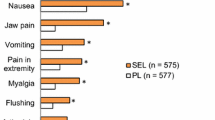
All participants experienced at least one AE during the OLE study (Table 4). The most common AEs included headache (64.4%), diarrhea (37.8%), and jaw pain (33.3%). Serious AEs occurred in 46.7% of participants overall (RAL to RAL, 53.3%; PBO to RAL, 33.3%), and severe AEs occurred in 46.7% overall (46.7% and 46.7%, respectively). In total, 82.2% experienced a treatment-related AE. Of these, 15.6% were classified as severe (RAL to RAL, 6.7%; PBO to RAL, 33.3%). The most common treatment-related AEs were headache (60.0%), jaw pain (33.3%), and diarrhea (28.9%). No serious AEs were considered to be related to the study drug.
The incidence rate of treatment-related AEs was lower for RAL to RAL participants compared with the PBO to RAL group (76.7% vs 93.3%), with a lower incidence of headache, diarrhea, and nausea for RAL to RAL participants.
AEs led to discontinuation in 7 (15.6%) participants (RAL to RAL, 5 participants; PBO to RAL, 2 participants). Other reasons for discontinuation included clinical worsening (4.4%), sponsor decision (2.2%), and consent withdrawal (4.4%). After study completion, participants could also transition into the ongoing phase III OLE study. In total, 25 participants from the phase II OLE study transitioned to the phase III program. There were a total of 6 (13.3%) deaths during the OLE study: 4 RAL to RAL participants and 2 PBO to RAL participants. No deaths were considered to be related to ralinepag exposure, rather the deaths were due to coronavirus disease 2019 (COVID-19) (n = 1), COVID-19 pneumonia (n = 1), cardiorespiratory failure (n = 1), and right heart failure (n = 3). Additionally, 2 participants who discontinued because of an AE in the RAL to RAL group died within the 28-day follow-up period (hemoptysis, n = 1; multiple organ dysfunction syndrome, n = 1).
Discussion
This phase II OLE study was conducted to evaluate the long-term safety and tolerability of continued therapy with orally administered ralinepag in participants with PAH who completed a preceding blinded phase II study. Participants who received ralinepag in the blinded phase II study continued on their current dose, and those who previously received placebo were transitioned to ralinepag and titrated until an MTD was reached.
The results of the OLE study show that ralinepag produced clinically relevant, sustained, and durable improvements over 24 months in 6MWD, with an overall median improvement from baseline of 41.0 m (P = 0.004), initially driven by PBO to RAL participants. Most participants remained stable in their WHO/NYHA FC (84.8%), including 92.3% of PBO to RAL participants. Notably, some RAL to RAL participants experienced ongoing improvements in their WHO/NYHA FC group through month 24 of the OLE study (FC shift from OLE baseline to month 24: I, 3.3% to 10.0%; II, 76.7% to 70.0%; III, 20.0% to 20.0%). Additionally, participants also experienced durable improvements in hemodynamic measures, with an overall median decrease from baseline of 52.2 dyn.s/cm5 in PVR. These long-term results support the initial blinded phase II data which reported a 20.1% reduction from baseline in PVR for participants on ralinepag (P < 0.0001) and suggest that ralinepag further decreased PVR after 22 weeks treatment in the preceding blinded study. In contrast, in the PBO group, PVR increased during the blinded study and did not decrease until ralinepag was administered in the OLE [9]. Additionally, more participants improved or had no change in their REVEAL Lite 2 and French non-invasive risk scores than deteriorated at month 24 in both groups.
Of note, these results were seen even though the majority of participants (57.7%) were on dual PAH-specific background therapy at baseline. In comparison, in the pivotal phase III study of selexipag, only 32.5% were on dual background therapy [14]. Dual background therapy with an endothelin receptor antagonist and a phosphodiesterase 5 inhibitor is currently regarded as standard of care for patients with PAH at intermediate risk [15,16,17]. It can be more difficult to assess the additive benefits of a study drug in participants taking one or two background therapies. The 6MWD test has been shown to have a ceiling effect, with additional treatments showing only modest or no improvement [18]. In the blinded phase, patients on placebo experienced an unexpected increase in 6MWD that precluded the ability to detect differences compared with the RAL group. This increase was attributed to the fact that patients on placebo had a shorter duration of PAH therapy before entering the study. The blinded phase II and OLE studies of ralinepag, however, showed a durable 6MWD treatment effect over the duration of both studies, despite a high number of participants on dual background therapy.
Our findings also support the favorable emergent safety profile of ralinepag with the IR formulation. The frequencies and severities of AEs were consistent with those seen in the preceding blinded phase II study. The incidence of treatment-related AEs was lower in RAL to RAL participants compared with PBO to RAL participants (76.7% vs 93.3%), leading to discontinuation in seven participants overall which was not unexpected given the duration of the study. This is likely due to improved tolerability to ralinepag treatment over time. The majority of AEs related to ralinepag were observed in participants who were treatment naïve, as is expected for those initiating treatment targeting the prostacyclin pathway, and no participants experienced a serious AE that was considered to be related to ralinepag treatment.
In PAH, the expression and function of prostacyclin are decreased, leading to diminished levels of cAMP and ultimately increased proliferation of vascular smooth muscle cells and decreased vasodilation [19]. Agents that target the prostacyclin pathway, such as ralinepag, stimulate the IP receptor, helping to prevent the downstream effects of the disease. The results of the blinded phase II and OLE studies show promising data regarding the efficacy and safety of IR formulation ralinepag treatment for patients with PAH and that the XR formulation has the potential to be a tolerable and easy-to-use prostacyclin therapy option for patients with PAH.
The phase III ADVANCE OUTCOMES study (NCT03626688) is currently evaluating the XR oral formulation of ralinepag. The ADVANCE OUTCOMES study has a 16-week titration period, with dosing starting at 50 µg and continuing up to the highest tolerated dose. The outcome of this ongoing phase III study will help to determine the efficacy, safety, and tolerability of XR ralinepag when added to PAH standard of care or PAH-specific background therapy. This extended-release formulation has the potential to improve tolerability by minimizing Cmax fluctuations and Cmin-related diminished efficacy while maintaining therapeutic levels of exposure throughout the day and minimizing the peak-to-trough fluctuations in drug plasma concentration that can occur with IR dosing [10,11,12,13, 20].
The analysis and conclusions obtained from this OLE study are limited by a lack of blinding or placebo control, so that bias cannot be eliminated. The sample size was relatively small with more participants from the ralinepag group in the preceding blinded phase II study having transitioned to the phase II OLE study. There is also a potential for selection bias for the cohort of participants who elected to continue in the OLE study. Long-term data are needed from participants exposed to the ralinepag XR formulation to evaluate sustained safety, tolerability, and treatment effects.
Conclusion
This phase II OLE study analysis showed that ralinepag produces durable hemodynamic improvements along with sustained, durable increases in 6MWD. Additionally, ralinepag had a predictable and manageable side effect profile.
Data Availability
United Therapeutics provides access to all individual participant data collected during the trial, after anonymization. Data are available to request after the indication studied has been approved in the USA and after primary publication acceptance, whichever is later. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data access should comply with local laws and regulations.
References
Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75.
Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension. Circulation. 2010;122(2):164–72.
D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9.
Hendriks PM, Staal DP, van de Groep LD, et al. The evolution of survival of pulmonary arterial hypertension over 15 years. Pulm Circ. 2022;12(4):e12137.
Clapp LH, Gurung R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015;120:56–71.
Adams J, Whittle B, Shen L, Patel J, Clapp LH. Potency, selectivity, and comparative platelet and vascular activity of ralinepag acting on prostacyclin receptors in human tissues. In: 6th World Symposium on Pulmonary Hypertension; February 27–March 1, 2018; Nice, France.
Adams J, Blackburn A, Parsley E, Tang Y, King C, Grundy J. 3022 Relative bioavailability and pharmacokinetic (PK) performance of a ralinepag extended-release (XR) tablet oral formulation and the effect of food and gender in healthy human subjects. Eur Heart J. 2018;39(suppl_1):ehy563-3022.
Grundy JS, King CD, Adams JW, Cabell CH. Safety, tolerability, and pharmacokinetics of the selective prostacyclin receptor agonist ralinepag in single and multiple dosing studies of an immediate-release oral formulation in healthy volunteers. Pulm Circ. 2020;10(2):1–13.
Torres F, Farber H, Ristić A, et al. Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: results from a phase 2 randomised, parallel group, placebo-controlled trial. Eur Respir J. 2019;54(4):1901030.
Adams J, Morgan M, Unett D, Whittle B. Pharmacokinetics and efficacy of ralinepag (APD811) in rats and humans. European Respiratory Society; September 9–13, 2017; Milan, Italy.
Grundy J, Blackburn A, Parsley E, Tang Y, King C, Adams J. Clinical pharmacokinetic (PK) performance of a ralinepag extended-release (XR) tablet. European Respiratory Soc; September 15–19, 2018; Paris, France.
Grundy J, Blackburn A, Tang Y, King C, Adams J. Clinical pharmacokinetic performance of a ralinepag extended-release tablet. J Heart Lung Transplant. 2019;38(4):S207–8.
Preston I, Adams J, Turner S, Christopher R. Safety and pharmacokinetics of ralinepag (APD811) in healthy adult subjects. Pulmonary Hypertension Professional Network Symposium; October 5–7, 2017; Bethesda, MD.
Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33.
Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25(142):408–17.
Burks M, Stickel S, Galiè N. Pulmonary arterial hypertension: combination therapy in practice. Am J Cardiovasc Drugs. 2018;18(4):249–57.
Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–44.
Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol. 2005;43(1):36–9.
Hassoun PM. Pulmonary arterial hypertension. N Engl J Med. 2021;385(25):2361–76.
Gidal BE, Clark AM, Anders B, Gilliam F. The application of half-life in clinical decision making: comparison of the pharmacokinetics of extended-release topiramate (USL255) and immediate-release topiramate. Epilepsy Res. 2017;129:26–32.
Acknowledgements
The authors extend our appreciation to Scott Seaman, PhD, Meredith Broderick, PharmD, JD, Brittany Davis, PhD, and Brooke Widner, PhD for their valuable insights and expertise in analyzing, interpreting data, and shaping the manuscript. Their collaborative efforts have significantly elevated the overall quality of this research. We would like to express our gratitude to Hyoshin Kim from North Carolina State University for her valuable contributions in conducting comprehensive data analyses.
Medical Writing/Editorial Assistance
The authors thank Dorothy Keine, PhD, of 3Prime Medical Writing, LLC for providing medical writing support, compensated by United Therapeutics Corporation.
Funding
This work and applicable Rapid Service and Open Access Fees was supported by United Therapeutics Corporation.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the work’s integrity as a whole, and have given their approval for this version to be published. Joan Barberà, Pavel Jansa, Elizabeth Klings, Arsen Ristić, Anne Keogh, Derek Soum, Youlan Rao, Rob Grover, Isil Saib, and Namita Sood contributed to the study conception, design, and manuscript preparation.
Corresponding author
Ethics declarations
Conflicts of Interest
Joan Barberà is a consultant and speaker for Janssen-Cilag, Merck Sharp & Dome and Ferrer, consultant for Acceleron Pharma, and has received grants from Janssen-Cilag, Merck Sharp & Dome and Ferrer. Pavel Jansa has received fees and grants from Janssen Pharmaceutical Companies of Johnson and Johnson, AOP Orphan, United Therapeutics, and Merck Sharp & Dohme. He has served as advisory boards member for Janssen Pharmaceutical Companies of Johnson and Johnson, AOP Orphan, and Merck Sharp & Dohme. Elizabeth Klings receives research support from United Therapeutics, Novartis, Bayer, Novo Nordisk/FORMA. She was an advisory board member for Vertex and Global Blood Therapeutics/Pfizer. She is the member of the safety review committee for a phase 1 trial for CSL Behring. Arsen Ristić and Anne Keogh have nothing to disclose. Namita Sood is a member of a speaker bureau for Bayer Pharmaceuticals and was a site PI for ADVANCE. Derek Solum, Youlan Rao, Rob Grover, and Isil Saib are employees of United Therapeutics.
Ethical Approval
Written informed consent was obtained from all study subjects, and protocols were approved by the institutional review board at each participating study site. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. A full list of the committees at each study site can be found in the supplement; the central IRB was Western Institutional Review Board (WIRB), which has since updated their name to WCG IRB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barberà, J., Jansa, P., Klings, E. et al. Ralinepag Phase II Open-Label Extension Study in Patients with Pulmonary Arterial Hypertension. Adv Ther 41, 1062–1074 (2024). https://doi.org/10.1007/s12325-023-02769-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02769-7