Abstract
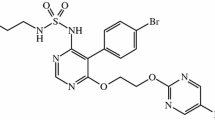
Selexipag (Uptravi®) is an orally active, first-in-class, selective prostacyclin IP receptor agonist. Selexipag was approved recently in the EU for the long-term treatment of pulmonary arterial hypertension (PAH) in adult patients with WHO functional class (FC) II or III as combination therapy in patients insufficiently controlled with an endothelin receptor antagonist and/or a phosphodiesterase type 5 inhibitor or as monotherapy in patients who are not candidates for these therapies, and in the USA for the treatment of PAH (WHO Group I) to delay disease progression and reduce the risk of hospitalization for PAH. Selexipag (200–1600 μg twice daily, as tolerated) significantly reduced the risk of the primary composite endpoint of all-cause death or a complication related to PAH (whichever happened first) versus placebo in patients with PAH (mainly WHO FC II or III) in the large, randomized, placebo-controlled GRIPHON study. The treatment effect was largely driven by significant reductions in disease progression and hospitalization for PAH. However, selexipag did not significantly reduce all-cause mortality. Additionally, the observed treatment effect was consistent in a broad range of prespecified subgroups, including treatment-naïve patients and those patients who were already receiving PAH-specific treatment at baseline. Exercise capacity was also improved with selexipag versus placebo. Selexipag was generally well tolerated, with an adverse event profile consistent with other therapies targeting the prostacyclin pathway. Thus, selexipag extends the treatment options available in patients with PAH.

Similar content being viewed by others
References
Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–75.
Pulido T, Zayas N, de Mendieta MA, et al. Medical therapies for pulmonary arterial hypertension. Heart Fail Rev. 2016;21(3):273–83.
Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50.
O’Connell C, Amar D, Boucly A, et al. Comparative safety and tolerability of prostacyclins in pulmonary hypertension. Drug Saf. 2016;39(4):287–94.
Safdar Z. Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med. 2011;105(6):818–27.
Actelion Pharmaceuticals US Inc. US prescribing information for Uptravi® (selexipag) tablets, for oral use. 2015. https://uptravi.com. Accessed 8 Nov 2016.
Actelion Pharmaceuticals Ltd. Uptravi: EU summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 8 Nov 2016.
Gatfield J, Menyhart K, Tunis M, et al. Selectivity of the selexipag active metabolite ACT-333679 for the IP receptor avoids DP1 or EP2-mediated inhibition of natural killer cell responses in vitro [abstract no. 2238 plus poster]. Am J Respir Crit Care Med. 2016;193.
Kuwano K, Hashino A, Asaki T, et al. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetamide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther. 2007;322(3):1181–8.
Kaufmann P, Okubo K, Bruderer S, et al. Pharmacokinetics and tolerability of the novel oral prostacyclin IP receptor agonist selexipag. Am J Cardiovasc Drugs. 2015;15(3):195–203.
Gatfield J, Menyhart K, Nayler O. Anti-contractile and anti-proliferative responses in human pulmonary arterial smooth muscle cells induced by ACT-333679, the active metabolite of selexipag, a non-prostanoid selective prostacyclin receptor agonist [abstract no. P2353]. Eur Respir J. 2014;44(Suppl 58).
Kuwano K, Hashino A, Noda K, et al. A long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl)acetam ide (NS-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}acetic acid (MRE-269), on rat pulmonary artery. J Pharmacol Exp Ther. 2008;326(3):691–9.
Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40(4):874–80.
Morrison K, Ernst R, Hess P, et al. Selexipag: a selective prostacyclin receptor agonist that does not affect rat gastric function. J Pharmacol Exp Ther. 2010;335(1):249–55.
Gatfield J, Menyhart K, Morrison K, et al. The non-prostanoid prostacyclin receptor agonist ACT-333679, the active metabolite of selexipag, is characterized by low beta-arrestin recruitment and receptor internalization activity [abstract no. A1542 plus poster]. J Am Coll Cardiol. 2015;65(10 Suppl).
Morrison K, Wanner D, Gatfield J, et al. Repeated oral administration of the selective prostacyclin receptor agonist selexipag does not cause tachyphylaxis in spontaneously hypertensive rats [abstract no. A1558 plus poster]. J Am Coll Cardiol. 2015;65(10 Suppl).
Bruderer S, Hurst N, Kaufmann P, et al. Multiple-dose up-titration study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of selexipag, an orally available selective prostacyclin receptor agonist, in healthy subjects. Pharmacology. 2014;94(3–4):148–56.
Bruderer S, Okubo K, Mukai H, et al. Investigation of potential pharmacodynamic and pharmacokinetic interactions between selexipag and warfarin in healthy male subjects. Clin Ther. 2016;38(5):1228–36.e1.
Hoch M, Darpo B, Remenova T, et al. A thorough QT study in the context of an uptitration regimen with selexipag, a selective oral prostacyclin receptor agonist. Drug Des Devel Ther. 2015;9:175–85.
EMA. European public assessment report (EPAR): Uptravi (selexipag). 2016. http://www.ema.europa.eu. Accessed 31 Oct 2016.
Kaufmann P, Cruz HG, Krause A, et al. Pharmacokinetics of the novel oral prostacyclin receptor agonist selexipag in subjects with hepatic or renal impairment. Br J Clin Pharmacol. 2016;82(2):369–79.
Kaufmann P, Niglis S, Bruderer S, et al. Effect of lopinavir/ritonavir on the pharmacokinetics of selexipag an oral prostacyclin receptor agonist and its active metabolite in healthy subjects. Br J Clin Pharmacol. 2015;80(4):670–7.
Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33.
Lang I, Gaine S, Galie N, et al. Effect of selexipag on long-term outcomes in patients with pulmonary arterial hypertension (PAH) receiving one, two or no PAH therapies at baseline: results from the GRIPHON study [abstract no. P2365 plus poster]. Eur Heart J. 2015;36(Suppl 1):381–2.
Channick R, Chin K, Di Scala L, et al. Individualized dosing of selexipag based on tolerability in the GRIPHON study shows consistent efficacy and safety in patients with pulmonary arterial hypertension (PAH) [abstract no. 961A plus poster]. Chest. 2015;148(4_MeetingAbstracts).
Gaine SP, Channick RN, Di Scala L, et al. Targeting the prostacyclin pathway in the treatment of connective tissue disease associated pulmonary arterial hypertension (PAH): insights from the randomized controlled GRIPHON trial with selexipag [abstract no. 283]. Am J Respir Crit Care Med. 2016;193:A6466.
Tapson VF, Channick R, Chin K, et al. Anticoagulant therapy is not associated with long term outcome in patients with pulmonary arterial hypertension (PAH): insights from the GRIPHON study [abstract no. 303]. J Heart Lung Transplant. 2016;35(4):S120.
Beghetti M, Channick R, Chin K, et al. Selexipag for pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD) after defect correction: insights from the randomized controlled GRIPHON study [abstract no. 6092]. In: The European Society of Cardiology Congress. 2016.
Preston IR, Chin K, Di Scala L, et al. Management of selexipag interruptions in the GRIPHON study [abstract no. 1870]. Eur Respir J. 2016;48(Suppl 60).
Medarov BI, Judson MA. The role of calcium channel blockers for the treatment of pulmonary arterial hypertension: how much do we actually know and how could they be positioned today? Respir Med. 2015;109(5):557–64.
Acknowledgements
During the peer review process, the manufacturer of selexipag was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Sean Duggan, Susan Keam, and Celeste Burness are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: L. J. Rubin, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, USA; O. Sitbon, Faculté de Médecine, Université Paris XI Sud, Paris, France.
Rights and permissions
About this article
Cite this article
Duggan, S.T., Keam, S.J. & Burness, C.B. Selexipag: A Review in Pulmonary Arterial Hypertension. Am J Cardiovasc Drugs 17, 73–80 (2017). https://doi.org/10.1007/s40256-016-0209-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-016-0209-9