Abstract
Introduction
In Australia, short-acting β2-agonists (SABA) are available both over the counter (OTC) and on prescription. This ease of access may impact SABA use in the Australian population. Our aim was to assess patterns and outcome associations of prescribed, acquired OTC and reported use of SABA by Australians with asthma.
Methods
This was a cross-sectional study, using data derived from primary care electronic medical records (EMRs) and patient completed questionnaires within Optimum Patient Care Research Database Australia (OPCRDA). A total of 720 individuals aged ≥ 12 years with an asthma diagnosis in their EMRs and receiving asthma therapy were included. The annual number of SABA inhalers authorised on prescription, acquired OTC and reported, and the association with self-reported exacerbations and asthma control were investigated.
Results
92.9% (n = 380/409) of individuals issued with SABA prescription were authorised ≥ 3 inhalers annually, although this differed from self-reported usage. Of individuals reporting SABA use (n = 546) in the last 12 months, 37.0% reported using ≥ 3 inhalers. These patients who reported SABA overuse experienced 2.52 (95% confidence interval [CI] 1.73–3.70) times more severe exacerbations and were 4.51 times (95% CI 3.13–6.55) more likely to have poor asthma control than those who reported using 1–2 SABA inhalers. Patients who did not receive SABA on prescription (43.2%; n = 311/720) also experienced 2.71 (95% CI 1.07–7.26) times more severe exacerbations than those prescribed 1–2 inhalers. Of these patients, 38.9% reported using OTC SABA and other prescription medications, 26.4% reported using SABA OTC as their only asthma medication, 13.2% were prescribed other therapies but not SABA OTC and 14.5% were not using any medication.
Conclusion
Both self-reported SABA overuse and zero SABA prescriptions were associated with poor asthma outcomes. The disconnect between prescribing authorisation, OTC availability and actual use, make it difficult for clinicians to quantify SABA use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
In Australia short-acting β2-agonists (SABAs) are available both over the counter (OTC) and on prescription. This ease of access may impact SABA use in the Australian population. |
We assessed SABA inhaler prescription, acquisition and usage patterns, the prevalence of SABA overuse (≥ 3 inhalers/year), both prescription and self-reported, and its relationship with asthma outcomes in persons aged 12 years and older, living with asthma in Australia. |
What was learned from the study |
The potential for SABA overuse was apparent from electronic medical records in many cases (92.9% of patients) but could also be hidden from medical view; SABA was over acquired OTC and over-used, by 37.5% and 37.0% of patients, respectively. |
Patients who self-reported overusing SABA and those who received zero SABA prescriptions in the last year experienced 2.52 (95% confidence interval [CI] 1.73–3.70) and 2.71 times (95% CI 1.07–7.26) more severe exacerbations respectively, than those prescribed or who used 1–2 SABA inhalers. |
Both zero SABA prescriptions and patient-reported overuse of SABA serve as a marker of higher exacerbation risk and should prompt a review of treatment needs. |
Introduction
Short-acting β2-agonists (SABAs) are the most widely prescribed asthma treatment today [1]. They provide effective relief from bronchoconstriction and its associated symptoms [2], engendering a strong emotional attachment by patients [3]. It is for this reason that patients often preferentially use SABAs when asthma symptom control begins to deteriorate [2], an approach which increases the potential for SABA overuse (defined as use of ≥ 3 inhalers/year), and risk of adverse outcomes [4,5,6,7,8,9,10,11]. High prescribing of SABAs has been identified as a key factor in over 40% of asthma deaths [12]. An increased risk of exacerbations with SABA overuse and associated systemic corticosteroid use also exposes patients to the risk of medication side-effects [13], further deteriorating asthma control and potential lung function decline [14]. The Australian Asthma Handbook (AAH) advises that regular low-dose ICS plus as needed SABA or as needed ICS/formoterol are suitable replacements for stand-alone SABA [15].
The SABA use IN Asthma (SABINA) studies have explored asthma treatment prescription patterns from around the world, and reported a global trend for over-prescribing SABA inhalers (defined as prescription of ≥ 3 SABA inhalers/year) [4,5,6], ranging from a low of 7.6% in South Korea, 52.6% in Australia, and up to > 70% in Kenya and South Africa [4,5,6]. Australia provides a unique perspective to investigate SABA use as it is available both over the counter (OTC) and authorised on prescription; a maximum of two inhalers may be issued on the initial prescription and an additional 10 inhalers on five automated prescription repeats can be issued by pharmacists without medical review in every 6-month period (i.e. potentially 24 inhalers in a 12 month prescription).
Although patterns of SABA use in Australia have previously been investigated, these studies have not captured the full picture, as they collected data from either electronic medical records (EMRs) or patient completed questionnaires, but not both, and the definition of SABA overuse varied [6, 8, 16]. Whilst EMRs record the intended medical treatments prescribed by primary care physicians, the Australian system does not capture the number of prescriptions dispensed at pharmacies or inhalers purchased OTC and thus do not necessarily equate to the number of inhalers a patient uses. Questionnaires are also susceptible to responder and recall bias. Thus, the combined use of EMRs and patient completed questionnaires permits a more comprehensive assessment of SABA prescription, acquisition, and usage patterns in the Australian population; allowing for a more in-depth assessment of the SABA treatment landscape, considering SABA accessibility in real life and true self-management behaviours which may be over-estimated using prescription data alone. It is anticipated that this multi-modal data collection could identify factors relevant to Australian patients and the wider healthcare sector contributing to SABA usage patterns in this population.
Our aims were to assess SABA inhaler prescription, acquisition and usage patterns (overall and by health card status), the prevalence of SABA overuse (both prescription and self-reported) and its relationship with self-reported asthma control and severe exacerbations in persons aged ≥ 12 years, living with asthma in Australia.
Methods
Study Design
This was an observational, cross-sectional study, using data derived from primary care EMRs and patient completed questionnaires contained within Optimum Patient Care Research Database Australia (OPCRDA). The dataset used for the present investigation comprised EMR data from the OPCRDA included in the SABINA III study which was conducted between March 2019 until January 2020 [6], plus additional OPCRDA EMR patient data (collected up to September 2021), and supplemented with patient-completed questionnaire data collected through Optimum Patient Care Australia’s (OPCA) primary care clinical audits delivered as part of quality improvement (https://optimumpatientcare.org.au/asthma/) (Fig. 1). The reference date for inclusion of a patient’s EMR and questionnaire was 12 months prior to the receipt of completed patient questionnaires. Information on regulatory and ethical approval is provided in the Supplementary Material.
Data Sources
Electronic Medical Records
Patient EMR data was obtained from OPCRDA, a non-for-profit research database, established and maintained by OPCA. Specifically, OPCRDA contains patient data from primary care practices and respiratory and allergy specialists across Australia, who have agreed to contribute de-identified patient data, and provides anonymised datasets for ethically approved studies. Individuals have the right to opt out of data sharing. OPCRDA currently contains data from 880,943 patients. The median retrospective period of medical records is 13 years.
Patient-Completed Questionnaires
Patients with asthma included in the OPCRDA were also asked to complete a questionnaire (available in Supplementary Material). These were sent via mail and could also be completed online.
Variables Collected
Table 1 provides a description of each study variable and the data source (either EMR or patient-completed questionnaire) used for their collection. The maximum number of SABA inhalers authorized on prescription annually, including any authorised repeats provided, was obtained from patient EMRs. As EMR prescription data is not linked to pharmacy dispensing software, this information provided the maximum of SABA inhalers which could be obtained on prescription, but not the actual number acquired or used. The patient-completed questionnaires captured information on the annual number of SABA inhalers acquired OTC, as well as self-reported SABA use (prescription or OTC), number of severe exacerbations in the last 12 months and asthma control status. In this manuscript the term prescription refers to SABA inhalers authorised on prescription by a clinician, including any authorised repeats; acquisition refers to SABA inhalers purchased by patients OTC without a prescription; and usage refers to patient-reported SABA inhaler use. A severe exacerbation was defined as the need for a course of acute OCS (≥ 20 mg/day), the need for emergency medical services for asthma or a hospital admission for asthma. The level of asthma symptom control was categorized using GINA control criteria [17].
Study Population
The study cohort consisted of male and female individuals (aged ≥ 12 years-old) with a documented diagnosis of asthma in their EMRs and who received asthma therapy at least once since the data of diagnosis. Individuals with a diagnosis of any chronic respiratory disease other than asthma (e.g. chronic obstructive pulmonary disease, cystic fibrosis) were excluded from the current study.
Study Outcomes
Primary outcomes included the assessment of SABA inhaler prescription, acquisition (OTC) and self-reported usage patterns in the last 12 months, and quantification of the proportion of patients who were over-prescribed, over-acquired, or over-used SABA (defined as ≥ 3 inhalers/year for each category). This limit was established using previously published assumptions [4, 6]. Secondary outcomes included the mean number of SABA inhalers prescribed and used according to health card status, and the association of SABA use (prescribed or patient-reported use) with self-reported severe asthma exacerbations and uncontrolled asthma symptoms.
Statistical Analysis
Demographic and clinical features were descriptively summarised and overall population and by asthma severity, categorised as AAH treatment steps. The proportion of patients with a SABA prescription, who acquired SABA OTC and who used SABA in the last 12 months were described categorically, overall and by AAH severity and compared within group using Chi squared test. The proportion of patients who were over-prescribed, over-acquired or over used SABA, and the mean number of SABA prescribed or used/year according to health card status were summarized using descriptive statistics. The number of SABA inhalers authorised on prescription were checked against self-reported rates of SABA use using a matrix table. Ordered logistic regression was used to examine the association of SABA authorised on prescription (as per EMR) and used (as self-reported by patients) on the level of self-reported asthma symptom control (odds ratio). Negative binomial regression was used to examine the effect of SABA authorised on prescription (as per EMR) and used (as self-reported by patients) on self-reported severe exacerbations (incident rate ratio). Data were adjusted for age, gender, education level, smoking status, AAH treatment intensity, health insurance, BMI and number of comorbidities.
The association of SABA inhalers acquired OTC among patients with 0 SABA inhalers authorized on prescription on self-reported severe exacerbations and asthma control was assessed post-hoc, and asthma medications used by patients with 0 SABA prescriptions stratified by OTC SABA acquisition. General demographic information and asthma medications used by patients with 0 SABA prescriptions, stratified by occurrence of self-reported severe asthma exacerbations, were also described post-hoc. All statistical analyses were performed using R statistical software (version 3.6.0), with all tests 2-sided and significance defined as 5%.
Results
Subject Disposition
Of 880,943 patients included in the OPCRDA, 53,050 had evidence in their EMR of asthma or COPD by diagnosis or treatment, and 21,319 were aged ≥ 12 with active asthma. Of these, 720 completed the patient questionnaire as part of a primary care clinical audit and were included in this study (Fig. 2).
Patient Demographics and Clinical Characteristics
The study population had a mean age of 53.1 (SD: 19) years and was predominantly female (69.3%), overweight/obese (72.2%), educated to university level (49.3%), did not hold a healthcare or concession card (62.6%) and had never smoked (66.6%) (Table 2). Most patients were at AAH treatment steps 3 or 4 (62.2%) and had 1–2 co-morbidities (42.2%).
Allergic rhinitis was the most common co-morbidity (84.4%), followed by obesity (52.4%) (Table 2). Of the co-morbidities mimicking or exacerbating asthma, depression and anxiety was the most common (38.8%) (Table 2). Co-morbidity prevalence patterns were similar in those with less severe (AAH steps 1–2) and with more severe disease (AAH steps 3–4). On average, patients experienced 0.8 exacerbations/year (SD 1.9) and 59.4% had partly- or un-controlled symptoms (Table 2).
EMR Recorded SABA Prescribing Patterns and Add-on Therapies
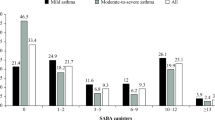
In the last 12 months, 56.8% (n = 409/720) of the study cohort received a prescription for SABA inhalers (Table 3). Of these individuals, 92.9% (n = 380/409) were issued with a prescription that permitted dispensing of ≥ 3 inhalers in the next 12 months and 87.5% (n = 358/409) were issued with prescriptions that permitted dispensing of ≥ 10 inhalers in the next 12 months (Table 3; Fig. 3). SABA authorization patterns were similar for males and females (S-Table 1). Individuals on AAH treatment steps 3–4, were more likely to have received a prescription for ≥ 3 SABA inhalers (97.5%, n = 268/275) than those at steps 1–2 (83.6%, n = 112/134, p < 0.001) (Table 3).
In relation to the 43.2% (n = 311/720) of individuals who were not prescribed SABA, 26.4% (n = 82/311) reported using OTC SABA as their only asthma medication, 13.2% (n = 41/311) were prescribed other therapies and did not use OTC SABA, 38.9% (n = 121/311) reported using OTC SABA and other prescription medications and 14.5% (n = 45/311) reported that they were not using any pharmacological interventions (Table 4). In the subset of individuals who did not receive a SABA prescription but were using other medications to control their asthma, ICS/LABA combinations used in isolation were most commonly prescribed (86.4%, n = 152/176) (Table 4).
Self-reported OTC SABA Acquisition Patterns
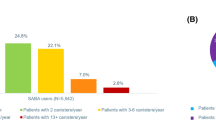
Thirty two percent of the total cohort (n = 208/650) reported acquiring SABA OTC in the last 12 months (Table 3). Whilst the majority of these individuals acquired 1–2 inhalers (62.5%, n = 130/208), more than one third (37.5%, n = 78/208) of people purchasing OTC SABA acquired ≥ 3 inhalers annually (Table 3; Fig. 3). Rates of self-reported OTC SABA acquisition were not statistically different (p = 0.075) across the asthma severity spectrum (Table 3), and were similar for males and females (S-Table 1).
Most individuals (70.2%, n = 203/289) who reported acquiring SABA OTC did not have an authorised prescription (Table 3). Among individuals who were not prescribed SABA but reported acquiring ≥ 1 inhalers/year OTC, the majority (59.6%, n = 121/203) were using both OTC SABA and other prescription medications to manage their condition. ICS/LABA combinations used alone were the most commonly prescribed medications (85.1%, n = 103/121) issued to this subpopulation (Table 4).
For patients who reported acquiring SABA by both means, OTC and authorised on prescription (n = 5), all were at AAH treatment intensity steps 3–4 and acquired ≥ 3 inhalers/year (Table 3).
Self-reported SABA Usage Patterns
Three quarters of the total study cohort (75.8%, n = 546/720) reported using SABA in the last 12 months. Of these individuals, the majority (63.0%, n = 344/546) reported using 1–2 inhalers, whilst the remainder (37.0%, n = 202/546) reported using ≥ 3 inhalers/year (Table 3; Fig. 3). Self-reported SABA usage patterns were similar for males and females (S-Table 1), but was statistically more likely to occur in individuals with more severe disease; 43.0% (n = 150/349) of patients at AAH steps 3–4 who obtained a SABA reported using ≥ 3 SABA inhalers/year compared to 26.4% (n = 52/197) of those at AAH steps 1–2 (p = 0.001) (Table 3).
Alignment of EMR Recorded SABA Prescribing Patterns with Self-reported SABA Usage Patterns
The number of EMR recorded SABA inhalers authorised on prescription in the last 12 months did not align with annual self-reported SABA usage (S-Table 2). For example, 94.6% of individuals (n = 331/350) who were authorised a maximum of 12 inhalers on prescription reported using less, with 66.0% (n = 231/350) reporting use of < 3 inhalers/year. Likewise, 69.5% of individuals (n = 216/311) who were NOT issued with an authorisation for SABA reported using ≥ 1 inhalers in the last 12 months, which had been acquired OTC, and 22.5% (n = 70/311) reporting use of ≥ 3 OTC SABA inhalers/year (S-Table 2).
SABA Prescription and Self-reported SABA Use According to Healthcare or Concession Card Status
Individuals with a healthcare or concession card received a higher number of SABA prescriptions/year compared to those without a health card irrespective of AAH treatment intensity (Fig. 4A). This pattern was not so apparent for SABA self-reported usage. Although individuals at AAH treatment step 2 with a health card reported using twice as many inhalers/year compared to patients at the same AAH step who did not hold a health card, individuals at AAH treatment step 4 without a health card, used more SABA inhalers than those with a health card (Fig. 4B).
Association of EMR Recorded SABA Prescribing Patterns and Asthma Outcomes
Compared to recommended SABA prescription (1–2 inhalers/year), prescription of ≥ 3 SABA inhalers/year was not associated with an increase in self-reported severe exacerbations (IRR 2.12; 95% CI 0.83–5.72) or lack of asthma symptom control (OR 1.68; 95% CI 0.82–3.6) (Fig. 5A, B). However, individuals prescribed zero SABA inhalers/year experienced 2.71 times (95% CI 1.07–7.26; p = 0.037) more self-reported severe exacerbations than those prescribed 1–2 inhalers (Fig. 5A).
Association of short-acting β2-agonist (SABA) inhalers authorised on prescription annually and self-reported SABA used (acquired either OTC or on prescription) on A self-reported severe exacerbations and B uncontrolled asthma symptoms. Data are adjusted for age, gender, education level, smoking status, AAH treatment intensity, health insurance, BMI and number of comorbidities. AAH Australian asthma handbook, BMI body mass index, CI confidence interval, IRR incident rate ratio, OR odds ratio
To better understand this observation, individuals who were prescribed zero SABA inhalers were classified into one of two groups based on the occurrence of self-reported severe exacerbations post-hoc (Table 5). Individuals who were prescribed zero SABA inhalers and experienced one or more self-reported severe exacerbations appeared more likely to be using an ICS/long acting β2-agonist combination as their maintenance therapy (63.1% vs 41.8%), purchase SABA OTC (81.6% vs 57.2%), and when doing so acquire ≥ 3 inhalers annually (44.7% vs 13.0%), than those prescribed zero SABA inhalers and who experienced 0 exacerbations in the last 12 months (Table 5).
Association of SABA OTC Acquisition and Asthma Outcomes
Individuals who reported acquiring ≥ 3 SABA inhalers/year OTC (and who had 0 SABA inhalers authorized on prescription; n = 73/289, 25.3%) experienced 3.05 more self-reported exacerbations (95% CI 1.63, 5.71; p < 0.001) and were 4.75 times (95% CI 2.61, 8.80; p < 0.001) more likely to have uncontrolled asthma, than those who acquired 1–2 inhalers (S-Table 3).
Association of Self-reported SABA use with Asthma Outcomes
Individuals who self-reported using ≥ 3 SABA inhalers/year experienced more than twice as many self-reported severe exacerbations (IRR 2.52; 95% CI 1.73–3.70; p < 0.001) and were over four times more likely to have uncontrolled asthma symptoms (OR 4.51; 95% CI 3.13–6.55; p < 0.001) than those who used 1–2 inhalers annually (Fig. 5A, B). Conversely, individuals who reported using zero SABA inhalers/year were less likely to have uncontrolled asthma symptoms (OR 0.42; 95% CI 0.28–0.62; p < 0.001) than those using 1–2 inhalers (Fig. 5B).
Discussion
Ours is the first study to use both EMR and patient completed questionnaire data to examine SABA prescription, acquisition, and usage patterns in Australia, enabling a comprehensive view of the Australian SABA landscape from both the physician and patient perspectives. Collecting data from both sources allowed us to: assess the mis-alignment between SABA prescription and usage; explore the impact of healthcare and concession card status on SABA prescribing patterns; investigate how patients acquire OTC SABA and use it in real life; and quantify the association between asthma outcomes when SABA is both prescribed appropriately and over-prescribed and used. We found that SABA was over-prescribed (92.9% of patients); over acquired OTC (37.5%) and over-used (37.0%) by many Australian patients living with asthma. The potential for SABA overuse was apparent from EMR records in many cases but could also be hidden from medical view; 43.2% of patients had zero SABA prescriptions in their EMR records, but 76% of patients reported using SABA. There was a strong association between both patient-reported SABA OTC acquisition and SABA overuse on poor asthma outcomes. A zero SABA prescription was also a red flag; these patients experienced more than twice as many severe exacerbations than those authorised a prescription for 1–2 inhalers and may be mostly hidden from clinical view. Even in patients receiving maintenance ICS-LABAs, OTC SABA purchases served as a marker of higher exacerbation risk and should prompt a review of treatment needs.
In agreement with previous studies we found a link between SABA over-prescription and poor asthma outcomes [4,5,6, 18], with SABA overuse likely the result of chronic poor asthma control rather than its cause. Assessment of SABA use represents an important tool for measuring the success of asthma management, informing treatment modification decisions [19], may encourage physicians and pharmacists to more carefully consider SABA prescription and recommendation practices, and prompt investigations of asthma control, adherence and inhaler technique. Importantly, we found that the rate of SABA authorisations did not agree with acquisition or usage; for example, 22.5% of patients did not have a SABA prescription, but reported acquiring 3 SABA inhalers/year OTC, and are essentially hidden from healthcare provider view. This may be unique to the availability of OTC SABA in Australia, as rates of self-reported SABA acquisition and usage (reported by just over one-third of the study cohort) were more indicative of consumer behaviour. This rate of SABA overuse is lower than previously published (70.1–73.9%), most likely due to differences in definition of high SABA usage (i.e. defined as > 2 occasions/week in the past 4 weeks in prior studies) [8, 16] and rigour of asthma diagnosis (i.e. previous investigations did not require participants to have a clinician-confirmed diagnosis of asthma).
The over-prescribing and overuse of SABA in Australia is likely a consequence of numerous factors including patient expectations, knowledge and behaviour, as well as patient-physician communications and physician resources and time [16, 20, 21]. In other countries, physician over-prescribing behaviours have been attributed to variability in thresholds for acceptable SABA use, questioning of the risk of morbidity and mortality with high SABA use, and a consideration that asthma guidelines are too ‘stringent’ [12]. Overuse of SABA by patients has been linked to a lack of knowledge that frequent usage would worsen asthma control, and strong psychological links to use of SABA, due to immediate relieving effects [22]. SABA overuse in Australia may also reflect specific peculiarities of the healthcare system and reimbursement practices [23, 24]. For example, the primary care prescription software used in Australian practices currently defaults to authorising a maximum of 12 inhalers on one prescription. A higher number of SABA prescriptions was also noted in those with a healthcare or concession card (irrespective of AAH treatment intensity) and in individuals in the general population classified as AAH treatment step 4. In these subsets of patients additional SABA authorisations and usage, respectively, may be viewed as a way to minimise the cost of repeat consultations and is a cheaper alternative to anti-inflammatory therapies.
OTC availability of SABA was established in Australia in the 1990’s to help curb mortality rates by ensuring patients could access relievers in an emergency [25, 26]. The message to patients of having constant access to relievers has since continued, particularly so after the epidemic thunderstorm asthma event that occurred in 2016 [27]. In the present investigation, acquisition patterns for OTC SABA were particularly worrisome in patients without a prescription and who self-reported experiencing ≥ 1 severe exacerbation. These individuals were more likely to purchase ≥ 3 SABA inhalers/year (44.7%), compared to patients with no SABA prescription and no self-reported exacerbations (13.0%). Again, it is possible these individuals view OTC SABA as a cheaper alternative than attending a medical consultation, but as a result may never had had their asthma fully assessed or received education on what to do in the event of deterioration of asthma symptom control.
In terms of clinical implications arising from this study, there is clearly a need to improve guideline directed treatment, and to align with recent evidence-based changes in both the AAH and GINA report [15, 28, 29]. GINA states clearly that “reducing and, ideally, eliminating the need for SABA reliever is both an important goal in asthma management and a measure of the success of asthma treatment” [28]. Several different strategies will be needed to achieve this—for example reducing maximum number of repeat prescriptions and the number of inhalers dispensed at any one time, and incentivizing regular review and treatment follow up (potentially in the form of remuneration for completion and review of asthma plans for primary care practitioners and respiratory medication reviews for pharmacists). Reducing the maximum number of inhalers allowed on any one prescription might have minimal impact on patients with asthma, but could help clinicians to more reliably estimate usage. This would create an opportunity for clinical review and patient education [16]. The recently published manifesto on SABA overuse also advocates for widespread education of health care providers on the revised GINA guidelines, appropriate patient education to stop self-medication and a transition to ICS/formoterol to reduce risk of exacerbations, mortality and hospitalizations [29]. Reducing OTC SABA acquisition could also be addressed at the pharmacy through the implementation of (1) pharmacist-led discussions around preventing high risk outcomes and behaviours to encourage change [30, 31], (2) education on the importance of ICS medications in managing asthma symptoms [30, 31], (3) implementation of programs to monitor acquisition patterns, potentially through an online system such as “My Health Record” which would overcome the challenge of individuals using multiple pharmacies.
A key strength of the current study was the dual data collection modalities (EMR and questionnaires), affording us the opportunity to identify how delivery of care can be improved for these patients. Collecting data on patient-reported OTC SABA acquisition and use has provided insight into patient behaviours not available via EMRs alone. Limitations include those associated with observational studies and patient reported data collection (e.g. recall bias, selection bias and missing data). Those with poorer asthma control may have been more motivated to complete our asthma questionnaire. The relatively small size of the study cohort may also have limited generalizability to the wider asthmatic population. In relation to the latter, despite having > 21,000 patients in the OPCRDA with a diagnosis of asthma and no other respiratory tract condition, only 3.4% of this population completed the questionnaire making them eligible for inclusion in the present study. This may have skewed our findings and should be considered when interpretating the data. The COVID-19 pandemic may have contributed to lower responder rate and may also have altered SABA usage patterns. Secondly, whilst a clinician’s diagnosis of asthma was required for inclusion in the present investigation, confirmation via spirometry was not mandated and there was no upper age limit for inclusion so there is potential for misdiagnosis in the study cohort. Finally, it should be noted that environmental factors at the time of the study may have contributed to the reported rates of SABA acquisition and usage. Data was collected during the 2019–2020 Australian bushfire season and individuals may have acquired and used or just acquired spare inhalers but not necessarily used more SABA compared to previous years.
Conclusion
In conclusion, despite the fact that GINA identifies the elimination of SABA reliever as a measure of treatment success, over prescription and overuse of SABA to treat asthma continues to be a problem in Australia. It is seen across the severity spectrum and is associated with worse asthma outcomes. Due to OTC availability of SABA in Australia, doctors may be unaware of the true extent of overuse in their patients. Removal of the default settings for repeat SABA prescriptions and limiting repeats enabling high numbers of canisters, monitoring OTC purchases, promotion of clinician and pharmacist review and patient education could be used to address excessive SABA use in Australia. It’s time to end the reign of SABA in Australia.
Data Availability
The authors do not have permission to give public access to the study dataset. However, researchers may request access to OPCRDA data for their own purposes. Access to OPCRDA can be made via the OPCRDA website (https://optimumpatientcare.org.au/contact-us/) or via the enquiries email audit@optimumpatientcare.org.
References
Crooks MG, Faruqi S. It is time to end our love affair with short-acting β(2)-agonists in asthma. ERJ Open Res. 2022;8:00353–2022.
Martin MJ, Harrison TW. Is it time to move away from short-acting beta-agonists in asthma management? Eur Respir J. 2019;53:1802223.
Blakeston S, Harper G, Zabala MJ. Identifying the drivers of patients’ reliance on short-acting β2-agonists in asthma. J Asthma. 2021;58:1094–101.
Bloom CI, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk RJP, et al. Asthma-related health outcomes associated with short-acting β(2)-agonist inhaler use: an observational UK study as part of the SABINA Global Program. Adv Ther. 2020;37:4190–208.
Quint JK, Arnetorp S, Kocks JWH, Kupczyk M, Nuevo J, Plaza V, et al. Short-acting Beta-2-agonist exposure and severe asthma exacerbations: SABINA findings from Europe and North America. J Allergy Clin Immunol Pract. 2022;S2213–2198(22):00285–9.
Bateman ED, Price DB, Wang H-C, Khattab A, Schonffeldt P, Catanzariti A, et al. Short-acting β(2)-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59:2101402.
FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–40.
Azzi EA, Kritikos V, Peters MJ, Price DB, Srour P, Cvetkovski B, et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9: e028995.
Price D, Wilson AM, Chisholm A, Rigazio A, Burden A, Thomas M, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1–12.
Blakey JD, Price DB, Pizzichini E, Popov TA, Dimitrov BD, Postma DS, et al. Identifying risk of future asthma attacks using UK medical record data: a respiratory effectiveness group initiative. J Allergy Clin Immunol Pract. 2017;5:1015-1024.e8.
Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872.
McKibben S, Bush A, Thomas M, Griffiths C. “Tossing a coin:” defining the excessive use of short-acting beta(2)-agonists in asthma-the views of general practitioners and asthma experts in primary and secondary care. NPJ Prim Care Respir Med. 2018;28:26.
Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling ZJJ, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204.
Soremekun S, Heaney L, Skinner D, Bulathsinhala L, Carter V, Chaudhry I, et al. Asthma exacerbations are associated with a decline in lung function: a longitudinal population based study. Thorax. 2023;78:643–52 (Epub ahdead of print).
National Asthma Council. Australian Asthma Handbook Version 2.2 [Internet]. 13th ed. 2022. https://www.asthmahandbook.org.au/. Accessed 9 Dec 2022.
Azzi E, Kritikos V, Peters M, Price D, Cvetkovski B, Alphonse PS, et al. Perceptions, attitudes, and behaviors of short-acting beta(2) agonist users: an Australian cross-sectional community pharmacy-based study. J Asthma. 2022;59:178–88.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2023. 2023; https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf. Accessed 9 Dec 2022.
Price D, Hancock K, Doan J, Taher SW, Muhwa CJ, Farouk H, et al. Short-acting β(2)-agonist prescription patterns for asthma management in the SABINA III primary care cohort. NPJ Prim Care Respir Med. 2022;32:37.
Domingo C, Singh D. The changing asthma management landscape and need for appropriate SABA prescription. Adv Ther. 2023;40:1301–16.
Sawyer SM, Fardy HJ. Bridging the gap between doctors’ and patients’ expectations of asthma management. J Asthma. 2003;40:131–8.
Chung LP, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust. 2018;209:S34-40.
Loh ZC, Hussain R, Balan S, Saini B, Muneswarao J, Ong SC, et al. Perceptions, attitudes, and behaviors of asthma patients towards the use of short-acting β2-agonists: a systematic review. PLoS One. 2023;18: e0283876.
Ampon RD, Reddel HK, Correll PK, Poulos LM, Marks GB. Cost is a major barrier to the use of inhaled corticosteroids for obstructive lung disease. Med J Aust. 2009;191:319–23.
Laba T-L, Jan S, Zwar NA, Roughead E, Marks GB, Flynn AW, et al. Cost-related underuse of medicines for asthma-opportunities for improving adherence. J Allergy Clin Immunol Pract. 2019;7:2298-2306.e12.
O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54:1900491.
Reddel HK, Cooper, S, Guevara-Rattray E, Ampon RD, Marks GB. Asthma control in Australia 1990–2011 21 years since the introduction of asthma managment guidelines—where are we now? [Internet]. Woolcock Institute of Medical Research; 2013. https://www.woolcock.org.au/pdf/downloads/Asthma-control-in-Australia-1990-2011.pdf. Accessed 13 Jul 2022.
Department of Health SG of V. Epidemic thunderstorm asthma [Internet]. Epidemic thunderstorm asthma. 2022. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/thunderstorm-asthma. Accessed 11 Nov 2022.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention [Internet]. 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf. Accessed 19 Oct 2022.
Canonica GW, Paggiaro P, Blasi F, Musarra A, Richeldi L, Rossi A, et al. Manifesto on the overuse of SABA in the management of asthma: new approaches and new strategies. Ther Adv Respir Dis. 2021;15:17534666211042534.
Putman B, Coucke L, Vanoverschelde A, Mehuys E, Lahousse L. Community pharmacist counseling improves adherence and asthma control: a nationwide study. BMC Health Serv Res. 2022;22:112.
Bridgeman MB, Wilken LA. Essential role of pharmacists in asthma care and management. J Pharm Pract. 2021;34:149–62.
National Asthma Council. Australian Asthma Handbook [Internet]. Managing Asthma in Adults. (2022). Available from: https://web.archive.org/web/20171129113442/, http://www.asthmahandbook.org.au/management/adults. Accessed 20 Sept 2022.
Acknowledgements
We wish to acknowledge and thank Marion Magee, Rob Campbell, Ying Liu, Nicole O’Sullivan, Ondrej Rejda, Lisa Sugg, Steph James, Kiran Dhillon, and Sophie Jones of the OPCA Improving Asthma outcomes in Australia Research Group for their valuable contributions in making this publication possible. We also thank all patients who provided consent for inclusion of their EMR data into the OPCRDA and for completing study questionnaires.
Medical Writing, and Editorial Assistance
Writing, editorial support, and/or formatting assistance in the development of this manuscript were provided by Shilpa Suresh, MSc, of the Observational and Pragmatic Research Institute, Singapore.
Funding
This study was conducted by Optimum Patient Care Australia (OPCA) and was partially funded by AstraZeneca and Optimum Patient Care Australia (OPCA). The cost of the Open Access Fee were provided by AstraZeneca. No funding was received by the Observational & Pragmatic Research Institute Pte Ltd (OPRI) for its contribution.
Author information
Authors and Affiliations
Consortia
Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors (David Price, Christine Jenkins, Kerry Hancock, Rebecca Vella, Florian Heraud, Porsche Le Cheng, Ruth Murray, Maarten Beekman, Sinthia Bosnic-Anticevich, Fabio Botini, Victoria Carter, Angelina Catanzariti, Joe Doan, Kirsty Fletton, Ata Kichkin, Thao Le, Chantal Le Lievre, Chi Ming Lau, Dominique Novic, John Pakos, Kanchanamala Ranasinghe, Alexander Roussos, Josephine Samuel-King, Anita Sharma, Deb Stewart, Bruce Willett, and Eric Bateman) made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. The first draft of the manuscript was written by Dr. Rebecca Vella and all authors took part in drafting, revising or critically reviewing the article. All authors gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work. All authors have given approval for the submission of this article. The authors received no direct compensation related to the development of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Kerry L. Hancock has received speakers’ fees, consulting honoraria and/or travel grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini Australia, Mylan and Novartis. Sinthia Bosnic-Anticevich has received honorarium for participation in expert advisory boards and given lectures for Teva Pharmaceuticals, AstraZeneca, GSK, Meda, Mundipharma, Sanofi, Mylan and received unrestricted research grants from Mylan, AstraZeneca, Teva and Mundipharma International. Angelina Catanzariti is an employee of AstraZeneca. Christine Jenkins, Joe Doan, Ata Kichkin, Chi Ming Lau, Dominique Novic, John Pakos, Kanchanamala Ranasinghe, Josephine Samuel-King, Bruce Willet, and Thao Le declares no conflict of interest. Anita Sharma is a practising Primary Care Physician and Senior Lecturer, School of Clinical Medicine-Primary Care Clinical Unit, University of Queensland. She supervises clinical training of primary care doctors and serves on advisory boards for Diabetes, Heart Failure and Osteoporosis for Novartis, Merck Sharp & Dohme and Boehringer Ingelheim, Eli Lilley and Amgen. Eric Bateman has received honorarium for participation in advisory boards from ALK, AstraZeneca, Novartis, Regeneron and Sanofi Aventis, and for giving lectures for AstraZeneca, Chiesi, Menarini, Novartis, Orion, Regeneron and Sanofi Aventis. He is a member of the Board and Science Committee of GINA. Maarten JHI Beekman was an employee of AstraZeneca at time of study conduct. Rebecca Vella, Florian Heraud, Porsche Le Cheng, Fabio Botini, Thao Le, Chantal Le Lievre, Alex Roussos are employees of Optimum Patient Care Australia. Ruth Murray is a consultant for the Observational and Pragmatic Research Institute. Victoria Carter is an employee of Optimum Patient Care Global and has 5% shareholding of Optimum Patient Care Australia. Kirsty Fletton is an employee of Optimum Patient Care United Kingdom. David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Inside Practice, GlaxoSmithKline, Medscape, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Medscape, Teva Pharmaceuticals.; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Ethical Approval
This study was approved by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee—the independent scientific advisory committee for the OPCRDA (ADEPT1819). The study was designed, implemented, and reported in compliance with the European Network Centers for Pharmacoepidemiology and Pharmacovigilance (ENCEPP) Code of Conduct (EMA 2014; EUPAS105682) All patients provided consent sharing their de-identified data with OPCRDA for research purposes. As noted, the dataset supporting the conclusions of this article was derived from the Optimum Patient Care Research Database Australia (https://optimumpatientcare.org.au/opcrda/). The OPCRDA has ethical approval from The Royal Australian College of General Practitioners (RACGP) National Research and Evaluation Ethics Committee (NREEC) to hold and process anonymised research data (NREEC Reference: 18-013).
Additional information
The Members of the OPCA Improving Asthma Outcomes in Australia Research Group are listed in acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Price, D., Jenkins, C., Hancock, K. et al. The Association Between Short-Acting β2-Agonist Over-Prescription, and Patient-Reported Acquisition and Use on Asthma Control and Exacerbations: Data from Australia. Adv Ther 41, 1262–1283 (2024). https://doi.org/10.1007/s12325-023-02746-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02746-0