Abstract
Introduction
Preliminary results from the SABINA (SABA use IN Asthma) program showed lower overuse of short-acting β2-agonist (SABA) in Italy compared to other European countries. The aim of the present study was to ascertain whether SABINA’s results might have been affected by the Italian National Health System and pharmaceutical market dynamics, by examining patients’ characteristics in relation to SABA prescription/purchase habits.
Methods
Multiple approaches were used: (1) a retrospective study using the General Practitioners’ (GPs) Italian IQVIA Longitudinal Patient Database (LPD) to assess SABA overuse (more than two canisters/year) and its association with exacerbation risk; (2) a survey conducted across 200 Italian pharmacies to calculate the proportions of SABA purchases without a prescription; (3) a cross-sectional study on the specialists’ IQVIA Patient Analyzer database to understand the SABA prescription habits of specialists.
Results
Among SABA users identified through IQVIA LPD, the proportion of patients having more than two SABA canisters/year was 32%. Overall, patients prescribed more than two SABA canisters/year by GPs had 30% higher risk of exacerbations than patients with a maximum of two SABA canisters/year. The joint evaluation of IQVIA LPD and survey’s findings revealed that IQVIA LPD tracks three out of four SABA canisters dispensed. The survey showed that, on average, SABA users purchased four canisters/year. Patients prescribed SABA by specialists were more frequently men, younger, thinner, and had higher spirometry values.
Conclusion
SABA overuse is common in Italy, with a share of consumption not regulated by medical prescriptions. Because SABA overuse increases exacerbation risk, changes to national guidelines should be encouraged to ensure implementation of global recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preliminary results from the SABINA (SABA use IN Asthma) program showed a lower proportion of SABA overuse in Italy compared to other European countries. |
This study’s objective was to examine whether SABINA’s results might have been due to the Italian National Health System and pharmaceutical market dynamics. |
SABA overuse is quite common among SABA users; the average number of SABA canisters purchased per year is four. |
More than two SABA canisters/year was associated with a 30% higher likelihood of experiencing exacerbations. |
Changes to national treatment guidelines should be encouraged to ensure successful implementation of the latest GINA recommendations. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14519592.
Introduction
Asthma is a heterogenous disease usually characterized by chronic airway inflammation, and defined by a history of respiratory symptoms (wheeze, shortness of breath, chest tightness, and cough) that vary over time and intensity, and by variable expiratory airflow limitation [1]. According to data from general practitioners (GPs), asthma prevalence in the Italian population (older than 15 years) is 6.1%, slightly higher in women than in men (6.6% versus 5.5%), and decreases with age [2]. The goal of asthma therapy, whose backbone is inhaled anti-inflammatory treatment, is to achieve and maintain disease control by minimizing symptoms and their impact on daily living activities and to reduce the risk of life-threatening exacerbations and long-term morbidity [3]. Despite evidence that overuse of short-acting beta-2 agonists (SABAs) and/or underuse of inhaled corticosteroids (ICS) appears to be predictive of exacerbations, studies have shown that SABA overuse is still common [4,5,6,7]. It is indeed under this premise that the SABINA (SABA use IN Asthma) program was recently implemented with the overarching goal of capturing the current burden of SABA use on a global scale [8]. The SABINA program includes four main pillars: SABINA I (a retrospective, observational database study with expanded objectives in the UK), SABINA II (a harmonized set of retrospective observational studies using country databases in Europe and Canada), SABINA III (a prospective collected cross-sectional multicountry study in 25 countries), and SABINA+ (multi-design studies conducted in other countries including Poland, Romania, Switzerland, USA, Hong Kong, Morocco, and China) [9]. Janson and colleagues published preliminary findings from SABINA I and the European SABINA II studies, which included more than one million individuals with asthma across five countries (UK, Germany, Italy, Spain, and Sweden) [9]. Overall, SABA overuse, defined as more than two canisters per year, was observed in approximately one-third of individuals with mild, moderate, and severe asthma across Europe. However, the prevalence of SABA overuse in Italy was just 9%, suggesting that Italy was an exception to the widespread use of SABA common to the other countries [9]. On the other hand, to have a more comprehensive overview on this somewhat discordant result, it is important to contextualize the data source used in light of the Italian National Health System (NHS) and pharmaceutical market regulations. The Italian data included in the SABINA II study [9] were obtained from the IQVIA Longitudinal Patient Database (LPD), a source of secondary data coming from about 900 Italian GPs, who collect patient records during their routine daily clinical practice. The IQVIA LPD captures only patients’ treatments from prescriptions written and recorded by GPs. Thus, there are two main constraints that should be considered: firstly, SABA may be dispensed by pharmacists without medical prescription if needed urgently [10]; secondly, a proportion of SABA prescriptions are directly dispensed by specialists, and do not require GPs to rewrite prescriptions. In light of this, the present study, as an extension of the Italian SABINA II data [9], integrated data from the Italian IQVIA LPD along with additional information coming from a survey conducted on a representative sample of Italian pharmacies. Finally, a cross-sectional study on specialists using the Italian IQVIA Patient Analyzer (PA) database was performed to understand how frequently specialists prescribe SABA to patients with asthma. The final aim of the study was to clarify whether an “Italian case” exists, or if the Italian results might have been affected by the peculiar dynamics of the Italian National Health System and pharmaceutical market.
Methods
Retrospective Study on the Italian IQVIA LPD
The first criterion for inclusion in the study was the registration of an asthma diagnosis, defined by the International Classification of Disease (ICD-9) code 493.xx (9th edition), during 2016 (i.e., selection period), by the GP. The date of the first diagnosis during this period represented the index date for each patient. The study period included a baseline (i.e., the 12-month period preceding the index date) and a follow-up (i.e., the 24-month period starting on the index date) period. The second inclusion criterion was an age of at least 12 years at the index date. The third inclusion criterion was the presence of an additional asthma diagnosis registration during the baseline period. Exclusion criteria were the presence of any registration of chronic obstructive pulmonary disease (COPD) (ICD-9 codes 491.xx, 492.xx, 496.xx) during the study period, and data unavailability for the study period. A description of IQVIA LPD, which has already been demonstrated to be a reliable source of data on respiratory diseases [11,12,13], a graphical representation of the study design, information extracted from the database, and a definition of comorbidities are available in Annex I of the supplementary material. Patients have been classified into treatment steps based on controller treatments prescribed by GPs during baseline and according to 2018 Global Initiative for Asthma (GINA) recommendations [14] (please see Annex II of the supplementary material for algorithm definition). The occurrence of exacerbations during follow-up was defined as asthma worsening that necessitated an antibiotic and/or short course of oral corticosteroids (OCS) and/or emergency room (ER) or hospital admission. In order to contribute to the definition of an exacerbation, antibiotic prescriptions and ER/hospital admission registrations had to be recorded as explicitly related to asthma by the GP. For OCS, as patients might be prescribed different OCS molecules, for each prescription, the total amount of active principle was converted into milligrams of prednisone equivalent. In order to contribute to the definition of an exacerbation, the total amount of active principle included in the prescription must not exceed 300 mg of prednisone equivalent. Patients were grouped on the basis of the number of SABA canisters received during the first year of follow-up, and demographic and clinical characteristics, healthcare resource utilization (HRU), and number of exacerbations were compared between patients with a maximum of two SABA canisters/year and patients with more than two SABA canisters/year by means of chi-square tests. The cutoff corresponding to two SABA canisters/year was adopted on the basis of 2018 GINA guideline recommendations [14] and in agreement with all SABINA studies [8, 9, 15, 16]. Asthma treatments included are detailed in Annex III of the supplementary material. An extension of the Cox proportional hazards model, the Andersen and Gill model for recurrent time-to-event data, was run to understand whether there was an association between the number of SABA canisters/year and the likelihood of experiencing exacerbations during follow-up, while adjusting for potentially confounding factors (i.e., age, sex, ICS mean daily dosage, number of comorbidities, and treatments step). The Anderson and Gill model accounted for the time between the index date and the exacerbation, and for the possibility of experiencing more than one exacerbation. Finally, a more conservative analysis was performed on the subgroup of patients with asthma who had at least one SABA prescription during the first year of follow-up, defined as “SABA users”. The mean number of SABA canisters/year as well as the proportion of patients prescribed more than two SABA canisters/year was provided for this subgroup. The latter analysis was intended to serve as a basis for comparison with results from the pop-up survey on pharmacies, which involved patients with asthma purchasing SABA (i.e., SABA users).
Pop-Up Survey on Pharmacies
IQVIA conducted a quantitative survey involving 200 Italian retail pharmacies between June and July 2019. Patients with asthma purchasing SABA were asked by collaborating pharmacists to provide information on the originator and reimbursability of the corresponding prescription, if available, and on the number of SABA canisters bought during the past year. Summary statistics on the number of SABA canisters purchased by patients during the past year and the percentages of SABA purchases without prescription or with a corresponding reimbursable prescription (either by a GP or a specialist) as well as with a corresponding non-reimbursable prescription (either by a GP or a specialists) were provided. Further information on the survey and the logical process which allowed to jointly evaluate information coming from IQVIA LPD and the pop-up survey are described in Annex IV of the supplementary material. The final aim of such a process was to understand LPD coverage in terms of SABA consumption.
Cross-Sectional Study on the Italian IQVIA Patient Analyzer
The study cohort was composed of patients with asthma examined by collaborating specialists (pulmonologists or allergologists) during 2018. Inclusion criteria were an age of at least 12 years and asthma as the reason for the visit. The only exclusion criterion was the presence of COPD among comorbidities. Patients were grouped in steps of treatment (from 1 to 4–5) according to the GINA report [14]. All patients’ characteristics were compared according to the presence or absence of a SABA prescription during the visit, and by means of chi-square or t test as appropriate. A description of IQVIA PA, which has been demonstrated to be a reliable source of information in the respiratory area [13, 14], is available in Annex V of the supplementary material.
General Considerations
All the statistical analyses on the IQVIA LPD and PA data were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). P values less than 0.05 were considered as statistically significant. All the analyses presented here were based on anonymized data that did not involve any clinical trial on human or animal subjects performed by any of the authors. Therefore, review board approval and patient consent were not necessary.
Results
Retrospective Study on the Italian IQVIA LPD
The retrospective study on the IQVIA LPD found 22,102 subjects who met inclusion criteria. Almost half of the patients were classified as step 4, and one-third as step 1, while individuals in steps 2, 3, and 5 accounted for 20% of the total cohort overall (Table 1). The stratification by number of SABA canisters received during first-year follow-up revealed that subjects with more than two SABA canisters numbered 1892, accounting for 9% of the cohort. When compared to patients with a maximum of two SABA canisters/year, subjects with more than two SABA canisters/year were more frequently men (52% versus 41%), younger (under 40 years, 39% versus 27%), thinner (under or normal weight patients, 47% versus 22%), and less frequently affected by comorbidities (no comorbidities, 69% versus 60%) (Table 1). While no differences were observed between the two groups in terms of treatment steps, a higher number of ICS canisters were prescribed and higher proportions of subjects with asthma-related specialist visits and examination requests were observed among patients with more than two SABA canisters/year. A higher proportion of patients with more than one GP visit per month was observed for the group with a maximum of two SABA canisters/year. Finally, a higher proportion of patients experiencing more than one exacerbation during the 2-year follow-up was observed for the group of subjects with more than two SABA canisters/year (Table 1).
Patients with more than two SABA canisters/year had approximately a 30% higher likelihood of experiencing exacerbations, even when accounting for covariates included in the model. In addition, patients who had an ICS mean daily dosage greater than 500 µg, were female, aged 65 or older, had comorbidities, and were GINA step 5 had a greater risk of experiencing exacerbations independently of the other model covariates. Individuals in steps 2–4 had a lower risk of exacerbations compared to those in step 1 (Table 2).
When patients with no SABA prescriptions during the first-year of follow-up were excluded from the analyses, 5942 (27%) patients with at least one SABA prescription during first-year follow-up were identified. Among those patients, the mean number of SABA canisters prescribed during first-year follow-up was three (data not shown), almost 10% had more than six SABA canisters/year (Fig. 1a), and the proportion of subjects with more than two SABA canisters/year was about 32% (Fig. 1b).
Pop-Up Survey on Pharmacies
The pop-up survey, which collected data from 1136 subjects, showed that the mean number of pharmacy dispensed SABA to patients with asthma was around four canisters per year. Fifty-two percent of these patients purchased more than two SABA canisters/year (data not shown). Overall, 15% of the patients who took part in the survey did not have a prescription for SABA, while reimbursable prescriptions represented 65% of SABA purchases. Non-reimbursable prescriptions by GPs and specialists accounted for 9% and 11%, respectively. As the mean number of SABA canisters/year prescribed to SABA users defined by the IQVIA LPD study was three, the mean number of SABA canisters/year we would observe in case the IQVIA LPD was able to track 100% of SABA consumption would be four. This fits with the finding that four canisters/year is the average number of SABA canisters purchased during 1 year resulting from the pop-up survey. LPD coverage in terms of SABA consumption tracking is 74% (40% + 9% + 25%) (Fig. 2).
Cross-Sectional Study on the Italian IQVIA Patient Analyzer
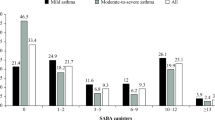
The cross-sectional study on the IQVIA PA included 4609 subjects meeting the asthma inclusion criteria. Almost half of the patients were classified as being in step 2, one-third were in step 3, while individuals in steps 1 and 4–5 accounted for about 25% of the total cohort overall (Table 3). The stratification by presence/absence of SABA prescription during the visit revealed that subjects prescribed a SABA accounted for about 15% of the total cohort. The frequency of patients with SABA prescriptions during the visit was inversely proportional to treatment intensity in different steps. Indeed, step 1 patients were those most frequently prescribed SABA (28%) and steps 4–5 those less frequently prescribed SABA (6%), and differences were statistically significant (data not shown). Patients with SABA prescription included a higher proportion of patients classified as step 1 and with controlled asthma and had higher values of FEV1 and PEF (Table 3).
Discussion
The aim of the present study was to investigate whether the results from the SABINA program [9] suggesting an “Italian case” of low SABA overuse compared to other European countries can be explained by the peculiar dynamics of the Italian NHS and pharmaceutical market. We found that SABA overuse is common among SABA users. Indeed, the average number of SABA canisters/year purchased by SABA users is four, thus higher than the cutoff defining high SABA use set by guidelines [1]. Prescription of more than two SABA canisters/year by GPs was associated with a 30% higher likelihood of experiencing exacerbations.
The joint evaluation of findings from IQVIA LPD study and the pop-up survey shows that in Italy patients with asthma using SABA collect an average of four canisters per year, and the proportion of patients with more than two SABA canisters per year should fall somewhere in the middle between 32% (i.e., the value resulting from the IQVIA LPD focus on SABA users) and 52% (i.e., the value resulting from the pop-up survey). Thus, we expect that the actual proportion of patients with more than two SABA canisters calculated on the basis of the total IQVIA LPD cohort of asthmatic patients is closer to values detected in other countries, which ranged from 16% for Germany to 38% for the UK [9]. Preliminary results from Italy included in the Janson et al. (2020) SABINA article [9] should now be read in light of the Italian NHS and pharmaceutical market situation, which corroborates the hypothesis that the “Italian case” is not realistic. A focus on the German SABINA study has been performed, similar to what had been done in the present study: the proportion of patients with more than two SABA canisters/year was calculated on the basis of the subgroup of subjects who had at least one SABA prescription during the study period, and resulted to be 36% and 38% based on GPs and specialists’ data, respectively [17]. Considering that in Germany SABA drugs are not available without a prescription [9], these findings are in line with the 32% observed in Italy, and that, as discussed above, is expected to be higher. Of note, both for Italy and Germany, the proportion of patients who had at least one SABA prescription represents about only 30% of the total cohort of patients with asthma [17]. This finding is in line with the XIII Report from HealthSearch, the Research Institute for the Società Italiana di Medicina Generale (SIMG), which showed a low prevalence of overall treatments in patients with asthma in primary care [18]. The results from the multivariate model run on the IQVIA LPD cohort showed that patients who had more than two SABA canisters/year have a 30% higher likelihood of experiencing exacerbations when compared to patients who had no more than two SABA canisters/year. The latter result is in total agreement with results from SABINA I study performed in the UK [15], which found a 20% increased risk of exacerbations for both step 1–2 and 3–5 patients who had a high level of SABA use (i.e., more than two canisters/year) [15]. Furthermore, GINA guidelines state that dispensing more than two SABA canisters/year is associated with an increased risk of emergency department visit or hospitalization independent of severity [1]. Results from the comparison of patients’ characteristics by number of SABA canisters show that individuals to whom GPs prescribe more than two SABA canisters/year are more frequently men, they are younger and thinner, and less frequently affected by comorbidities than patients with no more than two SABA canisters/year. Interestingly, the comparison of patient characteristics depending on the occurrence or not of a SABA prescription during the specialist visit are in line with data from GPs, confirming that physicians tend to prescribe SABA more frequently to patients they perceive as less health impaired. However, because SABAs have no anti-inflammatory effect [19, 20], the reliance on such relievers leaves individuals across all asthma severities at risk of preventable attacks [9, 21, 22]. Indeed, there is a continuously growing body of evidence showing how SABA overuse could affect health outcomes in patients with asthma, to which contributions from our study should be added [4, 6, 7, 23, 24]. Moreover, the current GINA guidelines recommend as-needed low-dose ICS–formoterol as the preferred reliever in step 1–2 patients and in step 3–5 patients prescribed ICS–formoterol maintenance and reliever therapy [1].
This study has some limitations. Firstly, the step of treatment for patients with asthma is not directly recorded by GPs into the IQVIA LPD, thus it was derived by means of an algorithm based on the medications received by patients during the year preceding the inclusion period. Secondly, exacerbations are not directly recorded in the database, thus requiring us to use an algorithm to detect their occurrence. However, our results, showing a higher risk of exacerbations for patients with more than two SABA canisters/year, are in line with the literature [4,5,6,7, 15], indicating the appropriateness of the rules set to detect exacerbations. Moreover, as observed previously [15, 25,26,27], the likelihood of experiencing exacerbations was increased with number of comorbidities, older age, and female sex. Thirdly, as in any study looking at inhaled treatment utilization, we cannot be sure that treatments prescribed/dispensed were actually used by patients. Automatic repeat prescriptions, or simultaneous prescriptions of multiple SABA canisters, may result in individuals having more SABA inhalers in their possession, which they may not necessarily use. Indeed, individuals with asthma typically have multiple SABA inhalers so that they have at least one inhaler in each of their surroundings (e.g., home, office, car). This is done so that patients have immediate access to their reliever in case of emergency [9].
Among the main strengths of the present study is the very large sample size of patients with asthma included in the analysis, as well as its representativeness, as the IQVIA LPD population characteristics are comparable to those of the Italian general population [18]. Both GPs and specialists’ data show higher proportions of women among patients with asthma, this being consistent with results from previous studies conducted in Italy [2, 13]. Similarly, the observed proportion of patients younger than 40 years accounting for more than half of the IQVIA PA cohort is in line with literature reporting that asthma prevalence decreases with age [2]. It is worth mentioning that the proportion of subjects younger than 40 years is lower when looking at the overall cohort of the IQVIA LPD patients with asthma. However, it should also be noted that the IQVIA LPD study only included patients with an additional asthma diagnosis during baseline, which implies exclusion of patients with newly diagnosed asthma, who are likely to be younger. In addition, patients’ characteristics are in line with those observed in the other countries that took part in the SABINA study, particularly Germany [9].
Finally, a very important strength of the present study is the opportunity to take advantage of very heterogeneous data sources. Using the IQVIA LPD and PA allowed us to gain insight from two different perspectives, those of GPs and respiratory specialists, covering the majority of potential prescribers of asthma treatments. The combined evaluation of findings from these diverse but complementary real-world data gave us a more comprehensive overview. In addition, insights offered by the pop-up survey allowed us to complete the picture on SABA consumption among patients with asthma in Italy.
Conclusion
Findings from this study suggest that, despite the fact that Italy had the lowest level of SABA consumption in the five-country SABINA study, an “Italian case” does not exist. Indeed, SABA overuse is quite common among SABA users, and both GPs and specialists tend to prescribe SABA more frequently to patients they perceive as less health impaired. It is fundamental to keep on encouraging changes to national treatment guidelines to align with GINA 2020 [1] in order to ensure successful implementation of these recommendations. Meanwhile, educational programs should be implemented to increase patients’ awareness towards the inflammatory nature of asthma, and to promote a conscious self-management of the disease.
References
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. https://www.ginasthma.org. Accessed 10 Mar 2021.
Cazzola M, Puxeddu E, Bettoncelli G, et al. The prevalence of asthma and COPD in Italy: a practice-based study. Respir Med. 2011;105(3):386–91.
Braido F, Baiardini I, Stagi E, Piroddi MG, Balestracci S, Canonica GW. Unsatisfactory asthma control: astonishing evidence from general practitioners and respiratory medicine specialists. J Investig Allergol Clin Immunol. 2010;20(1):9–12.
FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–40.
Laforest L, Licaj I, Devouassoux G, et al. Prescribed therapy for asthma: therapeutic ratios and outcomes. BMC Fam Pract. 2015;16:49.
Patel M, Pilcher J, Reddel HK, et al. Metrics of salbutamol use as predictors of future adverse outcomes in asthma. Clin Exp Allergy. 2013;43(10):1144–51.
Senthilselvan A, Lawson JA, Rennie DC, Dosman JA. Regular use of corticosteroids and low use of short-acting beta2-agonists can reduce asthma hospitalization. Chest. 2005;127(4):1242–51.
Cabrera CS, Nan C, Lindarck N, Beekman MJHI, Arnetorp S, van der Valk RJP. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J. 2020;55(2):1901858.
Janson C, Menzies-Gow A, Nan C, et al. SABINA: an overview of short-acting β2-agonist use in asthma in European countries. Adv Ther. 2020;37(3):1124–35.
Decreto Ministeriale 31 Marzo 2008. Gazzetta Ufficiale della Repubblica Italiana. https://www.gazzettaufficiale.it/eli/id/2008/04/11/08A02398/sg. Accessed 10 Mar 2021.
Di Marco F, Santus P, Terraneo S, et al. Characteristics of newly diagnosed COPD patients treated with triple inhaled therapy by general practitioners: a real world Italian study. NPJ Prim Care Respir Med. 2017;27(1):51.
Visentin E, Nieri D, Vagaggini B, Peruzzi E, Paggiaro P. An observation of prescription behaviors and adherence to guidelines in patients with COPD: real world data from October 2012 to September 2014. Curr Med Res Opin. 2016;32(9):1493–502.
Lavorini F, Bianco A, Blasi F, et al. What drives inhaler prescription for asthma patients? Results from a real-life retrospective analysis. Respir Med. 2020;166:105937.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2018. https://www.ginasthma.org. Accessed 10 Mar 2021.
Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: an observational UK study as part of the SABINA Global Program. Adv Ther. 2020;37(10):4190–208.
Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872.
Worth H, Criée CP, Vogelmeier C, et al. Overuse of short-acting beta-agonists (SABA) and risk factors among asthma patients in Germany as part of the SABINA framework. Eur Respir J. 2020;56(64):2633.
XIII Report Health Search (edizione 2020). Istituto di Ricerca della SIMG. Società Italiana di Medicina Generale e delle Cure Primarie. https://www.healthsearch.it/report/. Accessed 10 Mar 2021.
Hancox RJ, Aldridge RE, Cowan JO, et al. Tolerance to beta-agonists during acute bronchoconstriction. Eur Respir J. 1999;14(2):283–7.
Zhao H, Li R, Lv Y, et al. Albuterol inhalation increases FeNO level in steroid-naive asthmatics but not COPD patients with reversibility. Clin Respir J. 2017;11(3):328–36.
Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380(21):2020–30.
O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–76.
Bateman ED, Buhl R, O'Byrne PM, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135(6):1457–64.e4.
Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur Respir J. 1994;7(9):1602–9.
Patel M, Pilcher J, Reddel HK, et al. Predictors of severe exacerbations, poor asthma control, and β-agonist overuse for patients with asthma. J Allergy Clin Immunol Pract. 2014;2(6):751–8.
Bloom CI, Nissen F, Douglas IJ, Smeeth L, Cullinan P, Quint JK. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax. 2018;73(4):313–20.
Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1–12.
Acknowledgements
Funding
The present study and the journal’s Rapid Service Fees were funded by AstraZeneca.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All the named authors were involved in the conception and design of the study. Valeria Pegoraro was involved in data analysis and drafting the paper. Mariella D’Amato, Fabiano Di Marco, Francesco Lombardo, Claudio Micheletto, and Alberto Papi, were responsible for clinical validation of the study and review of the manuscript. All the named authors were involved in data interpretation and critically reviewed the paper. All the named authors read and approved the final manuscript.
Disclosures
Dr. Fabiano Di Marco reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from GSK, personal fees from Chiesi, personal fees from Zambon, grants and personal fees from Novartis, personal fees from Guidotti/Malesci, grants and personal fees from AZ, personal fees from Menarini, personal fees from Mundipharma, personal fees from TEVA, personal fees from Almiral, personal fees from Levante Pharma, personal fees from Sanofi, outside the submitted work. Dr. Mariella D’Amato reports grants from GSK, Sanofi Genzyme and AstraZeneca. Dr. Francesco P. Lombardo reports grants from AstraZeneca, Chiesi, and GSK. Dr. Claudio Micheletto has received a speaker honorarium from GSK, Sanofi, Astrazeneca, Novartis, Menarini, Guidotti, Chiesi, Zambon. Franca Heiman and Valeria Pegoraro have disclosed they are employees of IQVIA Solutions Italy S.r.l. Silvia Boarino, Giandomenico Manna, Francesca Mastromauro and Simona Spennato have disclosed they are employees of AstraZeneca S.p.A. Dr. Alberto Papi reports grants, personal fees from GlaxoSmithKline, AstraZeneca, Chiesi Farmaceutici and Sanofi/Regeneron; personal fees from Mundipharma, Zambon, Novartis; grants, personal fees from Roche and Edmondpharma, grants from Fondazione Maugeri and from Fondazione Chiesi.
Compliance with Ethics Guidelines
All the analyses here presented were based on anonymized data that did not involve any clinical trial on human or animal subjects performed by any of the authors. Therefore, review board approval and patient consent were not necessary.
Data Availability
The data that support the findings of this study are available from IQVIA, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the corresponding author upon reasonable request and with permission of IQVIA.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Di Marco, F., D’Amato, M., Lombardo, F.P. et al. The Burden of Short-Acting β2-Agonist Use in Asthma: Is There an Italian Case? An Update from SABINA Program. Adv Ther 38, 3816–3830 (2021). https://doi.org/10.1007/s12325-021-01772-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01772-0