Abstract
Introduction
Waldenström’s macroglobulinemia (WM) is a rare malignant B cell lymphoma which occurs in around 1–2% of all hematologic tumors. Ibrutinib was approved in China for WM on the basis of two global pivotal studies which enrolled no Chinese patients. The aim of this study was to determine the efficacy, safety, and pharmacokinetics of ibrutinib in Chinese patients with relapsed or refractory (r/r) WM.
Methods
This was an open-label, single-arm, multicenter phase 4 study conducted across five sites in China. Enrolled patients with clinicopathological confirmed WM received ibrutinib 420 mg once daily orally until disease progression or unacceptable toxicity. The primary endpoint was major response rate (MRR, partial response [PR], or better) according to the modified consensus criteria from the Sixth International Workshop on WM.
Results
Seventeen patients were enrolled; at data cutoff (March 19, 2022), MRR was 64.7% (90% confidence interval [CI] 42.0–83.4) and overall response rate was 100% (90% CI 83.8–100.0). One (5.9%) patient achieved very good PR, 10 (58.8%) achieved PR, and six (35.3%) achieved minor response. The median duration of response (PR or better) was 14.8 months (95% CI 10.8–not estimable [NE]). Median progression-free survival was 18.4 months (95% CI 12.9–NE). All patients experienced at least one treatment-emergent adverse event (TEAE) related to the study drug, and grade ≥ 3 TEAEs were reported in 13 (76.5%) patients. There were no TEAEs leading to dose reduction or death. The median model estimated maximum plasma concentration and area under the plasma concentration–time curve during 24 h after dosing at steady state were 40.5 ng/mL and 204 ng·h/mL, respectively.
Conclusions
Ibrutinib demonstrated durable responses in Chinese patients with r/r WM. Treatment was well tolerated with no new safety signals compared with the pivotal global studies. Ibrutinib exposure was also comparable between Chinese and non-Chinese patients.
Trial Registration
ClinicalTrials.gov identifier NCT04042376.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Waldenström’s macroglobulinemia (WM) is a rare lymphoma characterized by the infiltration of the bone marrow by lymphoplasmacytic cells that produce monoclonal immunoglobulin M. Ibrutinib was approved in China in November 2018 for the treatment of symptomatic WM on the basis of the PCYC-1118E and PCYC-1127-CA pivotal studies which included no Chinese patients. |
This phase 4 study was conducted to evaluate the efficacy, safety, and pharmacokinetics of ibrutinib in Chinese patients with relapsed or refractory (r/r) WM in a clinical trial setting. |
What was learned from this study? |
Single-agent ibrutinib showed durable clinical response in patients with r/r WM who were treated for a median duration of 14.3 months. |
The major response rate (defined as partial response or better) was 64.7%. Side effects were manageable. Overall, our data confirmed the clinical benefit of ibrutinib in the clinical trial setting, complementing that of real-world evidence in patients with r/r WM in China. |
Introduction
Waldenström’s macroglobulinemia (WM) is a rare malignant B cell lymphoma that is associated with an accumulation of clinical lymphoplasmacytic cells and monoclonal immunoglobulin M (IgM) secretion [1]. This disease accounts for around 1–2% of all hematologic tumors at an incidence of 3–4 per million people per year in the USA [2]. Treatment is recommended for symptomatic disease only [3, 4], and it includes chemotherapy, chemoimmunotherapy combinations, proteasome inhibitors, alkylating agents, and Bruton tyrosine kinase (BTK) inhibitors [3,4,5,6].
Ibrutinib is the first-in-class BTK inhibitor with proven efficacy in many B cell malignancies [7], including in treatment-naïve patients or those with relapsed or refractory (r/r) WM [6, 8,9,10]. Clinical data from two pivotal studies (PCYC-1118E and PCYC-1127-CA) demonstrated high response rates of up to 90.5% [6, 8], and this led to the approval of ibrutinib for WM by the US Food and Drug Administration and European Medicines Agency [11, 12]. In November 2018, the China National Medical Product Administration also approved ibrutinib for the treatment of WM on the basis of the results of the two pivotal studies, although no Chinese patients were enrolled [6, 8]. This made ibrutinib the first drug approved and recommended in the latest Chinese guidelines for WM, either as a monotherapy in the first-line setting for patients with WM who are not eligible to receive chemoimmunotherapy or who have received at least one prior treatment, or in combination with rituximab [4].
Before ibrutinib was approved for WM in China, most patients with symptomatic disease were treated with rituximab either as a monotherapy or in combination with chemotherapy. While rituximab-based regimens have been effective in WM, most patients will eventually develop resistance to rituximab because of widespread and repeated use [13, 14]. In addition to the tolerability issues arising from traditional systemic chemotherapies, treatment outcomes in the later-line settings have been modest, with the estimated median progression-free survival (PFS) reported in a large retrospective study in European patients with WM at 23.0 months and 16.0 months in the second- and third-line settings, respectively [13]. Therefore, ibrutinib represents a valuable treatment option that could potentially provide long-term disease control with reduced toxicity especially for those who do not respond to rituximab-based treatment.
The purpose of this post-approval phase 4 study was to evaluate the efficacy and safety and characterize the population pharmacokinetics (popPK) of ibrutinib monotherapy in Chinese patients with r/r WM.
Methods
Study Design
This study (WAL4001) was an open-label, single-arm, multicenter phase 4 study conducted at five study sites in China. Enrolled patients received ibrutinib orally at a dose of 420 mg once daily until disease progression or unacceptable toxicity. Patients who progressed on treatment were followed up on subsequent anticancer therapy, occurrence of other malignancy, survival status recorded until death, loss of follow-up, consent withdrawal, or study closure. The study protocol was amended once on March 25, 2020, to update the safety information regarding cerebrovascular incidence identified in the post-marketing setting and to clarify procedures and the collection of data.
This study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and local applicable regulatory requirements. All patients provided written informed consent according to the local requirements prior to enrollment. The trial protocol and any amendments were reviewed and approved by an independent ethics committee at each investigation site (Supplementary Table S1). This trial is registered with ClinicalTrials.gov (NCT04042376).
Patients
Key eligibility criteria included ≥ 18 years of age with centrally confirmed clinicopathological diagnosis of WM in accordance with the consensus panel of the Second International Workshop on WM (IWWM) [15]. Enrolled patients must also have at least one prior therapy, documented disease progression, or had no response to the most recent treatment regimen, a symptomatic disease meeting at least one criterion from the Second IWWM requiring treatment, measurable disease defined as serum monoclonal IgM > 0.5 g/dL, hematology and biochemical values within protocol-defined limits, and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of ≤ 2.
Patients were excluded from the study if they had known central nervous system involvement by WM, prior exposure to ibrutinib or other BTK inhibitors, hypersensitivity reaction to ibrutinib or to the excipients in its formulation, previously received WM-related therapy at most 30 days prior to first administration of the study treatment, received allogeneic hematopoietic stem cell transplant or plasmapheresis less than 35 days prior to the initiation of study treatment, history of stroke or intracranial hemorrhage within 6 months prior to enrollment, known bleeding disorders or hemophilia, or clinically significant cardiovascular disease, hepatic impairment, or active infection. A full account of the inclusion and exclusion criteria is described in Supplementary Table S2.
Outcomes and Assessments
The primary endpoint was major response rate (MRR, overall response rate was the term stated in the protocol) by investigator assessment, defined as the proportion of patients who achieved partial response (PR) or better according to the modified consensus criteria from the Sixth IWWM [16]. Secondary endpoints were overall response rate (ORR [clinical response rate was the term used in the protocol], defined as minor response [MR] or better), very good PR or better rate, duration of response (DOR), time to response (TTR), PFS, overall survival (OS), pharmacokinetics, and safety. All treatment responses were evaluated as per the Sixth IWWM by investigators [16]. The definitions of the efficacy endpoints are described in Supplementary Table S3.
Safety was evaluated on the basis of adverse events, clinical laboratory tests, physical examinations, and vital signs, summarized using frequencies and percentages. Adverse events were graded per National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 and assessed from informed consent to 30 days after last dose of study drug. Adverse events of special interest defined by the sponsor (major hemorrhage event) were also reported. Patients were observed for any adverse events until 30 days after the last dose of study drug.
Baseline tumor assessment was performed up to 42 days before the first dose of treatment. Computed tomography (CT) scans of the neck, chest, abdomen, and pelvis were performed. Magnetic resonance imaging was allowed if the CT scans could not be used to evaluate the sites of disease adequately. Radiographic imaging was performed every 16 weeks for the first 2 years, and thereafter every 24 weeks until disease progression. Bone marrow aspirate and biopsy were required at screening. Bone marrow was required to confirm complete response (CR), pre-dose at weeks 49 and 97 to assess response, and at time of progression or suspected progression due to progressive cytopenia.
Disease progression based on changes in IgM levels determined either by serum protein electrophoresis or by a quantitative IgM serum immunoglobulin test was confirmed by a subsequent evaluation within at least 4 weeks of the first finding.
Blood samples were collected from all patients for the measurement of plasma concentrations of ibrutinib and the metabolite PCI-45227 before treatment on day 1 of weeks 1, 5, and 9. The pharmacokinetic-evaluable patients were defined as patients who received at least one dose of study drug and had at least one quantifiable pharmacokinetic sample obtained post-treatment.
Statistical Analysis
Sample size was determined with the assumption that MRR by investigator is 69.8% in the study population, similar to the study by Treon et al. [6]. Hence, approximately 17 patients were recruited to obtain a power of 85% and to achieve a clinically meaningful MRR of 32% or higher with a one-sided significance level of 0.05.
Efficacy and safety analyses were performed on all patients who received at least one dose of ibrutinib. Pharmacokinetics analyses were performed on all patients who received at least one dose of ibrutinib and had at least one available pharmacokinetic sample after treatment. MRR, ORR, and very good PR or better rate were calculated using exact binomial distribution, and the exact (Clopper-Pearson) 90% confidence intervals (CIs) are presented. Time to event variables (DOR, PFS, and OS) and the corresponding 95% CIs were described using the Kaplan–Meier method. Time to major response was summarized descriptively for responders. Observed plasma concentrations of ibrutinib and metabolite PCI-45227 were summarized using descriptive statistics. Exposure matrices, including maximum plasma concentration at steady state (Cmax) and area under the plasma concentration–time curve during 24 h after dosing at steady state (AUCτ,ss), of ibrutinib from this study and from the two pivotal studies (PCYC-1118E and PCYC-1127-CA) were derived using the individually predicted ibrutinib concentrations from popPK modeling.
PopPK Modeling
A popPK analysis was conducted to characterize the PK of ibrutinib in Chinese patients with WM after oral administration. This analysis included data from the current study and the other two global pivotal trials (PCYC-1118E and PCYC-1127-CA) [6, 8]. PK-evaluable patients were defined as those who received at least one dose of study drug and had at least one quantifiable PK sample obtained post-treatment. The concentrations of metabolite PCI-45227 were not included in the popPK analysis.
A popPK model for ibrutinib was first developed using data from patients with B cell malignancies [17]. This initial popPK model was further updated by including additional internal clinical studies in patients with B cell malignancies (updated model, not published) and has been used for this work. In the previous popPK analysis, ibrutinib concentrations could be described using a two-compartmental model with sequential zero to first-order absorption and first-order elimination. The model was parameterized in terms of relative bioavailability and zero to first-order absorption (duration of zero order input [D1], temporal delay [lag time] before absorption process is started [ALAG1], and first-order absorption rate constant [ka]), linear clearance (CL), volume of distribution in the central compartment (V2), intercompartmental clearance (Q), and volume of distribution in the peripheral compartment (V3) (Supplementary Fig. S1). Interindividual variability was modeled with an exponential term. Residual variability was described using an additive model, as the plasma ibrutinib concentrations were log-transformed. The effects of CYP3A inhibitors (taken or not taken) and increasing age (continuous covariate) on F1 and meal condition (fast, modified fast, or fed) on D1 had been identified as statistically significant (but not clinically relevant) covariates for the final popPK model.
In this popPK analysis, the empirical Bayesian estimation (EBE) was performed using the previously developed model on the data from all three studies. In the EBE, basic goodness-of-fit plots were generated to assess the fit of the previous model on the current data, with the following graphs compared: observed concentrations versus population prediction (PRED), observed concentrations versus individual prediction (IPRED), conditional weighted residual (CWRES) versus PRED, and CWRES versus time since first dosing. In addition, a visual predictive check (VPC) was also performed to evaluate the agreement between the observed and predicted concentration generated by the previous popPK model. After we demonstrated that the previous model could describe the current data, the exposure metrics (Cmax and AUCτ,ss) at the recommended dose schedule (420 mg orally once daily) were derived from the predicted ibrutinib concentrations using the individual parameters obtained in the EBE for the three studies. The derived exposure metrics were compared between Chinese patients in the WAL4001 study and non-Chinese patients in the PCYC-1118E and PCYC-1127-CA studies. The analysis was conducted using a nonlinear mixed-effects model analysis in NONMEM® (Version 7.4, Icon Development Solutions, Ellicott City, MD).
In the final dataset, a total of 546 (WAL4001, 31; PCYC-1118E, 62; PCYC-1127-CA, 453) post-dose quantifiable samples of ibrutinib concentrations from 126 (WAL4001, 17; PCYC-1118E, 16; PCYC-1127-CA, 93) PK-evaluable patients were included in the popPK analysis.
Results
Patient Demographics and Baseline Characteristics
Seventeen patients were enrolled, and their detailed demographics and characteristics are summarized in Table 1. The median age was 64 (50–70) years; 10 (58.8%) were male. The majority of patients had an ECOG PS of 0 or 1 (n = 15, 88.2%), a serum IgM level of ≤ 70 g/L (n = 15, 88.2%), and extramedullary disease (n = 12, 70.6%). Median sum of products of diameters (SPD) for the target nodal lesions was 2363.0 mm2 (n = 11; 305.0–6417.0). Median baseline hemoglobin was 95.0 g/L (60–153), and 10 (58.8%) patients had hemoglobin ≤ 110 g/L. All patients received at least one prior systemic therapy for WM (1–4), and five (29.4%) received at least three prior systemic therapies. Glucocorticoids (n = 17, 100%) and nitrogen mustard analogues (n = 13, 76.5%) were the most common prior WM-related systemic therapeutic medication class used. Other systemic medication classes used included monoclonal antibodies (n = 12, 70.6%), proteasome inhibitors (n = 10, 58.8%), vinca alkaloids and analogues (n = 10, 58.8%), and other immunosuppressants (n = 9, 52.9%) such as thalidomide and lenalidomide. The complete list of systemic therapy used is presented in Supplementary Table S4. The most common prior treatment regimens were bortezomib, dexamethasone, and rituximab (BDR, n = 5); bortezomib and dexamethasone (BD, n = 3); dexamethasone, rituximab, and cyclophosphamide (DRC, n = 3); and cyclophosphamide, vindesine, prednisone, and rituximab (CVP-R, n = 3). One (5.9%) patient received prior WM plasmapheresis.
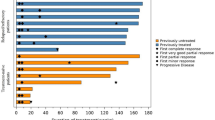
At data cutoff (March 19, 2022), eight patients remained on treatment (Fig. 1). Five (29.4%) patients discontinued treatment because of adverse events, three (17.6%) due to progressive disease, and one (5.9%) withdrew consent. Median duration of treatment was 14.3 months (2.5–22.2) and median follow-up time was 15.7 months (5.7–23.1).
Efficacy
At the time of data cutoff, the MRR was 64.7% (90% CI 42–83.4; Table 2). The primary efficacy endpoint of MRR prespecified as ≥ 32% of the lower limit of the 90% CI was met. One (5.9%) patient achieved very good PR and 10 (58.8%) achieved PR, six (35.3%) of whom achieved MR. All patients achieved MR or better response. The ORR was 100% (90% CI 83.8–100%). The median DOR among those who achieved PR or better was 14.8 months (95% CI 10.8–not estimable [NE]; Fig. 2a). Median time to major response was 3.7 months (95% CI 1.8–3.7) and median time to overall response was 0.95 months (95% CI 0.95–1.81). IgM improvement was observed in all patients (Supplementary Fig. S2). Treatment with ibrutinib resulted in a rapid reduction in serum IgM which continued over time throughout the study (Supplementary Fig. S3). At week 49, the median IgM levels were reduced by 68.4% (19.0–89.2%). The patient with very good PR recorded a maximum improvement from baseline in IgM levels by 91.4% at week 51. By week 33, SPD reduction was observed in the majority of patients (n/N = 9/11, 81.8%) with adenopathy at baseline. The median SPD was reduced by 57.8% (23.9–77.6%) and the maximum reduction in SPD from baseline was 80.0% at week 49, which was observed in a patient who achieved PR as best overall response.
Six (35.3%) patients had events of disease progression or death. Median PFS was 18.4 months (95% CI, 12.9–NE; Fig. 2b) and the 12 month PFS rate was 94% (95% CI 63–99). The median OS was not reached (95% CI 13.5–NE; Supplementary Fig. S4); 18-month OS rate was 84% (95% CI 49–96). Two deaths were reported during the study; one was due to pneumonia, which occurred more than 30 days after the last dose of study treatment and therefore was not considered related to treatment, and the reason for the other death was unknown (patient’s family declined to provide the cause of death).
Safety
All patients experienced at least one treatment-emergent adverse event (TEAE; Table 3) and treatment-related adverse event (Supplementary Table S5). Grade 3 or higher TEAEs and serious TEAEs were reported in 13 (76.5%) and eight (47.1%) patients, respectively. No TEAEs leading to death or dose reduction of ibrutinib were reported. Dose interruption was reported in three (17.6%) patients and due to arrhythmia (n = 1), ureterolithiasis (n = 1), and hypercalcemia and fracture (n = 1). Five (29.4%) patients discontinued treatment as a result of TEAEs.
The most frequently reported TEAEs (> 10% of patients) are presented in Table 3, and they included hyperuricemia (n = 11, 64.7%); neutrophil count decreased and white blood cell count decreased (both n = 5, 29.4%); hypoalbuminemia, upper respiratory tract infection, eczema, and anemia (all n = 4, 23.5%); and platelet count decreased, weight increased, and upper abdominal pain (all n = 3, 17.6%). Grade ≥ 3 TEAEs were reported in 13 (76.5%) patients; the most frequently reported grade ≥ 3 TEAEs were hyperuricemia (n = 5, 29.4%), neutrophil count decreased (n = 3, 17.6%), and anemia (n = 2, 11.8%). Serious adverse events (SAEs) were reported in eight (47.1%) patients. One (5.9%) patient reported an adverse event of special interest, which was grade 4 major hemorrhage intracranial, resulting in ibrutinib discontinuation. In terms of cardiac events, one patient experienced grade 2 cardiac failure which resulted in treatment discontinuation, and another had grade 2 cardiac arrhythmia which led to dose interruption. New malignancy was reported as an adverse event of clinical interest, and in our study, lung squamous cell carcinoma presented in one patient which also resulted in treatment discontinuation.
No clinically meaningful changes were observed in vital signs (blood pressure, weight, heart rate, respiratory rate, and body temperature). One patient reported abnormal values for electrocardiogram, reported as grade 2 arrythmia, resulting in dose interruption.
Pharmacokinetics Based on Observed Data
The median concentration of ibrutinib and its metabolite PCI-45227 on day 1 of week 5 was 1.71 ng/mL (range, below quantification limit to 12.7 ng/mL) and 7.79 ng/mL (1.74–85.8 ng/mL), respectively. The median concentration of ibrutinib and its metabolite PCI-45227 on day 1 of week 9 was 2.71 ng/mL (0.708–26.2 ng/mL) and 13.3 ng/mL (1.80–113 ng/mL), respectively.
PopPK Analysis
The goodness-of-fit plots for all three studies showed that CWRES values were generally scattered around the zero line across predicted range and time, while population predictions deviated from the identity line in WAL4001, likely as a result of the small sample size (Supplementary Fig. S5). VPC appeared to capture the central tendency and variability of the observed data (Supplementary Fig. S6). These results of external model evaluation indicated the consistency of ibrutinib PK profiles after oral administration in patients with WM and patients with other hematological malignancies, and between Chinese and non-Chinese patients with WM. The predicted median (range) Cmax and AUCτ,ss were 40.5 ng/mL (28.7–90.4 ng/mL) and 204 ng·h/mL (133–507 ng·h/mL), respectively, in WAL4001 (Supplementary Table S6).
Discussion
To our knowledge, this phase 4, single-arm, multicenter study is the first sponsor-initiated clinical trial to evaluate the clinical benefit of ibrutinib in Chinese patients with r/r WM, a population not included in previous pivotal ibrutinib clinical trials. Our study showed that the use of once-daily, single-agent ibrutinib afforded an MRR (defined as PR or better) of 64.7% per the modified Sixth IWWM criteria, with a median DOR of 14.8 months. One patient achieved very good PR and 10 had a PR. After a median follow-up of 15.7 months, median PFS was 18.4 months and median OS was not reached.
Our study results showed similar trends in terms of treatment responses and survival outcomes to previous pivotal studies. In a large phase 2 open-label study (PCYC-1118E), 63 previously treated patients who received ibrutinib monotherapy for a median duration of 19.1 months had a major response rate (defined as PR or better) of 73% and an ORR (defined as MR or better) of 90.5% [6]. After a median follow-up of 59 months, overall and major response rates were 90.5% and 79.4%, respectively [10]. The median 5-year PFS rate was 54%, and the 5-year OS rate was 87% [10]. In the phase 3 open-label substudy of the iNNOVATE trial by Dimopoulos et al. (PCYC-1127-CA), ibrutinib monotherapy demonstrated efficacy in 31 pretreated (rituximab-refractory) patients with WM, reporting an ORR (defined as MR or better) of 90% and major response rate (defined as PR or better) of 71% after a median follow-up of 18.1 months [8]. The estimated 18-month PFS and OS rates were 86% and 97%, respectively [8]. A more recent update on the final analysis of the iNNOVATE substudy with up to 5 years of follow-up indicated sustained treatment response, with ORR at 87% and 29% and 48% of patients achieving a very good PR and PR, respectively [14]. The median PFS and OS were 39 months and not reached, respectively [14]. In the present study, MRR, defined as PR or better, was similar to that observed in the previous pivotal studies. ORR, defined as MR or better, was higher, but the very good PR or better rate was lower than that in the pivotal studies, and this could be attributed to the higher disease burden of patients at baseline. Of note, patients enrolled in our study had higher serum IgM and lower hemoglobin levels at baseline compared with the other two pivotal studies; the incidence of extramedullary disease was similar but SPD among those with adenopathy was higher in the present study [6, 8].
Time to major response in the present study was 3.71 months, slightly longer than in both pivotal studies [6, 8]. Firstly, this could be attributed to the adoption of the latest Sixth IWWM guidelines on response criteria, requiring not only a reduction in IgM levels but also in extramedullary disease to be considered PR or better response. Unlike in the Third IWWM criteria that was adopted in the study by Treon and colleagues [6], a reduction of IgM levels by 50% would constitute a PR or better response. Moreover, 12 of the patients in our study who had extramedullary disease at baseline underwent their first image assessment on week 17 as per protocol, thereby delaying the confirmation of treatment response. Of note, time to overall response was 0.95 months, indicating an initial early response to ibrutinib of less than a month was achieved among Chinese patients.
Based on the results of this popPK analysis, the predicted median Cmax and AUCτ,ss at the recommended dose schedule (420 mg orally once daily) in Chinese patients in the WAL4001 study was numerically lower but generally within the range of the results in non-Chinese patients in global studies PCYC-1118E and PCYC-1127-CA (Supplementary Table S6 and Fig. S7). This indicates the consistency in the PK characteristics between Chinese and non-Chinese patients with WM after daily oral administration of ibrutinib.
Overall, ibrutinib was well tolerated, and no new safety signals emerged among Chinese patients. The incidence of TEAEs, drug-related TEAEs, and SAEs was similar across all three studies [6, 8]. However, TEAEs leading to treatment discontinuation occurred at a rate higher in our study than in the other two pivotal trials [6, 8]. We also observed a higher incidence of metabolism and nutritional disorders, but fewer gastrointestinal disorders. The incidence and severity of hyperuricemia observed in our study were also higher than in the other pivotal trials. Majority of those patients recovered at data cutoff without impacting the study. Rare cases of atrial fibrillation previously reported in other studies with ibrutinib were not observed here [6, 9, 10]. There was a single case of cardiac arrythmia which was resolved through dose interruption. Both cardiac conditions are not practice-altering as they could be managed with dose modification or the use of other pharmacological agents.
Small sample size is a limitation of our study, which was calculated on the basis of the objective of achieving a clinically meaningful MRR of 32% or higher. Patient genomic profiling was not assessed in our study, which could otherwise provide additional data on the impact of tumor genotypes on treatment outcomes in Chinese patients, given that the clinical response to ibrutinib could be influenced by MYD88 and CXCR4 genotypes [6, 14]. Further study is warranted with a longer duration of follow-up to understand the other prognostic factors, such as age, prior lines of systemic therapy, International Prognostic Scoring System for Waldenström Macroglobulinemia score, among others, to inform treatment outcomes. A study recently reported the long-term outcomes of ibrutinib monotherapy in WM [18]. Although the patient population was treatment-naïve, after a median follow-up of 50 months, MRR was 87% and 4-year PFS rate was 76%, signaling that ibrutinib was well tolerated and can provide long-term disease control [18].
Conclusion
Ibrutinib monotherapy was active and showed durable responses in Chinese patients with r/r WM, consistent with previous studies. The exposure of ibrutinib was generally comparable between Chinese and non-Chinese patients with WM. The study met its primary endpoint and treatment was well tolerated with no new safety signals, reaffirming ibrutinib as an ideal treatment option for WM in China.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
Dogliotti I, Jimenez C, Varettoni M, et al. Diagnostics in Waldenstrom’s macroglobulinemia: a consensus statement of the European Consortium for Waldenstrom’s Macroglobulinemia. Leukemia. 2022;37(2):388–95.
Steingrímsson V, Landgren O, Kristinsson SY. Epidemiology of Waldenström macroglobulinemia. In: Leblond V, Treon S, Dimoploulos M, editors. Waldenström’s macroglobulinemia. Cham: Springer; 2017. p. 97–109.
Kastritis E, Leblond V, Dimopoulos MA, et al. Waldenstrom’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(S4):iv41–50.
Hematology Oncology Committee of China Anti-Cancer Association, Chinese Society of Hematology Chinese Medical Association, Chinese Working Group of Walderstrom Macroglobulinemia. [Chinese guideline for diagnosis and treatment of lymphoplasmacytic lymphoma/Walderstrom macroglobulinemia (2022)]. Zhonghua Xue Ye Xue Za Zhi. 2022;43(8):624–30.
Ravi G, Kapoor P. Current approach to Waldenström macroglobulinemia. Cancer Treat Res Commun. 2022;31:100527.
Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–40.
Ayyildiz O, Demirkan F, Goker H, et al. Ibrutinib: from molecule to medicine. UHOD Uluslararasi Hematoloji-Onkoloji Dergisi. 2014;24:4–14.
Dimopoulos MA, Trotman J, Tedeschi A, et al. Ibrutinib for patients with rituximab-refractory Waldenstrom’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–50.
Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenstrom’s macroglobulinemia. N Engl J Med. 2018;378(25):2399–410.
Treon SP, Meid K, Gustine J, et al. Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström macroglobulinemia. J Clin Oncol. 2020;39(6):565–75.
Raedler LA. Imbruvica (ibrutinib): first drug approved for the treatment of patients with Waldenstrom’s macroglobulinemia. Am Health Drug Benefits. 2016;9:89–92.
IMBRUVICA® Summary of Product Characteristics. Janssen-Cilag International NV: Beerse, Belgium; 2022.
Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenstrom’s macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5(7):e299–309.
Trotman J, Buske C, Tedeschi A, et al. Single-agent ibrutinib for rituximab-refractory Waldenstrom macroglobulinemia: final analysis of the substudy of the phase III Innovate™ trial. Clin Cancer Res. 2021;27(21):5793–800.
Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):110–5.
Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenstrom macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160(2):171–6.
Marostica E, Sukbuntherng J, Loury D, et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol. 2015;75(1):111–21.
Castillo JJ, Meid K, Gustine JN, et al. Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenstrom macroglobulinemia. Leukemia. 2022;36(2):532–9.
Acknowledgements
We thank all participants and investigators for their participation in the study.
Medical Writing and Editorial Assistance
Medical writing support and editorial assistance was provided by Lawrence Law, MPH, of Parexel and funded by Janssen Global Services.
Funding
This study was sponsored by Janssen Research & Development, LLC. The publication of this study, including the journal’s Rapid Service and Open Access Fee, was funded by Janssen Global Services. Some local laboratory examinations per protocol requirements (for 10 of 17 participants) were supported by grants from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2022-I2M-1–022).
Author information
Authors and Affiliations
Contributions
Shuhua Yi and Lugui Qiu contributed to data acquisition and interpretation; drafted and critically revised the manuscript; critically reviewed and approved the final version to be published. Zhen Cai, Yu Hu, Aili He, and Sujun Gao contributed to data acquisition and interpretation; drafted the manuscript; critically reviewed and approved the final version to be published. Qian Li, Nating Zhang, and Yupeng Ren contributed to conception and design of the clinical study, data acquisition and interpretation; drafted and critically revised the manuscript; critically reviewed and approved the final version to be published. Linlin Sha contributed to conception and design of the clinical study, data acquisition and interpretation, and statistical analysis; drafted and critically revised the manuscript; critically reviewed and approved the final version to be published. Xue Gai contributed to conception and design of the clinical study, data acquisition and interpretation; critically revised the manuscript; critically reviewed and approved the final version to be published. Xue Yang and Rui Qin contributed to conception and design of the clinical study, data acquisition and interpretation, and statistical analysis; critically revised the manuscript; critically reviewed and approved the final version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the research and content.
Corresponding author
Ethics declarations
Conflict of Interest
Lugui Qiu receives consultation fees and honoraria from Xian Janssen Pharmaceutical Ltd, Pfizer, Beigene, and Roche. Qian Li, Linlin Sha, Nating Zhang, Yupeng Ren, Xue Gai, Xue Yang, and Rui Qin are employees of Johnson & Johnson. Xue Yang and Rui Qin report stock ownership from Johnson & Johnson. Shuhua Yi, Yu Hu, Zhen Cai, Aili He, and Sujun Gao have nothing to disclose.
Ethical Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and local applicable regulatory requirements. All patients provided written informed consent according to the local requirements prior to enrollment. The trial protocol and any amendments were reviewed and approved by an independent ethics committee at each investigation site.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yi, S., Cai, Z., Hu, Y. et al. Ibrutinib Efficacy, Safety, and Pharmacokinetics in Chinese Patients with Relapsed or Refractory Waldenström’s Macroglobulinemia: A Multicenter, Single-Arm, Phase 4 Study. Adv Ther 41, 672–685 (2024). https://doi.org/10.1007/s12325-023-02720-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02720-w