Abstract
Purpose
Ibrutinib is an oral Bruton’s tyrosine kinase inhibitor, recently approved for the treatment of mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) patients with at least one prior therapy. We developed a population pharmacokinetic (PK) model for ibrutinib in patients.
Methods
Ibrutinib PK data (3,477 observations/245 patients) were available from the following clinical studies: (1) A phase I dose-escalation study in recurrent B cell malignancies (dose levels of 1.25–12.5 mg/kg/day and fixed dose of 560 mg/day); (2) a phase II study in MCL (fixed dose level of 560 mg/day); (3) a phase Ib/II dose-finding study in CLL (fixed dose levels of 420 and 840 mg/day). Different compartmental PK models were explored using nonlinear mixed effects modeling.
Results
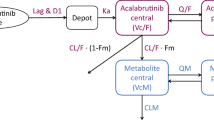
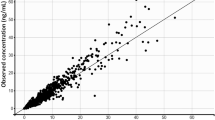
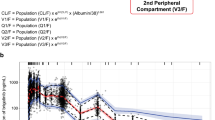
A two-compartment PK model with sequential zero–first-order absorption and first-order elimination was able to characterize the PK of ibrutinib. The compound was rapidly absorbed, had a high oral plasma clearance (approximately 1,000 L/h) and a high apparent volume of distribution at steady state (approximately 10,000 L). PK parameters were not dependent on dose, study, or clinical indication. The fasting state was characterized by a 67 % relative bioavailability compared with the meal conditions used in the trials and administration after a high-fat meal. Body weight and coadministration of antacids marginally increased volume of distribution and duration of absorption, respectively.
Conclusions
The proposed population PK model was able to describe the plasma concentration–time profiles of ibrutinib across various trials. The linear model indicated that the compound’s PK was dose independent and time independent.





Similar content being viewed by others
References
Byrd JC, Fuman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369:32–42
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS et al (2013) Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369:507–516
Woyach JA, Johnson AJ, Byrd JC (2012) The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 120:1175–1184
Wiestner A (2013) Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol 31:128–130
Herman SEM, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S et al (2011) Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-3276. Blood 117:6287–6296
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B et al (2013) Bruton tyrosine kinase inhibitor Ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 31:88–94
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B et al (2010) The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 107:13075–13080
Poggesi I, Sardu ML, Marostica E, Sukbuntherng J, Chang BY, de Jong J, Woot de Trixhe X, Vermeulen A, De Nicolao G, O’Brien SM, Byrd JC, Advani RH, James DF, Deraedt W, Beaupre D, Wang M (2014). Population pharmacokinetic-pharmacodynamic (PKPD) modeling of ibrutinib in patients with B-Cell malignancies. Accepted for AACR meeting “Hematological malignancies: Translating discoveries in novel therapies” September 20–23, 2014 Sheraton Philadelphia Downtown Philadelphia, PA, USA
Beal S, Sheiner LB, Boeckmann A, Bauer RJ, NONMEM (2009) User’s guides. Icon Development Solutions, Ellicott City
Ette Ene I, Williams Paul J (2007) Pharmacometrics: the science of quantitative pharmacology. Wiley-Interscience, New York
Oberg A, Davidian M (2000) Estimating data transformations in nonlinear mixed effects models. Biometrics 56:65–72
Bonate PL (2011) Pharmacokinetic-pharmacodynamic modeling and simulation, 2nd edn. Springer, New York
Bonate PL (1999) The effect of collinearity on parameter estimates in nonlinear mixed effect modeling. Pharm Res 16:709–717
Wahlby U, Jonsson EN, Karlsson MO (2002) Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci 4(4):27
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, (URL http://www.R-project.org)
DIDB: drug interaction DataBase: University of Washington (www.druginteractioninfo.org), Accessed 20 March 2013
Xu SX, Dunne A, Kimko H, Nandy P, Vermeulen A (2011) Impact of low percentage of data below the quantification limit on parameter estimates of pharmacokinetic models. J Pharmacokinet Pharmacodyn 38:423–432
Anderson BJ, Holford NHG (2008) Mechanism-based concepts of size and maturity. Annu Rev Pharmacol Toxicol 48:303–332
Jong de J, Sukbuntherng J, Skee D, Murphy J, O’Brien S, Byrd JC, et al (2014) Evaluation of the pharmacokinetics and food effect of oral ibrutinib in healthy subjects and chronic lymphocytic leukemia patients. AACR annual meeting. Abstract #4637: April 5–9
Imbruvica prescribing information (2013) http://www.imbruvica.com/downloads/Prescribing_Information.pdf. Accessed 24 February 2013
Sukbuntherng J, Jong de J, Skee D, Pak Y, Fardis M, O’Brien S et al (2014) Pharmacokinetics and safety of ibrutinib with concomitant use of CYP3A inhibitors in patients with B-cell malignancies. ACCP annual meeting
Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A et al (1997) Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 41:898–905
Montgomery DC, Peck EA (1982) Introduction to linear regression analysis. Wiley, London, p 277
Acknowledgments
The authors thank Purvi Jejurkar (Pharmacyclics) for bioanalytical support and Rishabh Pandey (SIRO Clinpharm Pvt. Ltd.) for additional editorial assistance. The authors also thank the study participants, without whom this study would not have been accomplished. The authors received financial support from Janssen Research and Development and Pharmacyclics.
Conflict of interest
Juthamas Sukbuntherng, David Loury, and Jesse McGreivy are employees of Pharmacyclics; Jan de Jong, Xavier Woot de Trixhe, An Vermeulen, and Italo Poggesi are employees of Janssen R&D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: Studies included in this analysis are registered on clinicaltrials.gov (NCT01105247; NCT01236391; NCT00849654).
Rights and permissions
About this article
Cite this article
Marostica, E., Sukbuntherng, J., Loury, D. et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol 75, 111–121 (2015). https://doi.org/10.1007/s00280-014-2617-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2617-3