Abstract
Introduction
Enhanced monofocal intraocular lenses (IOLs) represent a new type of lens, which should lead to a very good distance vision similar to monofocal IOLs and an improved intermediate vision without increasing the risk for photic phenomena.
Methods
The aim of this clinical observation/registry study was to directly compare two different IOL platforms (hydrophilic acrylic L-333 (group A) vs hydrophobic acrylic AN6Q (group B)) with the same enhanced monofocal optic principle but different material and haptic design in clinical routine. A total of 102 cataract cases (51:51) were included in the study. Groups A and B were similar regarding demographics, age (71.6 ± 9 years for L-333 and 73.6 ± 8 years for AN6Q) and their calculated IOL power (20.9 ± 2.0 D for L-333 and 21.5 ± 3.4 D for AN6Q). Spherical equivalent (SE), (un)corrected distance, intermediate visual acuity, the surgeons’ experience and patient feedback were assessed postoperatively.
Results
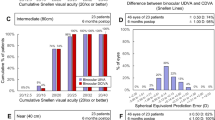
SE improved significantly in the AN6Q group, while the L-333 group showed a slightly smaller standard deviation postoperatively. In group A the uncorrected distance visual acuity (UDVA) improved from pre-op (0.43 ± 0.16 logMAR) to 1 month post-op (0.06 ± 0.04 logMAR) significantly and in group B from pre-op (0.54 ± 0.19 logMAR) to (0.05 ± 0.06 logMAR) postoperatively. Both groups showed excellent outcomes for distance without negative side effects. On testing uncorrected intermediate vision (80 cm) with Radner charts, 80% reached line 5 (0.0 logRAD) with fewer than one mistake and 10% reached line 4 (− 0.1 logRAD) in group A; 74% reached line 5 with fewer than one mistake and 4% reached line 4 in group B.
Conclusion
Both IOL models (groups A and B) provided satisfying results regarding implantation behaviour, refractive error, visual acuity and overall patient satisfaction. The haptic design might influence the outcome of refractive error. Long-term follow-up data should be considered in multicentre studies to further characterize both platforms and to optimize IOL power calculation (constants, surgeon factor). It was shown that the enhanced monofocal optic can provide good visual acuity for far distance and improve intermediate distance. This type of new monofocal optic design, which however must be strictly separated from typical refractive/diffractive multifocal, presbyopia-correcting lenses, could be a good option in standard cataract care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, a new type of intraocular lens (IOL), called enhanced monofocal IOL, has been introduced that is designed to provide excellent distance visual acuity (similar to a conventional monofocal lens) but better intermediate performance without increasing the risk to side effects traditionally caused by multifocality. |
However, these enhanced monofocal lenses should not be compared to classic refractive/diffractive premium lenses. Enhanced monofocals could be a good option in the standard care of patients with cataracts to provide high patient satisfaction in everyday life without increasing risks of side effects. |
The results of a registry study to evaluate a new enhanced monofocal optic in a hydrophilic and a hydrophobic IOL platform are promising and showed satisfied patients with improved intermediate visual acuity. |
However, patients must be informed in advance that this type of IOL does not provide absolute spectacle independence and that this is not the objective of the optic. |
Further studies and comparisons to conventional monofocal IOLs with high numbers of cases and longer follow-up are needed. |
Introduction
Today, patients increasingly expect excellent vision, not only for distance but also for intermediate and near distances, as many everyday tasks require this range of vision (computers, tablets, smartphones, reading monitors, operating vending machines or driving). Standard, monofocal intraocular lenses usually provide excellent uncorrected visual acuity for distance (UDVA), while the patient may achieve good intermediate and near visual acuity only with the help of spectacles. In the last decade, the number of presbyopia-correcting lenses (multifocal, refractive, diffractive, pinhole) has increased significantly in order to achieve spectacle independence even at near distance. However, the use of these premium lenses is not possible and reasonable in every individual case (possible side effects, costs).
Enhanced monofocal intraocular lenses (IOLs) represent a relatively new type of lens on the market, which should lead to an additional intermediate vision at up to 80 cm while retaining very good distance vision, without increasing the risk for photic phenomena [1,2,3]. These lenses must be distinguished from the classic enhanced depth of focus (EDoF) intraocular lenses, since the focus here is in a different range. In addition to choosing the optical principle, the surgeon has the task of selecting the fitting/best material. Currently, regarding market surveys, hydrophobic acrylic lenses are implanted most frequently, followed by hydrophilic acrylic IOLs [4].
As a result of the problem of calcification of some hydrophilic IOL materials in the past, there has recently been a kind of witch hunt against hydrophilic acrylic lenses. Recently, this was put into perspective in a review paper and with presentations by Auffarth and LaHood, stating the safety and state-of-the-art status of hydrophilic material and the possible advantages of hydrophilic lenses [5]. There are currently no uniform guidelines for the selection of hydrophilic or hydrophobic materials. Various secondary diagnoses have been discussed in the past, where the selection may play a role, like corneal dystrophy (Fuchs), endothelial grafts, diabetes, uveitis or pseudoexfoliation syndrome. Table 1 lists some of the stated advantages and disadvantages of IOL materials gathered from various publications [6,7,8,9,10,11,12,13,14,15].
Therefore, it is not possible to establish a purely scientific ranking of these materials. Both hydrophobic acrylate and hydrophilic acrylate materials in various models from different companies have proven their safety in scientific studies and in clinical practice. Problems like glistenings or calcifications have remained relatively rare side effects so far. The choice should be made individually and selected for each individual case.
The aim of this clinical observational study was to directly compare two different IOL platforms with the same enhanced monofocal optic but with different material (hydrophobic acrylate versus hydrophilic acrylate) and different haptic design in clinical routine.
The data was collected as part of a registry study, registered at clinicaltrials.gov under the registration number NCT05290870. The study adhered to the tenets of the Declaration of Helsinki and all patients gave their informed consent to participate in this study and for data about their case to be published. As all used devices/implants were already CE marked and the procedures followed the standard regime, no approval from an ethics board committee was required for this clinical observation.
Methods
Intraocular Lenses
Group A
The LENTIS Quantum L-333 (Teleon Surgical, Spankeren, the Netherlands) is an enhanced monofocal IOL, featuring the so-called Q-zone technology that should provide a smooth transition between the zones, facilitating distant or intermediate vision and, thus, reducing undesirable visual side effects such as halos and glare. The basic Quantum enhanced monofocal principle is to create a single elongated focal range to enhance depth of focus. The L-333 is a foldable, one-piece monofocal posterior chamber lens with plate-haptic design; the size of the spherical aberration neutral optic is 6.0 mm, and the overall diameter is 11.0 mm (Fig. 1, Table 2). The optic and haptics are equipped with a continuous posterior square edge design to achieve a 360° barrier effect. The material of the lens is called HydroSmart®, a copolymer consisting of hydrophilic acrylates with a hydrophobic surface (2-hydroxyethylmethacrylate). The biconvex optical design has a modified posterior aspherical surface. The lens is available from 10.0 to 30.0 diopters (D), in 0.5-D increments and is equipped with an UV filter. The material has a refractive index of 1.46, an Abbe number of 58 and a water content of around 25.5%. According to the manufacturer’s specifications, the L-333 provides patients with more vision in the intermediate distance (80 cm) compared with traditional monofocal IOLs, but with comparable contrast sensitivity and distance vision. The recommended injector is Medicel’s Viscojet Bio 1.8 or 2.0 (Medicel AG, Altenrhein, Switzerland) and the lens can be implanted through a 2.0-mm clear corneal incision.
Group B
The ACUNEX Quantum AN6Q (also by Teleon Surgical) is a foldable, hydrophobic acrylic, one-piece intraocular lens (IOL) with classic C-loop design. It features the same Q-Zone technology as the L-333 that subtly modifies the central part of its optics to increase depth of focus. The lens has a step vaulted C-loop design with an optical diameter of 6.0 mm and an overall diameter of 12.5 mm. The lens is available from 10.0 to 30.0 D, in 0.5-D increments, has a refractive index of 1.54 and an Abbe number of 41. It has a blue light filter and UV-absorbing material with a water content of 4%. According to the manufacturer, the lens enables significantly better vision at intermediate distance (80 cm) with comparable contrast sensitivity to a standard monofocal lens. The recommended injector is Medicel’s Accuject BL 2.1 and the lens can be implanted through a 2.2- to 2.4-mm clear corneal incision.
A subjective and objective performance assessment is important to undertake to better judge the overall outcome of the lenses in clinical routine. Furthermore, the clinical evaluation tried to elaborate on the different benefits and disadvantages of hydrophilic and hydrophobic materials.
Clinical Performance
In the scope of the prospective registry study, demographics (year of birth, comorbidities), pre-op and 1-month post-op data (power, sphere, cylinder, axis, uncorrected and corrected distance visual acuity; UDVA and CDVA, uncorrected intermediate visual acuity UIVA, respectively) were assessed (Table 3). Autorefraction was assessed with the Zeiss Visuref 100 (Carl Zeiss, Oberkochen, Germany) and the Oculus Park 1 (OCULUS Optikgeräte GmbH, Wetzlar, Germany). Visual acuity was assessed with Early Treatment of Diabetic Retinopathy Study (ETDRS) charts and Radner reading charts.
In terms of demographics, both groups were similar regarding age (71.6 ± 9 years for L-333 and 73.6 ± 8 years for AN6Q) and their calculated IOL power (20.9 ± 2.0 D for L-333 and 21.5 ± 3.4 D for AN6Q). No relevant comorbidities were reported for any patients.
Statistical Analysis
For the statistical analysis and figure generation of the clinical performance data, Python 3.9 with the matplotlib (3.7.1) and scipy (1.10.1) packages was used. A Shapiro–Wilk test was used to test if the data was drawn from a normal/standard distribution. Depending on the outcome of the test, either a t test (normal distribution) or a Mann–Whitney U test (no normal distribution) was performed. An α of 0.05 was chosen as significance value.
Results
Implantation Behaviour
All surgeries were performed by the same surgeon between March and December 2022. The same instruments for each implantation were used and a 2.4-mm clear corneal incision was performed in all cases using BVI Beaver safety knives. A Viscojet Bio 2.0 was used as injector to implant the L-333 and the Accujet BL 2.1 for the ACUNEX AN6Q. An exemplary snapshot from the operating room shows the successfully implanted lenses immediately after unfolding (Fig. 2). Both the hydrophilic and hydrophobic models could be easily implanted directly into the capsular bag without additional manipulation. As a result of the different materials and geometry of the two lenses, the unfolding time and behaviour were different. No complications regarding implantation process or unfolding were documented.
Spherical Equivalent (SE)
The SE derived from spherical and cylinder power is shown in Fig. 3. The Shapiro–Wilk test showed that both the pre-op (W = 0.649, p < 0.001 for L-333) and post-op (W = 0.953, p = 0.044 for L-333 and W = 0.948, p = 0.031) data were not drawn from a standard distribution. Only the pre-op data from the L-333 model seemed to be normally distributed (W = 0.956, p = 0.058). However, as most of the data was not normally distributed, a Mann–Whitney U test with the exact method to calculate the significance value was performed to investigate if the spherical equivalent improved 1 month after implantation compared to the pre-op state. No significant difference was found for the L-333 model (U = 1161, p = 0.353), while a significant difference was found for the AN6Q model (U = 2074.5, p < 0.001). This indicated that SE of the AN6Q improved from pre-op (1.47 ± 1.82 D) to 1 month post-op (− 0.01 ± 0.59 D) significantly.
Table 4 shows the number of eyes below a certain spherical equivalent refractive error. It should be noted that these were the very first clinical cases and only after this first evaluation were further optimizations applied to further increase the accuracy (A-constant optimization, surgeon factor).
Visual Acuity
The UDVA is shown in Fig. 4. The Shapiro–Wilk test showed that both the pre-op (W = 0.821, p < 0.001 for L-333 and W = 0.909, p < 0.001 for AN6Q) and post-op (W = 0.857, p < 0.001 for L-333 and W = 0.714, p < 0.001) data were drawn from a standard distribution. Therefore, a t test was performed to investigate if UDVA improved 1 month after implantation compared to the pre-op state. For both cases a significant difference was found (t = 15.807, p < 0.001 for L-333 and t = 17.657, p < 0.001 for AN6Q). This indicates that for L-333, UDVA improved from pre-op (0.43 ± 0.16 logMAR) to 1 month post-op (0.06 ± 0.04 logMAR) significantly. Such an improvement was also found for the AN6Q model, with 0.54 ± 0.19 logMAR pre-op to 0.05 ± 0.06 logMAR post-op.
The same principle was applied to the CDVA data shown in Fig. 5. The Shapiro–Wilk test showed that both the pre-op (W = 0.925, p = 0.003 for L-333 and W = 0.891, p < 0.001 for AN6Q) and post-op (W = 0.486, p < 0.001 for L-333 and W = 0.418, p < 0.001) data were drawn from a standard distribution. For both cases, the t test revealed a significant difference (t = 19.397, p < 0.001 for L-333 and t = 20.641, p < 0.001 for AN6Q). This indicates that for L-333, CDVA improved significantly from pre-op (0.27 ± 0.09 logMAR) to 1 month post-op (0.01 ± 0.02). Again, an improvement was found for the AN6Q model, with 0.30 ± 0.10 logMAR pre-op to 0.01 ± 0.03 logMAR post-op.
Spherical equivalent and visual acuity results were also compared between the two models (Fig. 6). For the non-normally distributed SE data, the Mann–Whitney U test revealed no significant differences between the hydrophilic and hydrophobic platform (U = 990, p = 0.052). This was also the case for the t test with UDVA (t = 0.938, p = 0.351) and CDVA (t = 0.325, p = 0.746).
Adverse Events (AE)
During the 1-month time frame of the registry study, no adverse events were reported by the patients. And no AEs were documented over a longer observational period of 6 months (in addition to the registry study’s required observation period).
Subjective Assessments and Patient Feedback
The handling of both injectors and the handling of the two IOL models were rated as uncomplicated. There were no complications reported in all cases (n = 102). The workflow in the operating room was rated as very good by the physician and the scrub nurse. Unfolding of the hydrophilic and hydrophobic lenses differed according to the geometry of the IOL and as a result of the material. In all cases, the IOLs could be positioned in the capsular bag. In the follow-up period of 4 weeks, no cases of glistenings were observed in the hydrophobic IOL group and no cases of calcifications were observed in the hydrophilic group. However, it must be mentioned that the follow-up period for this was very short and further long-term evaluations are necessary and planned. In an additional postoperative survey of patient (questionnaire) satisfaction was rated “very high” in 92% of cases and “high” in 8% of cases. There were no dissatisfied patients and all patients affirmed that they would repeat the procedure because the quality of life was improved.
Discussion
Laboratory Experiments
The L-333 IOL was recently analysed in an optical bench study [16]. The laboratory study investigated the enhanced monofocal IOL (L-333) and the monofocal counterpart (L-313), using OptiSpheric IOL PRO2 (Trioptics, Germany) in order to assess the optical quality according to ISO standards. The two IOLs were evaluated through frequency modulation transfer function (MTF), Strehl ratio (SR) and focus MTF at 50/lp/mm using a 3.0-mm and a 4.5-mm aperture. Tilt and decentration were also measured and wavefront measurements were obtained using WaveMaster IOL 2 (Trioptics, Germany). The study confirmed some enlarged depth of focus of the L-333 by combining spherical aberration of different order and opposite sign. The enhanced monofocal Lentis Quantum performed very well even with large aperture sizes, indicating that the lens could also be a good option in refractive procedures in younger patients (with wide pupils). Moreover, the L-333 showed a kind of tolerance to decentration and tilt due to its optical design compared to typical multifocal (diffractive, high add) IOLs. Nevertheless, the L-333 as an enhanced monofocal IOL should be very clearly differentiated from a typical multifocal IOL as the focus is in a different range. A clear distinction should also be made from typical EDoF IOLs like the Lentis Comfort (LS-313 MF15, Teleon Surgical) as these EDoF IOLs have a higher near addition of 1.5 D and therefore better UIVA performance at intermediate distances up to 60 cm. Interesting possibilities would also be a kind of mix/match selection of different optic designs (EDoF and enhanced monofocal) in the future.
Another laboratory study objectively analysed the hydrophobic enhanced monofocal ACUNEX Quantum (AN6Q) and compared it with the monofocal ACUNEX AN6 [17]. The results confirmed that the ACUNEX AN6 can provide a sharp contrast and distinct image focus, while the enhanced monofocal AN6Q provided an extended range of focus with only a minor, neglectable decrease in contrast quality. The study showed that the AN6Q effectively generates the depth of focus by combining spherical aberration (Z 4–0) and secondary spherical aberration (Z 6–0) of opposite sign.
Subjective Performance
In the scope of the registry study, all performance measures showed a satisfying outcome. In the case of SE, only group B (AN6Q) showed a significant improvement after implantation. However, the post-op standard deviation of SE for group A (L-333) was much smaller (0.24 D) than pre-op SE (2.86 D), indicating that most of the patients improved, nonetheless. The bigger standard deviation of post-op SE for group B compared to group A is worth noting as it might be explained by the different haptics used for the models. The C-loop design of the AN6Q could be slightly less stable than the plate-haptic design of the L-333 in the first postoperative period, resulting in a slightly higher standard deviation for the AN6Q. Significant improvements in all groups were found regarding distance visual acuity outcomes, both uncorrected and corrected with excellent post-op performance and a very low standard deviation.
Outside the scope of the prospective registry study, Radner reading table No. 4 and the Radner-Götlinger reading table were used to assess near visual acuity monocularly at 80 cm distance at 1 month post-op [18]. The results are given in Reading Acuity Determination (logRAD) and are equivalent to the distance acuity in logMAR. Out of the 51 eyes of group A, 41 (80%) reached line 5 (0.0 logRAD) with fewer than one mistake, 5 eyes (10%) reached line 4 (− 0.1 logRAD) and the remaining 5 eyes were below line 5. For group B, 38 eyes of the 51 in total (74%) reached line 5 with fewer than one mistake, 2 eyes (4%) reached line 4 and 11 eyes (22%) reached line 6. These results are consistent with the high overall satisfaction in the survey. It must be emphasized that only monocular vision for intermediate range was tested here, and that further improvement could be achieved by testing binocular vision for reading at 70–80 cm. However, this was not the subject of this study. It will be assessed in further evaluations and long-term observations.
Hydrophilic vs Hydrophobic
Teleon offers lenses on hydrophilic and hydrophobic platforms with various different optics. This fact makes it possible for the surgeon to choose the best option in the individual case or to follow a mix and match concept; this seems important to achieve high patient satisfaction. It is currently not scientifically clear which haptic concept (C-loop versus plate haptic) and which material properties and water content (phob vs phil) are fundamentally better, as there are advantages and disadvantages to both. In our study, there was a slight indication that the C-loop haptic of AN6Q was less stable and resulted in a slightly higher standard deviation of SE than the plate-haptic design of L-333. But further evaluations with higher numbers of cases are necessary. There was a different behaviour of the tested IOLs during implantation and unfolding due to material properties (water content) and also shape/design. However, no advantages and disadvantages could be scientifically documented because no complications occurred in all cases (n = 102). The unfolding behaviour and speed of unfolding were slightly different as a result of material properties of the hydrophilic and the hydrophobic IOLs, but might play a minor role.
Limitations of This Study
The follow-up period in this registry study is relatively short. The main goal of this evaluation was to provide initial clinical data and verify if the lens is safe and shows good postoperative results. Lens models with different optics but the same material and haptic design have been implanted in clinical routine many times and have been observed over a long period of time and proved their safety in clinical routine and in studies [19,20,21]. The aim of the present manuscript is to provide first clinical data for a new variant of a monofocal lens and discuss the optic design and laboratory results of the optical bench analysis. Further in-depth studies (multicentre, high numbers of cases) and direct comparisons to standard monofocal IOLs, EDoF and premium lenses (diffractive, refractive) are necessary.
Enhanced Monofocal Optic Design
This relatively new group of lenses (called enhanced monofocals) seems to be another interesting step to meet the individual needs of patients with cataracts. Other manufacturers also offer IOLs that can be counted in this category, e.g. Eyhance (Johnson & Johnson Vision), LuxSmart (Bausch & Lomb), Isopure (BVI), Vivinex Impress (Hoya Surgical) and IC-8 (AcuFocus). It is important to emphasize that these enhanced monofocal lenses should not claim to be “spectacle-free” and should therefore not be included in the group of typical “premium refractive lenses”. These IOLs are monofocal lenses that aim to offer additional advantages without increasing the risk of disadvantages and could therefore represent a good further alternative in the standard care of patients with cataracts.
Conclusion
The data of the registry study confirm that the lenses are safe to use. Patient satisfaction was very high in both groups and no adverse side effects were reported. The postoperative results were satisfactory in both groups. Glistenings or calcifications did not occur in any case in the time of observation (albeit relatively short). Thus, both tested IOL materials can be considered as very safe. Results of the registry study confirmed distance visual acuity comparable to a standard monofocal IOL but improved intermediate visual acuity at approximately 80 cm for both platforms (L-333 and ANQ6). This resulted in a relatively high level of patient satisfaction. No interfering effects like halo/glare were reported postoperatively. Further evaluations (high numbers of cases, multicentre study design) with a longer follow-up period are recommended.
References
Choi WK, Han HJ, Son HS, Khoramnia R, Auffarth GU, Choi CY. Clinical outcomes of bilateral implantation of new generation monofocal IOL enhanced for intermediate distance and conventional monofocal IOL in a Korean population. BMC Ophthalmol. 2023;23(1):157. https://doi.org/10.1186/s12886-023-02897-2.
Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg. 2021;47(2):184–91. https://doi.org/10.1097/j.jcrs.0000000000000399.
Mencucci R, Morelli A, Cennamo M, Roszkowska AM, Favuzza E. Enhanced monofocal intraocular lenses: a retrospective, comparative study between three different models. J Clin Med. 2023;12(10):3588. https://doi.org/10.3390/jcm12103588.
Market Scope. 2020 IOL market report: mid-year update (Market Scope). https://www.market-scope.com/. Accessed 10 June 2023.
Auffarth GU, LaHood B. IOL materials: what we know today. (Peer2Peer: The Podcast). https://www.youtube.com/watch?v=8TckITPX2AA. Accessed 2023 Jun 19.
Chang DF. Single versus three piece acrylic IOLs. Br J Ophthalmol. 2004;88(6):727–8. https://doi.org/10.1136/bjo.2004.040063.
Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35(7):1281–97. https://doi.org/10.1016/j.jcrs.2009.04.017.
Davis G. The evolution of cataract surgery. Mo Med. 2016;113(1):58–62.
Kumari R, Srivastava MR, Garg P, Janardhanan R. Intra ocular lens technology—a review of journey from its inception. Ophthalmol Res Int J. 2019;2:1–9.
Nejima R, Miyata K, Honbou M, et al. A prospective, randomised comparison of single and three piece acrylic foldable intraocular lenses. Br J Ophthalmol. 2004;88(6):746–9. https://doi.org/10.1136/bjo.2003.037663.
Nilsson SE, Textorius O, Andersson BE, Swenson B. Clear PMMA versus yellow intraocular lens material. An electrophysiologic study on pigmented rabbits regarding “the blue light hazard.” Prog Clin Biol Res. 1989;314:539–53.
Özyol P, Özyol E, Karel F. Biocompatibility of intraocular lenses. Turk J Ophthalmol. 2017;47(4):221–5. https://doi.org/10.4274/tjo.10437.
Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004;30(4):873–8. https://doi.org/10.1016/j.jcrs.2004.01.031.
Topete A, Serro AP, Saramago B. Dual drug delivery from intraocular lens material for prophylaxis of endophthalmitis in cataract surgery. Int J Pharm. 2019;10(558):43–52. https://doi.org/10.1016/j.ijpharm.2018.12.028.
Zhou KY. Silicon intraocular lenses in 50 cataract cases. Chin Med J (Engl). 1983;96(3):175–6.
Borkenstein AF, Borkenstein EM, Schmid R. Evaluating optical quality of a new hydrophilic enhanced monofocal intraocular lens and comparison to the monofocal counterpart: an optical bench analysis. Ophthalmol Ther. 2022;11(6):2045–56. https://doi.org/10.1007/s40123-022-00561-4.
Borkenstein AF, Borkenstein EM, Schmid R. Analysis of a novel hydrophobic acrylic enhanced monofocal intraocular lens compared to its standard monofocal type on the optical bench. BMC Ophthalmol. 2022;22(1):356. https://doi.org/10.1186/s12886-022-02584-8.
Radner W, Willinger U, Obermayer W, Mudrich C, Velikay-Parel M, Eisenwort B. Eine neue Lesetafel zur gleichzeitigen Bestimmung von Lesevisus und Lesegeschwindigkeit [A new reading chart for simultaneous determination of reading vision and reading speed]. Klein Monbl Augenheilkd. 1998;213(3):174–81. https://doi.org/10.1055/s-2008-1034969.
Oshika T, Arai H, Inoue Y, Fujita Y. Five-year clinical outcomes of low-add-power segmented rotationally asymmetrical intraocular lens. Ophthalmol Ther. 2023;12(3):1649–56. https://doi.org/10.1007/s40123-023-00703-2.
Oshika T, Arai H, Fujita Y, et al. One-year clinical evaluation of rotationally asymmetric multifocal intraocular lens with +1.5 diopters near addition. Sci Rep. 2019;9(1):13117. https://doi.org/10.1038/s41598-019-49524-z.
Son HS, Khoramnia R, Yildirim TM, Baur I, Labuz G, Auffarth GU. Functional outcomes and reading performance after combined implantation of a small-aperture lens and a segmental refractive bifocal lens. J Refract Surg. 2019;35(9):551–8. https://doi.org/10.3928/1081597X-20190806-02.
Acknowledgements
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contribution
Andreas F Borkenstein and Eva-Maria Borkenstein both made substantial contributions, reviewed and approved the final manuscript.
Funding
Teleon Surgical B.V. and MaganaMed GmbH sponsored parts of the registry study but these sponsors had no involvement in the analysis of the results, writing or publication of this article The journal’s Rapid Service and Open Access fees were paid by the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethical Approval
The data was collected as part of a registry study, registered at clinicaltrials.gov under the registration number NCT05290870. The study adhered to the tenets of the Declaration of Helsinki and all patients gave their informed consent to participate in this study and for data about their case to be published. As all used devices/implants were already CE marked and the procedures followed the standard regime, no approval from an ethics board committee was required for this clinical observation.
Conflict of Interest
The authors have no financial or proprietary interest in any material or method mentioned. There was no funding for the analysis of the data and production of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Borkenstein, A.F., Borkenstein, EM. Clinical Performance of New Enhanced Monofocal Intraocular Lenses: Comparison of Hydrophobic C-loop and Hydrophilic Plate-Haptic Platform. Adv Ther 40, 4561–4573 (2023). https://doi.org/10.1007/s12325-023-02635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02635-6