Abstract
Introduction
This study aimed to compare remimazolam to propofol in psychomotor recovery after total intravenous anesthesia (TIVA) using the Trieger dot test.
Methods
Sixty-six patients who were scheduled to undergo endoscopic sinus surgery with American Society of Anesthesiologists (ASA) physical status I or II were randomly allocated to the remimazolam (group R) or propofol group (group P). In group R, all patients received flumazenil postoperatively. After discontinuation of anesthetic agents, the time to eye opening, response to verbal commands, extubation, and discharge from the operation room were measured. Psychomotor recovery was assessed using the Trieger dot test before induction and at 0, 30, 60, 90, 120, 150, and 180 min after anesthesia.
Results
The time to eye opening, response to verbal commands, extubation, and discharge from the operation room were significantly longer in group P compared to group R (group P: 9.8 ± 3.2 min, 11.5 ± 3.4 min, 12.7 ± 3.4 min, 18.1 ± 4.2 min; group R: 6.5 ± 2 min, 7.3 ± 2.6 min, 8.4 ± 2.9 min, 13.2 ± 3.2 min; respectively, p < 0.05). In the Trieger dot test, the number of dots missed was significantly increased in group R compared to group P at 30, 60, 90, and 120 min after discharge from the operation room (group R: 20.5 ± 9.3, 16 ± 8.8, 14.9 ± 11.1, 14.3 ± 10.8; group P: 14.6 ± 7.8, 10 ± 7.1, 8.7 ± 7.3, 7.3 ± 5.7; respectively, p < 0.05). The maximum distance of dots missed was significantly increased in group R compared to group P at 30 min after discharge from the operation room (group R: 3.9 ± 2.8; group P: 2.7 ± 1.6; p < 0.05).
Conclusion
Our results suggest that remimazolam with flumazenil leads to rapid recovery following anesthesia; however, it may cause delayed psychomotor decline.
Clinical Trial Registration
This trial is registered with the University Hospital Medical Information Network (registration number UMIN000044900).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Remimazolam is a novel benzodiazepine that has a rapid onset and recovery with fewer adverse effects. |
No comparison of remimazolam and propofol in psychomotor recovery from total intravenous anesthesia (TIVA) has been reported. |
To clarify the efficacy and safety of remimazolam, we compared remimazolam with propofol for psychomotor recovery after TIVA using the Trieger dot test. |
What was learned from the study? |
Remimazolam with flumazenil resulted in faster recovery immediately after anesthesia compared to propofol. |
In contrast, the psychomotor function of remimazolam 30–120 min after anesthesia was lower than that of propofol. |
Our findings emphasize the need for careful observation for at least 2 h after remimazolam infusion. |
Introduction
Remimazolam, an ultrashort-acting anesthetic agent, is an ester-based benzodiazepine with a high affinity to the binding site of γ-aminobutyric acid (GABA) receptor [1,2,3]. Remimazolam is metabolized by tissue esterases distributed throughout the body; therefore, the metabolite is independent of the liver and renal function [1]. Owing to its rapid onset, predictable recovery, and minimal hemodynamic instability, remimazolam is increasingly used for anesthesia [4, 5]. Several studies have demonstrated the efficacy and safety of remimazolam for general anesthesia and sedation [6,7,8]. Recently, this novel benzodiazepine was approved for general anesthesia in Japan [9]. It has been reported that psychomotor recovery after sedation with remimazolam and flumazenil was faster than that with midazolam [10].
Propofol, a short-acting intravenous anesthetic agent, is commonly used for sedation and general anesthesia owing to its rapid onset and emergence from anesthesia. Total intravenous anesthesia (TIVA) with propofol is one of the most common options because of its predictable recovery time, safety, and efficacy. In spinal surgery, TIVA with propofol is preferable to volatile anesthetics to avoid the attenuation of motor-evoked potential (MEP) monitoring [11]. Our previous work clarified that the combination of propofol and remifentanil achieved rapid and reliable recovery of psychomotor function compared with propofol–fentanyl after TIVA [12].
Remifentanil, ultrashort-acting μ-opioid receptor agonist, achieves predictable recovery in TIVA owing to its fast emergence from anesthesia. The elimination half-life of remifentanil metabolized by tissue and plasma nonspecific esterases is shorter compared to fentanyl which is metabolized by the liver [12, 13]. Thus, remifentanil could achieve a manageable TIVA, surprisingly. Similar to propofol, remimazolam is a novel TIVA option.
Although the efficacy and safety of remimazolam and propofol have been established, a comparison of these agents in terms of recovery remains to be elucidated. In fact, the effects of remimazolam and propofol on psychomotor recovery remain controversial. Further studies are needed to determine the safety and efficacy of these anesthetic agents during the perioperative period.
In the current study, we sought to compare the effects of remimazolam and propofol on psychomotor recovery 0–180 min after the discontinuation of general anesthesia using the Trieger dot test.
Methods
This study was approved by the Ethics Committee of the Dokkyo Medical University (R-49-3) and registered with the University Hospital Medical Information Network (UMIN, registration number UMIN000044900). All procedures were performed in accordance with the ethical standards of the Institutional and National Research Committee and the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. After written informed consent was obtained, 66 patients with American Society of Anesthesiologists (ASA) physical status I or II, aged 20–65 years, who were scheduled to undergo endoscopic sinus surgery were studied. Patients with cardiovascular, respiratory, hepatic, renal, or cerebrovascular diseases, body mass index (BMI) > 30 kg/m2, or history of allergy to any study drugs were excluded. Participants were randomly assigned to two groups: patients in group R (n = 33) received a continuous infusion of remimazolam, while those in group P (n = 33) received a continuous infusion of propofol. None of the patients received premedication.
In the operation room, standard monitoring of 12-lead electrocardiogram (ECG) signals (FDX-4521L; Fukuda Denshi Co. Ltd., Tokyo, Japan), noninvasive measurement of arterial blood pressure, pulse oximetry, and assessment of bispectral index, an index for measuring the depth of anesthesia (Bispectral Index™ Monitoring System; Medtronic, Minneapolis, MN, USA), were performed. After adequate preoxygenation, anesthesia was conducted with 0.5–1 μg/kg/min of remifentanil (a potent and short-acting opioid agonist), an effect-site target propofol concentration of 3–4 μg/ml propofol in group P or 12 mg/kg/h remimazolam in group R. Propofol was administrated using a target-controlled infusion (TCI) system (TE-SS830T; Terumo Medical Corp., Shibuya, Japan). After loss of consciousness, 0.6 mg/kg of rocuronium (a non-depolarizing neuromuscular blocking agent) was administered, followed by manual ventilation with 100% oxygen using a face mask. Tracheal intubation was performed by an experienced anesthesiologist using a video laryngoscope (McGRATH™ MAC Video Laryngoscope; Medtronic, Minneapolis, MN, USA). Anesthesia was maintained with remifentanil (0.25–0.5 μg/kg/min), air–oxygen mixture, 2–5 μg/ml effect-site target propofol concentration in group P or 1–2 mg/kg/h remimazolam in group R. The ventilator settings were adjusted to an end-tidal carbon dioxide tension (PETCO2) of 35–40 mmHg during the study. The anesthetics doses were adjusted to maintain BIS values between 40 and 60 during surgery. All patients received a continuous infusion of acetate Ringer’s solution at a rate of 5 ml/kg/h during the surgery. At the end of surgery, all patients were given 20 mg/kg body weight of acetaminophen for analgesia.
After the operation, all intravenous anesthetics were immediately discontinued and then 4 mg/kg body weight of sugammadex was administered to reverse the neuromuscular blockade. In group R, all patients received 0.2–0.5 mg of flumazenil as an antidote after surgery. After discontinuation of the anesthetic agents, the time to eye opening, response to verbal commands, extubation, and discharge from the operation room were measured. In addition, to assess the recovery of psychomotor function following general anesthesia, a psychomotor function test known as the Trieger dot test was conducted prior to induction and at 0, 30, 60, 90, 120, 150, and 180 min after discharge from the operation room. This test consists in joining together a dotted line which was represented in the form of a figure. The number of dots missed (NDM), which represents the total number of dots unconnected, and maximum distance of dots missed (MDDM: millimeters), which represents the longest distance between the drawn line and missed dots, were evaluated.
The primary outcome of the present study was the quality of psychomotor recovery after infusion of remimazolam compared with propofol. Therefore, the primary endpoints of psychomotor recovery were defined as NDM and MDDM in Trieger dot test after anesthesia. The secondary endpoint of our study was the time to recovery from general anesthesia evaluated by eye opening, response to verbal commands, extubation, and discharge from the operation room after anesthesia. In addition, we evaluated the heart rate (HR), systolic blood pressure (sBP), diastolic blood pressure (dBP), and oxygen saturation (SpO2) as secondary endpoints.
Statistical Analyses
Statistical analysis was performed using Prism 6 (GraphPad, La Jolla, CA, USA). Data are expressed as mean ± standard deviation. Patient characteristics were analyzed using Student’s t test and Fisher’s exact test as appropriate. The time to eye opening, response to verbal command, extubation, discharge from the operation room, NDM, and MDDM were analyzed by two-way analysis of variance. When a significant overall effect was detected, Bonferroni’s post hoc test was conducted. In all analyses, the probability of detecting a significant difference was set at the 5% level (p < 0.05). On the basis of a previous study [12], a sample size of 25 subjects in each group was considered adequate to detect a difference of 12 mm in MDDM between the two groups at a power of 80%, with α = 0.05.
Results
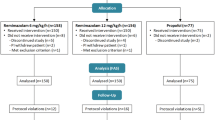
Sixty-nine patients were screened and 66 were enrolled in the present study. However, two patients were excluded from this analysis as a result of postoperative delirium (Fig. 1).
Table 1 shows the patient characteristics for the present study. There were no significant differences in age, sex, ASA physical status, or BMI between the two groups. In this study, no significant difference was found in the duration of anesthesia or surgery between the two groups. No complication was observed in this study.
Table 2 shows the hemodynamic variables before induction, before intubation, immediately after intubation, after extubation, and at discharge from the operation room. There were no significant differences between two groups in HR, sBP, dBP, and SpO2 throughout the present study. However, our findings indicated that the perioperative hemodynamic variables in group R tended to be more prominent in comparison to group P.
Table 3 shows the time to recovery from general anesthesia (eye opening, response to verbal command, extubation, and discharge from operation room) after discontinuation of anesthetic agents. The time to eye opening (group P: 9.8 ± 3.2 min; group R: 6.5 ± 2 min, p < 0.05), response to verbal commands (group P: 11.5 ± 3.4 min; group R: 7.3 ± 2.6 min, p < 0.05), extubation (group P: 12.7 ± 3.4 min; group R: 8.4 ± 2.9 min, p < 0.05), and discharge from the operation room (group P: 18.1 ± 4.2 min; group R: 13.2 ± 3.2 min, p < 0.05) were decreased in group R compared to group P.
Table 4 shows the NMD and MDDM in the Trieger dot test before and after general anesthesia. The NMD in group R was significantly increased at 30 min (group P: 14.6 ± 7.8; group R: 20.5 ± 9.3, p < 0.05), 60 min (group P: 10 ± 7.1; group R: 16 ± 8.8, p < 0.05), 90 min (group P: 8.7 ± 7.3; group R: 14.9 ± 11.1, p < 0.05), and 120 min (group P: 7.3 ± 5.7; group R: 14.3 ± 10.8, p < 0.05) after discharge from the operation room compared to group P. Furthermore, the MDDM in group R was significantly increased at 30 min (group P: 2.7 ± 1.6 mm; group R: 3.9 ± 2.8 mm) after discharge from the operation room compared to group P.
Discussion
The combination of propofol and remifentanil has been an indispensable option for TIVA. Recently, remimazolam, a new benzodiazepine, in combination with flumazenil has garnered recognition for its ability to achieve efficient and safe anesthetic effect owing to its pharmacological characteristics. Remimazolam is recognized as an ultrashort-acting and rapid offset drug compared to midazolam [9, 10]. In addition, the quality of recovery assessed by QoR-15 on postoperative day 1 or 2 showed similar results between remimazolam and propofol [14]. However, in our study, although remimazolam with flumazenil resulted in rapid recovery compared to propofol, psychomotor recovery from remimazolam was inadequate compared to propofol 1–2 h after discharge from the operation room. In terms of recovery from anesthesia, anesthesiologists must pay special attention to delayed psychomotor decline after the use of remimazolam, especially with flumazenil.
Assessment of Psychomotor Function
The Trieger dot test has commonly been used to assess psychomotor function and is a widely recognized as a validated measure of anesthetic recovery [12, 15]. This objective measurement is thought to be more sensitive than other tests for assessing intermediate and late recovery of psychomotor function following general anesthesia [12]. In the previous study, to evaluate psychomotor recovery after remimazolam sedation with flumazenil, Chen et al. measured body sway, choice reaction test, saccadic eye movement, smooth pursuit eye movement, and word recall test [10]. The present study is the first to compare psychomotor recovery between remimazolam and propofol anesthesia using the Trieger dot test.
Comparison of Remimazolam and Propofol in Hemodynamic Variables
Hemodynamic stability during anesthesia is also a benefit of remimazolam. Typically, propofol suppresses hemodynamic changes during anesthesia compared to remimazolam. Several studies have reported that the incidence of hypotension was lower under remimazolam than under propofol anesthesia [6, 14, 16,17,18]. In the present study, there were no statistically significant differences in perioperative hemodynamic variables including HR, sBP, dBP, and SpO2 between the two groups. However, a slight inhibitory effect on hemodynamic changes was detected in the propofol group, but not in the remimazolam group. In addition, there was no adverse cardiovascular event such as lethal arrhythmia or refractory hypotension in either group. Our findings are consistent with those of previous reports for the adverse hemodynamic events.
Comparison of Propofol and Remimazolam in Recovery from Anesthesia
Chen et al. showed that the effect of a single dose of midazolam on psychomotor function was much stronger than that of remimazolam [10]. However, they empathized that although rapid psychomotor recovery rapid psychomotor recovery could be achieved, the residual effect of remimazolam was likely to induce delayed psychomotor decline 2 h after infusion. They proposed that careful attention must be paid to psychomotor assessment for 2 h following remimazolam sedation. In the present study, the time to eye opening, response to verbal commands, extubation, and discharge from the operation room were shorter in group R than those in group P. In contrast, NMD at 30, 60, 90, and 120 min and MDDM at 30 min after discharge from the operation room were significantly increased in group R compared to group P. These results imply that recovery from remimazolam may be prolonged after discharge from the operation room rather than immediately after anesthesia. In terms of delayed psychomotor recovery from remimazolam, our results are consistent with the results reported by Chen et al. These results emphasize that anesthesiologists must pay special attention to delayed anesthetic recovery at least 2–3 h following remimazolam infusion.
Doi et al. reported a significant increase in recovery time as defined by eye opening, date of birth, extubation, and discharge from the operation room following remimazolam anesthesia at a dose of 6 and 12 mg/kg/h compared to propofol [6]. In their study, flumazenil was given when no awakening was observed within 30 min after the discontinuation of remimazolam. Remimazolam antagonized by flumazenil is commonly thought to produce more rapid and reliable recovery than propofol [8, 19]. A previous retrospective study showed that there was no significant difference in the incidence of extubation recall between the remimazolam–flumazenil and propofol groups [20]. However, several studies have reported the reappearance of the effect of remimazolam antagonized by flumazenil [21,22,23]. To avoid re-sedation or respiratory depression, careful observation for at least 1–2 h is recommended after remimazolam anesthesia [22]. In our observation, flumazenil was administered to all patients; therefore, recovery in group R was facilitated immediately after the termination of agent compared to that in group P. However, a delayed psychomotor decline was observed in group R at 30–120 min after the discontinuation of remimazolam. Our findings emphasize the risk of residual effect of remimazolam for at least 2 h after anesthesia, especially with flumazenil.
Limitations
Our study has several limitations. First, the study conducted by Choi et al. compared the quality of recovery (QoR) between remimazolam and propofol anesthesia 1 or 2 days postoperatively [14]. They concluded that the QoR of remimazolam was non-inferior to that of propofol during this time period. However, our observation associated with psychomotor recovery was observed for 180 min following the general anesthesia. Consequently, our findings were unable to exclude the possibility of the remimazolam effect reappearing the day following anesthesia, particularly in cases involving high-dose remimazolam. Thus, a longer observation period for psychomotor recovery might be preferable.
Second, our results were evaluated solely using the Trieger dot test. There are several methods available to assess psychomotor function, such as the digit symbol substitution test and choice reaction test [10, 24]. Therefore, further methods for evaluating psychomotor recovery could have been used in our observation.
Third, all patients in our study were administered flumazenil for the reversal of the remimazolam. Basically, remimazolam is reversed by flumazenil in case of delayed recovery. In our results, immediate recovery (0–30 min after anesthesia), but not late recovery (30–120 min after anesthesia), from remimazolam was facilitated by flumazenil. In contrast, immediate recovery with propofol was prolonged; however, late recovery was superior compared to that of remimazolam. This discrepancy may be attributable to the administration of flumazenil. Careful observation is required when administering a large bolus injection of flumazenil to avoid reappearance of remimazolam’s effect after anesthesia [22]. Further studies without flumazenil should be conducted to clarify the safety and efficacy of remimazolam.
Conclusions
In the present study, TIVA with remimazolam and flumazenil resulted in faster recovery compared to TIVA with propofol. In contrast, remimazolam combined with flumazenil caused delayed psychomotor decline. Careful observation for a moderate to lasting residual effect of remimazolam is required following anesthesia, especially when antagonized by flumazenil.
References
Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–51.
Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107:60–6.
Rogers WK, McDowell TS. Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs. 2010;13:929–37.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501.
Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127:41–55.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–53.
Yao Y, Guan J, Liu L, Fu B, Chen L, Zheng X. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomised, double-blind trial. Eur J Anaesthesiol. 2022;39:911–7.
Lee HJ, Lee HB, Kim YJ, Cho HY, Kim WH, Seo JH. Comparison of the recovery profile of remimazolam with flumazenil and propofol anesthesia for open thyroidectomy. BMC Anesthesiol. 2023;23:147.
Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020;34:479–82.
Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–24.
Shida Y, Shida C, Hiratsuka N, Kaji K, Ogata J. High-frequency stimulation restored motor-evoked potentials to the baseline level in the upper extremities but not in the lower extremities under sevoflurane anesthesia in spine surgery. J Neurosurg Anesthesiol. 2012;24:113–20.
Takayama A, Yamaguchi S, Ishikawa K, et al. Recovery of psychomotor function after total intravenous anesthesia with remifentanil-propofol or fentanyl-propofol. J Anesth. 2012;26:34–8.
Coskun D, Celebi H, Karaca G, Karabiyik L. Remifentanil versus fentanyl compared in a target-controlled infusion of propofol anesthesia: quality of anesthesia and recovery profile. J Anesth. 2010;24:373–9.
Choi JY, Lee HS, Kim JY, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82: 110955.
Mishra SK, Chandrasekaran A, Parida S, Senthilnathan M, Bidkar PU, Gupta SL. Time course of psychomotor recovery after intravenous dexmedetomidine infusion as a part of balanced anaesthetic technique: a randomised, double-blind study. Indian J Anaesth. 2019;63:623–8.
Dai G, Pei L, Duan F, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021;87:1073–9.
Chen S, Wang J, Xu X, Zhang J, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12:4594–603.
Fan S, Zhu Y, Sui C, Li Q, Jiang W, Zhang L. Remimazolam compared to propofol during hysteroscopy: a safety and efficacy analysis. Pain Ther. 202312(3):695–706.
Pan Y, Chen M, Gu F, et al. Comparison of remimazolam-flumazenil versus propofol for rigid bronchoscopy: a prospective randomized controlled trial. J Clin Med. 2022;12:257.
Sato T, Mimuro S, Kurita T, et al. Recall of extubation after remimazolam anesthesia with flumazenil antagonism during emergence: a retrospective clinical study. J Anesth. 2022;36:688–92.
Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021;35:322.
Masui K. Caution!! Reappearance of remimazolam effect after a flumazenil bolus: a larger bolus of flumazenil and a lower total remimazolam clearance are higher risks. J Anesth. 2023;37:1–5.
Takemori T, Oyama Y, Makino T, Hidaka S, Kitano T. Long-term delayed emergence after remimazolam-based general anesthesia: a case report. JA Clin Rep. 2022;8:86.
Parida S, Badhe AS. Comparison of cognitive, ambulatory, and psychomotor recovery profiles after day care anesthesia with propofol and sevoflurane. J Anesth. 2014;28:833–8.
Acknowledgements
We thank the participants of this study.
Author Contributions
Shigeki Yamaguchi made substantial contributions to the conception, design of the work and revision of the manuscript drafts. Toshifumi Takasusuki made substantial contributions to the data analysis and manuscript drafting. Takahito Shimizu made substantial contributions to data acquisition. All the authors have approved the submitted version of the manuscript and agreed to be accountable for any part of the work.
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors.
Medical Writing/Editorial Assistance
Writing assistance for the preparation of this article was provided by Editage (www.editage.jp). Financial support for this assistance was provided by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the Ethics Committee of the Dokkyo Medical University (R-49-3) and registered with the University Hospital Medical Information Network (UMIN, registration number UMIN000044900). All procedures were performed in accordance with the ethical standards of the Institutional and National Research Committee and the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Takahito Shimizu, Toshifumi Takasusuki and Shigeki Yamaguchi declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shimizu, T., Takasusuki, T. & Yamaguchi, S. Remimazolam Compared to Propofol for Total Intravenous Anesthesia with Remifentanil on the Recovery of Psychomotor Function: A Randomized Controlled Trial. Adv Ther 40, 4395–4404 (2023). https://doi.org/10.1007/s12325-023-02615-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02615-w