Abstract
iGlarLixi is a fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide used in the treatment of type 2 diabetes. iGlarLixi has proven clinical benefits in terms of glycemia, weight control, and safety, defined by the risk of hypoglycemia. It simultaneously targets many pathophysiologic abnormalities which are at the root of type 2 diabetes and thus presents a complementary mode of action. Finally, it may also address diabetes treatment burden, and, by decreasing the complexity of treatment, it may improve patient adherence and persistence and fight against clinical inertia. This article reviews the results of major randomized controlled trials in people with type 2 diabetes that compared iGlarLixi to other therapeutic regimens, representing different intensification strategies, such as basal supported oral therapy, oral antidiabetic drugs, and a combination of the latter with glucagon-like peptide 1 receptor agonists. Moreover, as a supplement to randomized trials, data from real-world evidence have also been included.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
iGlarLixi demonstrated greater efficacy in terms of glycemic control in people with type 2 diabetes (T2D) previously treated with basal supported oral therapy (BOT), glucagon-like peptide 1 receptor agonists (GLP-1 RAs), or oral antidiabetic drugs (OADs). |
iGlarLixi has shown a greater reduction in the HbA1c value than other evaluated therapies (basal insulin, premix insulin, or GLP-1 RAs). Moreover, a higher proportion of people with T2D treated with iGlarLixi achieved an HbA1c value < 7.0% at the end of the trials compared with people with T2D treated otherwise. |
iGlarLixi has a favorable safety profile in terms of hypoglycemia when compared to other therapeutic regimens using insulin. iGlarLixi only had a higher risk of hypoglycemia compared with Lixi, which is associated with having insulin as a component. |
iGlarLixi fixed-ratio combination simplifies insulin therapy regimens, with fewer daily insulin injections, a lower risk of hypoglycemia, and a more favorable effect on weight. Therefore, it seems a safe and effective treatment option for people with T2D. |
Introduction
According to the most recent data announced by the International Diabetes Federation, diabetes is one of the fastest-growing health emergencies in the world. It is estimated that nowadays 537 million people have diabetes, and this number might rise up to 783 million in the next 25 years. Over 90% of people with this disease have type 2 diabetes (T2D), and it unfortunately goes undiagnosed in about 45% of individuals [1].
The latest approach to T2D management recommended by the common position of American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) is holistic and patient-centered. Besides the most important aspects of glycemic and weight control, it also includes the management of cardiovascular risk factors and emphasizes the significance of cardiorenal protection. Beyond multifactorial lifestyle modifications, a suitable individually tailored pharmacotherapy is the key component of successful diabetes management that prevents or postpones its complications and preserves a satisfactory quality of life [2]. Moreover, an issue of utmost importance is that cardiovascular protection should always be accompanied by the early and intensive management of hyperglycemia [3, 4].
Several years ago, Ralph DeFronzo indicated eight key pathophysiological pathways (known as “the ominous octet”) which contribute to carbohydrate metabolism disorders: progressive beta cell failure; insulin resistance in the liver, muscles, and brain; accelerated lipolysis in the adipocytes; impairment of the incretin effect; hyperstimulation of alpha cells; and increased renal glucose reabsorption [5]. The greater the number of these abnormalities targeted by the treatment (at best all of them), the more effective the treatment becomes. Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are one such treatment. They act via hyperglycemia-induced and glucose-dependent insulin secretion augmentation and simultaneous glucagon suppression, improving the incretin effect impaired in diabetes. Moreover, they slow down gastric emptying, limit postprandial glycemic excursions, and reduce appetite, and therefore caloric intake, with a beneficial effect on body weight [6]. Since 2005, when exenatide administered twice daily was approved for treatment, GLP-1 RAs consolidated their position in T2D treatment. This was especially so since some of them, based on the results of the cardiovascular outcome trials (CVOTs), beyond improving metabolic control have also been approved for reducing the risk of major adverse cardiovascular events in people with T2D and established cardiovascular disease or multiple cardiovascular risk factors [7,8,9,10]. On the basis of this potential action, GLP-1 RAs are currently recommended as the preferred first injectable therapy, before the initiation of insulin therapy [11]. GLP-1 RAs improve glycemic control with lower risk of hypoglycemia and beneficial efficacy in body weight compared to monotherapy with insulin [12]. However, we should be aware that, when blood glucose meaningfully exceeds target ranges, the introduction of insulin might be the best therapeutic option for an individual as insulin, not GLP-1 RAs, can address almost any level of blood glucose and for this reason should not be delayed if needed. Basal insulin therapy is a critical part of effective disease management for many people with T2D. Aversion to basal insulin therapy may lead to unnecessary hyperglycemia and suboptimal outcomes [13]. There are, of course, some commonly known challenges of insulin therapy and its intensification that should be considered in daily practice, like weight gain, the risk of hypoglycemia, and the need for titration for optimal effect [14, 15]. Taking the above into account, a combined therapy of GLP-1 RAs and insulin is recommended. This kind of therapy guarantees simultaneous targeting of multiple pathophysiologic processes that characterize T2D, an improved effectiveness, and a long-term effect with less weight gain and lower rates of hypoglycemia than observed with intensified insulin regimens [11]. At the same time, in many cases, it simplifies the treatment regimen, improving patient adherence and avoiding the burden and complexity of treatment and clinical inertia. To meet these needs, preparations combining both GLP-1 RAs and insulin as fixed-ratio combinations (FRCs) have been introduced into clinical practice. To date, two FRCs have been approved for treatment—insulin glargine 100 U/mL with lixisenatide (iGlarLixi) and insulin degludec with liraglutide (iDegLira) [2].
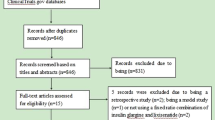
In this review, we present the results of the randomized control trials (RCTs) in people with T2D (predominantly white) comparing iGlarLixi to other relevant treatment intensification strategies relevant from the point of view of the frequency of use in everyday practice: basal supported oral therapy (BOT), oral antidiabetic drugs (OADs) combined with GLP-1 RAs, and OADs only. Results from Japanese and Asian Pacific populations are not included in this review because we focused on the Caucasian population. Moreover, we included data from real-world evidence (RWE) as a supplement to RCTs. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
People with T2D Previously Treated with BOT Model
LixiLan-L Randomized Trial [16]
In the LixiLan-L study, Aroda et al. sought to answer whether FRCs or the correctly titrated dose of a long-acting basal insulin analogue is a more effective and beneficial treatment for people with suboptimally controlled T2D. LixiLan-L was an open-label, randomized, multinational, multicenter, parallel-group clinical trial assessing the efficacy and safety of iGlarLixi compared with insulin glargine (iGlar). The study was conducted among people with T2D with inadequate glycemic control previously treated with the BOT model. Apart from basal insulin treatment for at least 6 months before screening (with at least a 3-month period of a stable regimen), admissible OADs included up to two oral glucose-lowering agents like metformin, glinide, a sulfonylurea, dipeptidyl peptide 4 inhibitor (DPP4i), and/or sodium-glucose cotransporter 2 inhibitor (SGLT2i). During a 6-week run-in period, OADs other than metformin were discontinued and the basal insulin—if not used before—was switched to iGlar, with a dose that was titrated and/or stabilized for all people with T2D. At the end of this run-in period, the mean HbA1c value decreased to 8.1% (from the initial 8.5%). Subsequently, people with T2D were randomized 1:1 into iGlar (n = 365) or iGlarLixi subgroups (n = 366) for a 30-week treatment period, with the primary outcome defined as the change in the HbA1c value. Individuals treated with iGlarLixi achieved a greater reduction in HbA1c (− 1.1%) than those in the iGlar arm (− 0.6%; p < 0.0001). The glycemic target of HbA1c < 7.0% was achieved by 55.0% of participants treated with iGlarLixi compared to 30.0% of iGlar-treated individuals. It is important to emphasize that participants using iGlarLixi noted weight loss (mean reduction of 0.7 kg), whereas those in the iGlar group experienced weight gain (+ 0.7 kg; p < 0.0001). There was no statistically significant difference in symptomatic hypoglycemia (≤ 70 mg/dL) between groups. As for other adverse effects (AEs), gastrointestinal complaints (mild to moderate) were observed more frequently in the iGlarLixi group. The results demonstrated the greater efficacy in terms of glycemic control and weight management of iGlarLixi compared to iGlar.
SoliMix [17, 18]
In everyday practice, when BOT treatment does not result in proper metabolic control, a common strategy to intensify the treatment is to include a mealtime insulin injection in the basal-plus model, or multiple insulin injections as basal-bolus, or a premix insulin therapeutic scheme. Therefore, Rosenstock et al. performed a 26-week, open-label, randomized, multicenter study designed to prove the non-inferiority of iGlarLixi in reducing the HbA1c value and its superiority in body weight control compared to a premix insulin injected twice daily [18]. The study included 887 people with T2D from 17 countries, with the disease previously insufficiently controlled with BOT. The baseline HbA1c value was 7.5–10.0%, and previous treatment was based on basal insulin combined with metformin and/or SGLT2i for at least 3 months. The participants were randomized 1:1 into an iGlarLixi group (n = 403) and a premix insulin arm (30% insulin aspart and 70% insulin aspart protamine, BIAsp 30) injected twice daily as an active comparator (n = 404). OADs were continued after necessary clinical adjustments. At the end of the trial, both primary efficacy endpoints were met. The non-inferiority primary endpoint was achieved, as iGlarLixi reduced the HbA1c value by 1.3%, whereas BIAsp 30 reduced it by 1.1% (p < 0.001). The superiority of iGlarLixi over BIAsp 30 was demonstrated in relation to body weight change: participants in the iGlarLixi arm slightly reduced their weight (baseline weight of 80.7 ± 16.5 kg vs 80.2 ± 16.6 kg at week 26) whilst those treated with BIAsp 30 gained weight (baseline weight of 82.2 ± 18.5 kg vs 83.4 ± 19.0 kg at week 26; p < 0.001). The study secondary endpoints were the HbA1c reduction, the proportion of participants achieving HbA1c < 7.0% without weight gain, and the proportion of participants achieving HbA1c < 7.0% both without weight gain and hypoglycemia (< 70 mg/dL), changes in daily insulin dose, and fasting plasma glucose (FPG). The superiority of iGlarLixi compared to BIAsp 30 with respect to HbA1c, weight management, and the risk of hypoglycemia was demonstrated. Moreover, iGlarLixi treatment was simpler (only once-daily injections) and characterized by fewer hypoglycemic episodes than BIAsp 30, avoiding not only the burden and complexity of treatment but also fulfilling the safety criteria regarding hypoglycemia. Considering other AEs, all of them were mild to moderate, with nausea as the most common. In the iGlarLixi group, 32.6% of participants experienced at least one AE, whereas 27.7% of participants in the BIAsp30 arm reported AEs. These data indicate that once-daily treatment with iGlarLixi is simpler and leads to a better glycemic control and body weight improvement with fewer hypoglycemic episodes than treatment with BIAsp 30 injected twice daily. As such, it might contribute to better therapeutic adherence, treatment persistence, and less clinical inertia.
People with T2D Previously Treated with GLP-1 RAs
LixiLan-G Study [19]
According to the latest diabetes management guidelines, GLP-1 RAs are recommended as the first injectable therapy in T2D [2, 11]. However, there are some people with T2D who do not reach individual target HbA1c value with this therapy and an intensification towards basal insulin, preferably in the form of FRCs therapy, is therefore considered. Following these considerations, Blonde et al. designed a 26-week, randomized, controlled, open-label, parallel-group trial named LixiLan-G to compare the treatment switch from GLP-1 RAs to iGlarLixi versus the continuation of therapy with the current GLP-1 RAs. People with T2D enrolled in the study were characterized by an HbA1c value of 7.0–9.0% and treated with the maximum tolerated doses of GLP-1 RAs. Of the eligible people, 60% had GLP-1 RAs administered daily (liraglutide once a day or exenatide twice a day) and 40% injected the GLP-1 RAs once a week (dulaglutide, exenatide extended release, or albiglutide). Concomitant medications included metformin and/or pioglitazone and/or SGLT2i. Participants were randomized 1:1 into an iGlarLixi arm (n = 257) and a GLP-1 RAs group (n = 257). The primary endpoint was the change in HbA1c. Starting from the baseline HbA1c value of 7.8% in both groups, there was a greater reduction in the iGlarLixi arm (to 6.7%) than in the GLP-1 RAs arm (to 7.4%; p < 0.001) after 26 weeks. Secondary endpoints included the percentage of people with T2D achieving an HbA1c value < 7.0% and < 6.5% at the end of the trial as well as the change in fasting plasma glucose, post-prandial plasma glucose after a standard meal, self-monitored plasma glucose profile, body weight, and iGlarLixi dose from baseline to week 26. As for body weight, participants switching from GLP-1 RAs to insulin-based iGlarLixi reported weight gain, while those continuing with GLP-1 RAs treatment reported weight loss. A higher proportion of people with T2D treated with iGlarLixi reached the composite endpoint of HbA1c < 7.0% without documented symptomatic hypoglycemia (< 70 mg/dL; 43% in the iGlarLixi arm vs 25% in the GLP-1 RAs arm). The difference was even higher (57.0% vs 25.0%, respectively) once the hypoglycemia threshold of < 54 mg/dL was considered. The percentage of people with T2D who required rescue therapy was lower in the iGlarLixi group (5.0%) compared to the GLP-1 RAs group (15.0%). Regarding AEs, nasopharyngitis, nausea, and diarrhea were the most common, and most of them were classified as mild to moderate. AEs were generally more common among participants treated with iGlarLixi (63.9%) than those in the GLP-1 RAs group (47.3%), and this trend also applied to symptomatic hypoglycemia. As expected, when comparing an insulin-based therapy with continued GLP-1 RAs, more people in the iGlarLixi group experienced documented hypoglycemic events for the thresholds of ≤ 70 mg/dL (≤ 3.9 mmol/L; 27.8% vs 2.3% of people with T2D, respectively) and < 54 mg/dL (< 3.0 mmol/L; 9.4% vs 0.4% of people with T2D, respectively).
Only one case of severe hypoglycemia was reported, and it occurred in the iGlarLixi arm. The LixiLan-G trial provided evidence to support the claim that in people with suboptimally controlled T2D, switching from GLP-1 RAs to FRCs iGlarLixi can improve diabetes management, and this therapeutic procedure is not only effective but also safe.
Since T2D is a progressive disease, and the therapeutic effect of some drugs may decrease over time, a 26-week extension of the iGlarLixi arm was continued by Blonde et al. It aimed to determine the safety and efficacy of iGlarLixi over a total period of 52 weeks. The endpoints from week 26 of the LixiLan-G study were compared to the results obtained at week 52 (i.e., week 26 of the extension). The participants maintained the same glycemic control (mean HbA1c = 6.7% both at week 26 and 52), a comparable proportion of individuals achieved the HbA1c target value < 7.0% (62.0% at week 26 and 64.0% at week 52, respectively), and similar results were obtained in the proportion of people with T2D reaching this target without symptomatic hypoglycemia (< 54 mg/dL; 57.0% at week 26 vs 58.0% at week 52, respectively). Moreover, which is equally important, the safety results were also similar, with low rates of hypoglycemic episodes and gastrointestinal AEs. Overall, these results provide further evidence suggesting persistent efficacy of iGlarLixi over time [20].
People with T2D Previously Treated with OADs Only
LixiLan-O Study [21]
Basal insulin therapy improves glycemic management by lowering, first of all, fasting and nocturnal plasma glucose values [22]. However, it also normalizes postprandial glycemia through indirect influence. Treatment with basal insulin enables proper glycemic control in about 50–60% of people with T2D uncontrolled by OADs only [23]. In the remaining cases, we consider adding prandial insulin injections or exploring the full potential of combining “new” glucose-lowering agents, like GLP-1 RAs among them, with basal insulin [24, 25]. On the basis of these facts, Rosenstock et al. performed an open-label, multinational, multicenter, randomized, parallel-group clinical trial (LixiLan-O study) in a group of 1170 people with T2D receiving a suboptimal treatment (mean baseline HbA1c = 8.1%) with metformin and/or other oral antidiabetic agents [21]. Their aim was to compare the efficacy and safety of iGlarLixi with each of its components (iGlar and lixisenatide (Lixi)) administered separately. During the first 4 weeks, metformin therapy was optimized, and other glucose-lowering drugs were discontinued. Subsequently, the participants were randomized into three arms: iGlarLixi (n = 469), iGlar (n = 467), and Lixi (n = 234). The change in HbA1c at week 30 was the primary outcome. The mean HbA1c value was 6.5% (least squares [LS] mean change − 1.63%) in the iGlarLixi arm, 6.8% (LS mean change − 1.34%) in the iGlar arm, and 7.3% (LS mean change − 0.85%) in the Lixi group. Moreover, the percentage of people with T2D achieving an HbA1c value < 7.0% was highest for the iGlarLixi group (74.0% vs 59.0% for iGlar and 33.0% for Lixi; p < 0.0001 for all). As for body weight change, people with T2D treated with Lixi and iGlarLixi reduced their weight (by 2.3 kg and 0.3 kg, respectively), and those treated with insulin (iGlar) experienced weight gain (mean increase of + 1.1 kg). Symptomatic hypoglycemia (≤ 70 mg/dL) occurred more often in the arms treated with insulin as a component of the therapy (1.4 and 1.2 events/patient-year for iGlarLixi and iGlar, respectively) and less often in participants using Lixi (0.3 events/patient-year). Most of the AEs were classified as mild to moderate. Nausea and diarrhea were the most frequent AEs among participants in both the iGlarLixi (9.6% and 9.0%, respectively) and the Lixi (24.0% and 9.0%, respectively) groups, yet they subsided during treatment. The results of this trial showed that iGlarLixi, which combines the therapeutic effects of iGlar and Lixi, improves glycemic control without increasing the hypoglycemic episode rate or weight gain (compared with iGlar) and with less gastrointestinal AEs (compared with Lixi).
Graphical Summary of Major Efficacy and Safety Outcomes of RCTs
HbA1c Reduction
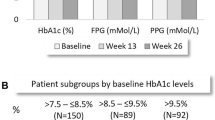
In all the studies we analyzed, iGlarLixi achieved a greater reduction in the HbA1c value compared with the other evaluated therapies (basal insulin, premix insulin, and GLP-1 RAs) (Fig. 1).
Proportion of People with T2D Achieving HbA1c < 7%
In all of the presented RCTs, a higher proportion of people with T2D treated with iGlarLixi had achieved an HbA1c value < 7.0% at the end of the trial than people with T2D treated otherwise (Fig. 2).
Body Weight Changes
The outcomes of these RCTs revealed a beneficial effect in weight reduction after using iGlarLixi compared with iGlar. In studies with Lixi as a comparator, body weight reduction was not in favor of the iGlarLixi because insulin is associated with weight gain as a side effect of the therapy (Fig. 3).
Risk of Hypoglycemia
All the analyzed RCTs evaluated the safety of iGlarLixi. Since iGlarLixi has an insulin component, it was not surprising that its comparisons with Lixi (in the LixiLan-G and LixiLan-O studies) revealed a higher risk of hypoglycemia. Compared to other therapeutic regimens using insulin, the safety profile in terms of hypoglycemia was in favor of iGlarLixi (LixiLan-L and SiloMix studies). The percentage of people with T2D with symptomatic hypoglycemia is presented in Fig. 4.
Real-World Evidence Studies
Results coming from RCTs are considered the most reliable type of evidence, and clinical guidelines are created on their basis. Yet, RCTs are conducted among highly selected, highly controlled, and rather small groups of people with T2D in terms of the randomized population, even including large CVOTs [7,8,9,10, 26, 27]. In contrast, RWE refers to data collected from large cohorts and reflects the everyday reality of medical practice, but it is less reliable. Nonetheless, RWE illustrates what is the real correlation between the theoretical clinical recommendations and following these recommendations in everyday medical practice. Taking all the above into account, RCTs and RWE could be considered mutually complementary [28] and therefore it is also worth presenting evidence from real life. In this review, we present the RWE on the largest cohorts of people with T2D published to date concerning the effectiveness and safety of iGlarLixi.
In order to determine the influence of other antihyperglycemic therapies on the safety and efficacy endpoints of iGlarLixi treatment, Guja et al. performed a 6-month, retrospective, observational study of people with T2D treated with iGlarLixi and divided them into users (n = 320) and non-users of SGLT2i (n = 1285) at baseline. Medical data were obtained from the Optum-Humedica electronic medical records database and changes in HbA1c, hypoglycemic episode frequency, and other AEs were assessed. Treatment with iGlarLixi was effective and safe regardless of SGLT2i therapy, as the decrease in the HbA1c value and the prevalence of hypoglycemia and other AEs were similar in both groups [29].
Recently, the results of one more RWE constructed on the basis of medical databases were published. McCrimmon et al. presented data from the SoliSimplify real-world study comparing the effectiveness of iGlarLixi and a multiple-injection basal-bolus regimen in people with T2D previously suboptimally treated with basal insulin. In this study, they retrospectively analyzed the medical records of people with T2D obtained from the Observational Medical Outcomes Partnership database. The HbA1c value of people with T2D was compared at baseline and after a 6-month follow-up period to determine if iGlarLixi was non-inferior compared with multiple insulin injections. The comparison of the study cohort (n = 814 for each group) revealed a similar HbA1c reduction (the non-inferiority criteria were met), with less weight gain among people treated with iGlarLixi. Hypoglycemic episodes were rare in both groups, probably as a result of underreporting [30].
Among RWE studies, there were two prospective studies in which iGlarLixi treatment was initiated at the discretion of the investigator in accordance with the local and international recommendations on diabetes management. One of them was a 26-week non-interventional study conducted by Kis et al. that evaluated treatment with iGlarLixi in a real-life setting including people with T2D suboptimally controlled with either OADs (n = 282) or BOT (n = 71) who initiated iGlarLixi treatment. Almost 61% of people had a reduction in HbA1c of at least 1.0% from baseline till week 26, which was the primary efficacy endpoint. Compared with baseline measurements, a mean weight loss of 2.3 kg was reported at the end of the study. Safety outcomes were assessed on the basis of hypoglycemic episodes and other AEs. The hypoglycemia incidence rate was 0.5 events/person-year, and there were no severe hypoglycemic episodes reported during the study. As for other AEs associated with the use of iGlarLixi, five out of all 13 reported AEs were gastrointestinal. Two of them resulted in study discontinuation, and none were serious [31].
Another of these prospective studies was performed among 901 suboptimally controlled Romanian people with T2D by Bala et al. during an open-label study named STAR.Ro. People with T2D who were previously treated with OADs and/or basal insulin initiated iGlarLixi treatment. Subsequently, participants were observed for 24 weeks. At the end of the trial there was a reduction of 1.3% in HbA1c compared with the baseline value. Moreover, the mean fasting plasma glucose level and the mean body weight decreased significantly during the observation period (− 3.1 mmol/L and − 1.6 kg, respectively) [32].
Finally, another RWE study compared the drug persistence, adherence, and therapy cost of iGlarLixi (n = 952 people with T2D) with that of GLP-1 RAs and basal insulin in free-dose combinations (n = 932 participants). Data from people with T2D with a HbA1c value ≥ 8% who received one of these treatments were obtained from the US Optum Clinformatics database. The participants were followed for 12 months. Compared with people with T2D treated with GLP-1 RAs and basal insulin in free-dose combinations, significantly more individuals treated with iGlarLixi were persistent (44.8% vs 36.3%; p < 0.001) and adherent to the therapy (41.3% vs 18.7%; p < 0.001). The calculated time difference between groups in terms of persistence and adherence to therapy was approximately 30 days. There was no difference in terms of HbA1c reduction, probably because the same agents were compared. However, treatment with iGlarLixi reduced the number of visits to outpatient clinics [33].
In summary, all the currently available results from RWE studies on the effectiveness and safety of iGlarLixi performed to date confirm, in a real-life setting, the observations from RCTs.
The review is limited solely to the Caucasian population; however, it must be noted that there are trial results concerning other ethnic groups which were not included in this manuscript.
Summary
Cardiovascular disease remains the leading cause of mortality and morbidity in people with T2D [34]. Recent results from the EMPA-REG OUTCOME study [26] and other landmark CVOTs using SGLT2i and GLP-1 RAs have revealed significant and clinically meaningful reductions in cardiorenal risk, changing the current paradigm of care in diabetes [7,8,9,10, 35,36,37,38,39,40]. We have witnessed a shift in the therapeutic approach from being glucocentric to targeting cardiovascular and renal protection. Currently, glycemic, weight, and cardiovascular risk factor management are equally important principles of diabetes care, along with the use of glucose-lowering agents with cardio- and renoprotective properties [2]. GLP-1 RAs and SGLT2i have a proven cardiovascular and renal protective potential that is independent of the HbA1c value [38,39,40]. For this reason, their use should be considered people with T2D at high risk of cardiovascular events and with pre-existing cardiovascular disease, heart failure, or chronic kidney disease, regardless of metformin use. Previous studies aiming to assess the relationship between intensive normalization of glycemia and cardiovascular risk, such as ACCORD [41, 42], VADT [43], and ADVANCE [44], showed that treatment intensification is not necessarily beneficial for all people with T2D. On the other hand, clinical inertia should be avoided because elevated HbA1c values correlate with the occurrence of vascular complications of diabetes, especially macrovascular complications [45]. It proved the basis for the individualization of the glycemic target and considerations about the importance of metabolic legacy effects. According to recently announced results of the UKPDS 44 study [3], the glycemic legacy effects seen in T2D may be explained largely by historical HbA1c values having a greater impact than recent values on clinical outcomes. This is especially so for macrovascular outcomes, which are the most spectacular from the patient’s point of view. Regarding these observations, an assumption should be made that early detection of diabetes and intensive glucose control from the time of diagnosis are essential to maximize the reduction of the long-term risk of glycemic complications, not only microvascular as was believed but also macrovascular. Moreover, in the diabetes treatment conception, like in the hypertension management guidelines [46], administering a combined therapy from diagnosis surpasses a stepwise treatment intensification in terms of better glycemic durability, the subsequent onset of secondary treatment failure, and the postponing of insulin initiation. This last point is especially beneficial because insulin is associated with weight gain and the potential risk of hypoglycemia, which could reduce the benefits of glycemic control [47]. The VERIFY study illustrates this point, since it shows that early combination therapy leads to better glycemic durability than a stepwise approach in the treatment of people with newly diagnosed T2D and delays both primary and secondary treatment failure without tolerability issues [47]. Moreover, since the absolute effectiveness of most oral antidiabetic drugs rarely exceeds 1%, commencing treatment with combination therapy should be considered in all people with T2D presenting with HbA1c values 1.5–2.0% above target [48]. Taking the above observations into account, it is mandatory nowadays in diabetes that cardiovascular and renal protection is accompanied by early intensive management of hyperglycemia which necessitates the harmonization of the gluco- and cardiorenal-centric approach of disease management. In addition, according to current consensus report by the ADA/EASD regarding management of hyperglycemia in T2D, hypoglycemia risk and weight control are also given great importance [2]. According to the ADA/EASD, the combination of a GLP-1 RAs and a long-acting basal insulin like iGlarLixi FRCs can be considered as a first injectable therapy, depending on patient profile (including HbA1c level). This combination therapy is very promising given the complementary mechanisms of action targeting many pathophysiologic abnormalities that are at the root of T2D. GLP-1 RAs improve glycemic control by stimulating insulin release, suppressing glucagon secretion, and delaying gastric emptying [6]. This last point is an important factor in reducing postprandial glucose, whereas basal insulin primarily reduces fasting plasma glucose mainly by suppressing hepatic glucose production [49]. iGlarLixi FRCs are a clinically relevant alternative treatment option for people with uncontrolled T2D who are insulin-naïve or already treated with insulin. Translating theory into actual numbers, it is estimated that up to 200 million people with T2D all over the world require insulin therapy, and those numbers might be underestimated [50]. The majority of them use insulin in at least two injections per day, whether as premix insulin or as the basal-plus or basal-bolus model. Compared with premix insulin, which is the insulin therapy model that is most often overused, iGlarLixi FRCs were more effective (HbA1c value reduction and weight management) and safer in terms of the risk of hypoglycemia (SoliMix study [17]). According to evidence-based medicine, a combination such as FRCs increases the durability of the glycemic effect and has a beneficial effect on body weight compared with insulin therapy alone. Moreover, it has a potential impact on medication burden, adherence, and treatment persistence, with both its components being at least neutral on cardiovascular risk [51, 52]. iGlarLixi FRCs help simplify insulin therapy regimens with fewer daily insulin injections, lower insulin doses compared with premix insulin, a lower risk of hypoglycemia, and a more favorable effect on weight. It therefore helps decrease clinical inertia, which is defined as the failure to advance therapy [53] or to de-intensify [54] therapy when appropriate to do so. Recommendations, however, are not always reflected in everyday clinical practice. Real-world data indicate that the initiation of injectable therapy (either GLP-1 RAs or insulin) is postponed until there is a significant metabolic decompensation [55], and this ought to be modified in the near future.
Conclusion
We are witnessing dynamic changes in recommendations regarding the treatment of diabetes, which include both a paradigm shift in the field of cardiovascular and renal protection, but also a new approach to antihyperglycemic therapy and the concept of clinical inertia. The results of the UKPDS 44 study [3] clearly indicate the need to move away from stepwise intensification in favor of earlier intensive two-drug therapy and the recommendations include fixed-ratio combinations as well. According to current guidelines, it is recommended, especially in older people with T2D, to de-intensify insulin therapy from premix insulin and multiple injections in the basal-plus scheme and to use the potential of basal insulin with new classes of drugs, including GLP-1 RAs. In 2011 Nauck et al. heard the sound of wedding bells [56], and more than a decade later, we still hear them. Therefore, FRCs can be likened to a perfect marriage in which the partners complement each other, and it seems an effective and safe treatment option for people with T2D.
References
International Diabetes Federation. IDF diabetes atlas, 10th edn. Brussels, Belgium: 2021. https://www.diabetesatlas.org. Accessed 20 April 2023
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Lind M, Imberg H, Coleman RL, Nerman O, Holman RR. Historical HbA(1c) values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care. 2021;44(10):2231–7.
Prattichizzo F, La Sala L, Ceriello A. Two drugs are better than one to start T2DM therapy. Nat Rev Endocrinol. 2020;16(1):15–6.
DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol Metab. 2021;46: 101102.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Caruso I, Cignarelli A, Sorice GP, et al. Cardiovascular and renal effectiveness of GLP-1 receptor agonists vs. other glucose-lowering drugs in type 2 diabetes: a systematic review and meta-analysis of real-world studies. Metabolites. 2022;12(2):183.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Nauck MA, Quast DR. Cardiovascular safety and benefits of semaglutide in patients with type 2 diabetes: findings from SUSTAIN 6 and PIONEER 6. Front Endocrinol (Lausanne). 2021;12: 645566.
American Diabetes Association. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S1–2.
Anderson SL, Trujillo JM. Lixisenatide in type 2 diabetes: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2016;7(1):4–17.
Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25(6):1413–20.
Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):232.
Edelman S, Pettus J. Challenges associated with insulin therapy in type 2 diabetes mellitus. Am J Med. 2014;127(10 Suppl):11–6.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972–80.
McCrimmon RJ, Al Sifri S, Emral R, et al. Advancing therapy with iGlarLixi versus premix BIAsp 30 in basal insulin-treated type 2 diabetes: design and baseline characteristics of the SoliMix randomized controlled trial. Diabetes Obes Metab. 2021;23(6):1221–31.
Rosenstock J, Emral R, Sauque-Reyna L, et al. Advancing therapy in suboptimally controlled basal insulin-treated type 2 diabetes: clinical outcomes with iGlarLixi versus Premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care. 2021;44(10):2361–70.
Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G randomized clinical trial. Diabetes Care. 2019;42(11):2108–16.
Blonde L, Rosenstock J, Frias J, et al. Durable effects of iGlarLixi up to 52 weeks in type 2 diabetes: the LixiLan-G extension study. Diabetes Care. 2021;44(3):774–80.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–35.
Charbonnel B, Bertolini M, Tinahones FJ, Domingo MP, Davies M. Lixisenatide plus basal insulin in patients with type 2 diabetes mellitus: a meta-analysis. J Diabetes Complic. 2014;28(6):880–6.
Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough–what next? Diabetes Metab Res Rev. 2007;23(4):257–64.
Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia. 2006;49(5):846–54.
Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30(2):263–9.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34): e213.
Guja C, Giorgino F, Blonde L, et al. Concomitant iGlarLixi and sodium-glucose co-transporter-2 inhibitor therapy in adults with type 2 diabetes: LixiLan-G trial and real-world evidence results. Diabetes Ther. 2022;13(1):205–15.
McCrimmon RJ, Cheng AYY, Galstyan G, et al. iGlarLixi versus basal plus rapid-acting insulin in adults with type 2 diabetes advancing from basal insulin therapy: the SoliSimplify real-world study. Diabetes Obes Metab. 2023;25(1):68–77.
Kis JT, Nagy G, Kovacs G. Effectiveness of IGlarLixi, a fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, in people with type 2 diabetes. Diabetes Ther. 2021;12(9):2517–29.
Bala C, Cerghizan A, Mihai BM, Moise M, Guja C. Real-world evidence on the use of a fixed-ratio combination of insulin glargine and lixisenatide (iGlarLixi) in people with suboptimally controlled type 2 diabetes in Romania: a prospective cohort study (STAR.Ro). BMJ Open. 2022;12(5):e060852.
Edelman S, Cassarino D, Kayne D, Dex T, Li X, Pasquel FJ. Treatment persistence and adherence in people with type 2 diabetes switching to iGlarLixi vs free-dose combinations of basal insulin and glucagon-like peptide 1 receptor agonist. J Manag Care Spec Pharm. 2022;28(9):958–68.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–27.
Masson W, Lavalle-Cobo A, Lobo M, Masson G, Molinero G. Novel antidiabetic drugs and risk of cardiovascular events in patients without baseline metformin use: a meta-analysis. Eur J Prev Cardiol. 2021;28(1):69–75.
Crowley MJ, McGuire DK, Alexopoulos AS, et al. Effects of liraglutide on cardiovascular outcomes in type 2 diabetes patients with and without baseline metformin use: post hoc analyses of the LEADER trial. Diabetes Care. 2020;43(9):108–10.
Neuen BL, Arnott C, Perkovic V, et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab. 2021;23(2):382–90.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Buse JB, Bigger JT, Byington RP, et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12a):21–33.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–40.
Aroda VR, Eckel RH. Reconsidering the role of glycaemic control in cardiovascular disease risk in type 2 diabetes: a 21st century assessment. Diabetes Obes Metab. 2022;24(12):2297–308.
Whelton PK, Carey RM, Mancia G, Kreutz R, Bundy JD, Williams B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: comparisons, reflections, and recommendations. Circulation. 2022;146(11):868–77.
Matthews D, Del Prato S, Mohan V, et al. Insights from VERIFY: early combination therapy provides better glycaemic durability than a stepwise approach in newly diagnosed type 2 diabetes. Diabetes Ther. 2020;11(11):2465–76.
American Diabetes Association. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38.
Edgerton DS, Kraft G, Smith M, et al. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2(6):e91863.
Garg SK, Rewers AH, Akturk HK. Ever-increasing insulin-requiring patients globally. Diabetes Technol Ther. 2018;20(S2):21–4.
Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57.
Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–34.
Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12.
Peng XV, McCrimmon RJ, Shepherd L, et al. Glycemic control following GLP-1 RA or basal insulin initiation in real-world practice: a retrospective, observational, longitudinal cohort study. Diabetes Ther. 2020;11(11):2629–45.
Nauck MA, Meier JJ. Pharmacotherapy: GLP-1 analogues and insulin: sound the wedding bells? Nat Rev Endocrinol. 2011;7(4):193–5.
Acknowledgements
Funding
The Open Access was funded by Medical University of Silesia and the Rapid Service Fee by Sanofi Aventis, Poland.
Medical Writing and Editorial Assistance
Editorial assistance was performed by Proper Medical Writing (Karolina Beda-Maluga) and funded by Sanofi Aventis, Poland.
Author Contributions
Conceptualization: Hanna Kwiendacz, Janusz Gumprecht; Literature search: Hanna Kwiendacz; Data analysis: Hanna Kwiendacz; Writing—original draft preparation: Hanna Kwiendacz, Katarzyna Nabrdalik, Janusz Gumprecht; Writing—review and editing: Hanna Kwiendacz, Katarzyna Nabrdalik, Leszek Czupryniak, Tomasz Klupa, Maciej Małecki, Małgorzata Myśliwiec, Krzysztof Strojek, Janusz Gumprecht; Funding acquisition: not applicable; Supervision: Janusz Gumprecht. All authors read and approved the final manuscript.
Disclosures
Hanna Kwiendacz received remunerations/fees for activities on behalf of Sanofi-Aventis, Eli Lilly, and Novo Nordisk. Leszek Czupryniak, Janusz Gumprecht, Tomasz Klupa, Maciej Małecki, Małgorzata Myśliwiec, Katarzyna Nabrdalik, and Krzysztof Strojek received remunerations/fees for activities on behalf of Sanofi-Aventis, Eli Lilly, Novo Nordisk, Bioton, Polfa Tarchomin, Servier, Astra-Zeneca, Boehringer-Ingelheim, Medtronic, Roche, and Abbott.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kwiendacz, H., Nabrdalik, K., Czupryniak, L. et al. The Wedding Bells Sound Really Good! iGlarLixi Fixed-Ratio Combination in the Treatment of Type 2 Diabetes: A Narrative Review. Adv Ther 40, 3395–3409 (2023). https://doi.org/10.1007/s12325-023-02567-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02567-1