Abstract
Introduction
As new glucagon-like peptide 1 receptor agonist (GLP-1 RA) formulations are available, the aim of this study was to understand dulaglutide and subcutaneous (s.c.) semaglutide dosing patterns in people with type 2 diabetes mellitus (T2DM) in the UK and Germany as well as oral semaglutide in the UK.
Methods
Adults with evidence of T2DM and a prescription of dulaglutide or semaglutide between August 2020 and December 2021 were identified using the IQVIA Longitudinal Prescription Data (LRx). Patients were divided into cohort 1 (incident users) and cohort 2 (prevalent users) based on previous exposure to GLP-1 RAs and were followed up to 12 months post-index.
Results
During the patient selection window in Germany and the UK, 368,320 and 123,548 patients respectively received at least one prescription of a study GLP-1 RA. Among dulaglutide users in Germany at 12 months post-index, the 1.5-mg dosage formulation was the most common for both cohort 1 (65.6%) and 2 (71.2%). Among s.c. semaglutide users at 12 months post-index, 39.2% and 58.4% of cohort 1 received 0.5 mg and 1.0 mg, respectively. In the UK, at 12 months post-index, the most common dulaglutide dosage formulation was 1.5 mg (71.7% cohort 1 and 80.9% cohort 2). Among s.c. semaglutide users at 12 months post-index, 0.5- and 1.0-mg formulations were the most common for both cohort 1 (38.9% and 56.0%, respectively) and cohort 2 (29.5% and 67.1%, respectively). Prescribing of the more recently introduced 3.0- and 4.5-mg formulations for dulaglutide and oral semaglutide was also reported in the study.
Conclusion
Dosing patterns of GLP-1 RAs, although similar between the UK and Germany, were heterogeneous over time. Given that the higher dulaglutide doses and oral semaglutide were recently introduced to the market, additional real-world evidence studies which include clinical outcomes is required.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Type 2 diabetes mellitus (T2DM) is a complex and progressive metabolic disease; therefore, an individualized treatment approach is recommended |
As new glucagon-like peptide 1 receptor agonist (GLP-1 RA) formulations are available, the overall aim of this study was to understand dulaglutide and subcutaneous semaglutide prescribing patterns in people with T2DM in the UK and Germany as well as oral semaglutide in the UK |
Dosing patterns of GLP-1 RAs, although similar between the UK and Germany, were heterogeneous over time. Co-occurring diabetes medications varied in both number and type, and there were some notable differences between the two countries. These prescribing patterns could indicate that treatments are tailored to patient’s needs |
This study provides real-world evidence (RWE) of current GLP-1 RA dose distributions in Germany and the UK but given that higher dulaglutide doses were recently introduced to the market in Germany and the UK, as well as oral semaglutide in the UK, additional RWE including clinical outcomes is needed |
Digital Features
This article is published with digital features, including an infographic to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.22717597.
Introduction
In 2021, the prevalence of diabetes in adults aged 20–79 was estimated at 10.0% in Germany and at 8.2% in the UK [1]. Type 2 diabetes mellitus (T2DM) is a complex and progressive metabolic disease which increases cardiovascular risk, including coronary, peripheral, and carotid artery disease. In addition, prolonged hyperglycemia can lead to irreversible complications such as diabetic retinopathy, nephropathy, and neuropathy[2].
In Germany, the German Diabetes Society (DDG) and the German Society of Internal Medicine (DGIM), and in the UK, the National Institute for Health and Care Excellence (NICE), recommend an individualized approach to T2DM care [3, 4]. The details of these guidelines are presented in the Supplementary Material, Table S1.
In the UK, the initiation of an injectable medication such as basal insulin or GLP-1 RA is recommended as third- or fourth-line therapy for patients with T2DM, respectively, in delay compared to Germany [3, 4].
Dulaglutide [5,6,7], subcutaneous (s.c.) semaglutide [8,9,10], and oral semaglutide [11, 12] are among the GLP-1 RAs approved by the European Medicines Agency, but at the time of the study, oral semaglutide was not available in Germany. The characteristics of these three medications are summarized in Supplementary Material, Table S2.
In Germany, dulaglutide package sizes 4 and 12 are sold at 103.43€ and 287.72€ [13], respectively, while semaglutide package size 1 costs 80.86€ and size 3 costs 220.03€ [14]. These costs are covered by the statutory health insurance (SHI).
In the UK, the NHS indicative price for any dulaglutide dosage size 4 is £73.25 [15], for any oral semaglutide size 30 is £78.48 [16], and for any s.c. semaglutide dosage size 1 is £73.25 [16]. Given the limitation or lack of data on the real-world use of the above-mentioned medications, the overall aim of this study was to understand dulaglutide and s.c. semaglutide prescribing patterns in people with T2DM in the UK and Germany, as well as oral semaglutide in the UK.
Methods
Study Design and Data Source
This retrospective cohort study identified adults with evidence of T2DM and a recorded prescription of dulaglutide or semaglutide using the IQVIA Longitudinal Prescription Data (LRx) for Germany and the UK.
IQVIA LRx data receive approximately 4 billion prescription claims per year [17], which includes approximately 80.0% of all prescriptions reimbursed by SHI funds in Germany and covers approximately 64.0% of all dispensations made in the UK.
The LRx Germany contains information from nationwide pharmacy data centers which process prescription data of German SHI patients. Diagnosis information is not captured but may be inferred based on treatments received. Data are generally entered at the point of sale by retail pharmacies, and dispensed medications are logged using a unique patient identifier. Data are uploaded from sites to a vendor where all data are collated and pseudonymized to ensure patient information is unidentifiable according to data privacy laws.
The LRx UK is a patient dispensation dataset, based on retail pharmacy data, which enables longitudinal tracking of patient prescription activity. Dispensed medications are logged using a pharmacy-level unique patient identifier, and a new identifier is assigned if the patient switches pharmacies.
Within both the LRx Germany and LRx UK, data collected include year of birth (Germany) or age group (UK), gender, geographic information in addition to information on co-prescribing and new, switch, and repeat dispensations. For each dispensation, details such as date, strength, pack size, and number of packages are also captured. Diagnosis information is not captured but may be inferred based on treatments received.
The authors had permission to access and use the data from both the IQVIA LRx databases, and ethical approval was not required for secondary use of these data.
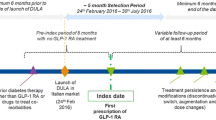
The study design is presented in Fig. 1. The patient selection window spanned from 1 August 2020 to 31 December 2021 to include the earliest market launch dates of the dulaglutide higher doses and of oral semaglutide (January 2021 and August 2020, respectively).
The index date was defined as the first prescription of dulaglutide, s.c. semaglutide, or oral semaglutide registered during the patient selection period. The index date for Cohort 1 (Incident GLP-1 RA Users) was the first prescription of a GLP-1 RA of interest with a lookback period of 6 months (please see Fig. 1). The index date for Cohort 2 (Prevalent GLP-1 RA Users) was the first prescription of a GLP-1 RA of interest during the patient selection time window. There was no minimum follow-up, and estimates were provided at pre-specified time points of index and 3, 6, 9, and 12 months post-index. Patients who had shorter follow-up only contributed to the analysis at the time points when they were under observation. End of available follow-up was defined for each patient as earliest of last date of prescription activity (for any medication) in the database, the end of study period, or switch from index medication. Switching was defined as dispensation of a new non-index (not used in the pre-index period or the index date) GLP-1 RA or any other anti-diabetic agent within the period of 30 days prior to or after discontinuation, i.e., discontinuation date ± 30 days.
Furthermore, co-occurring medications prescribed 4 months before and 4 months after the index date were analyzed.
Variables
All variables used in the analysis are presented in the Supplementary Material, Table S3.
Patient Population
Patients were deemed eligible for cohort 1 (Incident GLP-1 RA Users) if they met all the following criteria: aged ≥ 18 years at the start of lookback period (defined as a minimum of 6 months pre-index of observation time); first prescription for GLP-1 RA of interest (either as monotherapy or part of a combined therapy) during the patient selection window; minimum of 6 months of prescription activity in the lookback period; evidence of T2DM as indicated by at least one oral anti-diabetic medication in the lookback period (including index date). Patients were excluded from cohort 1 if they met any of the following criteria: prescription of any GLP-1 RA during the lookback period; unknown age; prescription of > 1 GLP-1 RA at first prescription.
Patients were included in cohort 2 (Prevalent GLP-1 RA Users) if they met all the following criteria: aged ≥ 18 years at the start of lookback period; treatment with GLP-1 RA medication in the lookback period; dispensation of a GLP-1 RA of interest during the selection window; minimum of 6 months of prescription activity during the lookback period. Patients were excluded from cohort 2 if they met any of the following criteria: first time being prescribed a GLP-1 RA, defined as no dispensations for GLP-1 RAs prior to index date; unknown age; prescription of > 1 GLP-1 RA at first prescription.
Statistical Analyses
Descriptive analyses were conducted using number and percentage for categorical variables, while mean and standard deviation (SD) were used for continuous variables. Absolute number and percentage of patients of dulaglutide and s.c. semaglutide users at index and at 3, 6, 9 and 12 months post-index (stratified by dosage formulations) for cohort 1, cohort 2, and overall (cohort 1 and cohort 2) were calculated in Germany and the UK. Results were not pooled across countries.
In the UK, for oral semaglutide users, absolute number and percentages of patients by dosage formulation are provided at index and 3, 6, 9, and 12 months post-index. The data on oral semaglutide have been presented as a single cohort because of smaller numbers in cohort 2 (N = 4).
Results
Germany
In Germany, 368,320 patients had at least one dispensation of one of the study GLP-1 RAs between 1 August 2020 and 31 December 2021. Of those, 160,292 (43.5%) and 160,538 (43.6%) were deemed eligible for cohort 1 (incident users) and cohort 2 (prevalent users), respectively (see Supplementary Material, Table S4A and S4B).
Baseline characteristics
The baseline characteristics of the two cohorts are presented in Table 1. In both cohorts more patients received dulaglutide (52.9%, cohort 1; 68.2%, cohort 2) at index date compared to s.c. semaglutide (47.1%, cohort 1; 31.8%, cohort 2). Moreover, at least 82.0% in all the treatment groups were aged ≥ 50 years.
The mean available follow-up time for cohort 2 was twice that of cohort 1 (mean [median], 11.0 months [14.0] and 5.1 months [4.0], respectively).
Dose Distributions over the Follow-Up Period
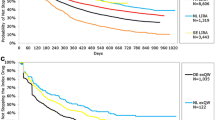
The dose distributions of dulaglutide and s.c. semaglutide at the pre-specified time points for cohort 1, cohort 2, and overall are presented in Fig. 2 (see Supplementary Material, Table S4C and S4D).
At index, a large proportion of dulaglutide users in cohort 2 received 1.5 mg (88.0%), while less than half of the population in cohort 1 received dulaglutide 1.5 mg (49.1%). At 3 months post-index, the proportion of patients receiving the 1.5-mg dulaglutide dose was 68.8% in cohort 1, remaining the same in cohort 2 (88.6%). At 12 months post-index date, the 1.5-mg dulaglutide dose remained the most common dosage formulation dispensed for both cohorts (65.6% cohort 1 and 71.2% cohort 2), while dulaglutide 0.75 mg was dispensed to 14.5% if cohort 1 and to 7.7% of cohort 2.
Notably, the 3.0- and 4.5-mg dosage formulations were introduced to the market relatively recently in January 2021 in both countries. Dispensation of 3.0-mg dulaglutide at index was less common for both cohorts, with only 1.4% in cohort 1 and 0.5% in cohort 2. However, at 12 months post-index the 3.0-mg dulaglutide dose reached a maximum dose distribution of 15.4% in cohort 1 and 17.3% in cohort 2. The 4.5-mg dulaglutide registered the lowest percentage in both cohorts 1 and 2 at index (0.2% and 0.1%), with a slight increase to 4.5% and 3.8%, respectively, at 12 months post-index.
Among s.c. semaglutide users, over three quarters (78.0%) of cohort 1 recorded a dose of 0.25 mg at first dispensation. The most common dosage formulations for s.c. semaglutide cohort 2 were 0.5 mg (46.9%) and 1.0 mg (41.0%). At 3 months post-index, a large drop in 0.25-mg semaglutide was recorded in cohort 1 (8.2%), as users received 0.5 mg (54.0%) and 1.0 mg (37.8%) more commonly. At the same time point, most of cohort 2 (61.5%) was dispensed 1.0 mg. At 12 months post-index, 2.4%, 39.2%, and 58.4% of cohort 1 received doses of 0.25 mg, 0.5 mg, and 1.0 mg, respectively, while 73.3% of cohort 2 received the 1.0-mg dose, 25.8% received the 0.5-mg dose, and 0.9% received the 0.25-mg dose.
Co-occurring Anti-diabetic Medications
Overall, the most common co-occurring anti-diabetic medication dispensed was biguanide in cohort 1 (69.4%) and cohort 2 (62.7%). However, the second most dispensed co-occurring anti-diabetic medication differed between cohort 1 (SGLT-2i: 37.7%) and cohort 2 (basal insulins: 46.8%).
Cohort 1
The majority of cohort 1 received one (dulaglutide, 28.2%; s.c. semaglutide, 26.9%), two (dulaglutide, 34.2%; s.c. semaglutide, 31.7%), or three (dulaglutide, 25.5%; s.c. semaglutide, 27.3%) co-occurring anti-diabetic medications. Biguanide was the most common co-occurring anti-diabetic medication for dulaglutide (67.8%) and s.c. semaglutide users (71.2%). The second most common co-occurring anti-diabetic medications were SGLT-2i for dulaglutide users (38.0%) and basal insulins for s.c. semaglutide users (39.6%).
Cohort 2
Co-occurring anti-diabetic medications were similar between dulaglutide and s.c. semaglutide users. Both were commonly dispensed one (29.6% and 26.8%), two (35.4% and 34.5%), or three (21.7% and 24.1%) co-occurring anti-diabetic medications.
The most common co-occurring anti-diabetic medication for dulaglutide and s.c. semaglutide users was biguanide followed by basal insulins (61.8% vs. 64.6% and 45.2% vs. 50.1%, respectively).
Switching
Switching from dulaglutide or s.c. semaglutide to another anti-diabetic class was not common; only 5.1% of cohort 1 and 6.3% of cohort 2 switched class during the study period (Table 2).
Among those who switched in cohort 1 (N = 8101) and cohort 2 (N = 10,190), the most common switch was to non-index GLP-1 RAs for cohort 1 (32.0%) and cohort 2 (58.0%). The second most common medications were basal insulins (cohort 1, 22.5%; cohort 2, 11.5%) and SGLT2i (cohort 1, 17.2%; cohort 2, 11.4%). Moreover, the majority of the overall cohort was not dispensed additional anti-diabetic medications post-index.
United Kingdom
In the UK, 123,548 patients received at least one prescription of a study GLP-1 RA during the patient selection window (1 August 2020–31 December 2021). Of those, 51,953 (42.1%) and 57,602 (46.6%) were deemed eligible for cohort 1 and 2, respectively (see Supplementary Material, Table S5A and S5B).
Unlike Germany, at the time of the study oral semaglutide was available in the UK; therefore, patients who were prescribed it were included in the cohorts. The data of patients prescribed oral semaglutide are presented as a single cohort.
Dulaglutide and Subcutaneous Semaglutide
Baseline characteristics
The baseline characteristics of dulaglutide and s.c. semaglutide patients are shown in Table 3.
At index, the proportion of patients dispensed dulaglutide (49.2%) or s.c. semaglutide at index (50.8%) was similar in cohort 1. In contrast, a higher proportion of patients in cohort 2 received dulaglutide (67.4%) than s.c. semaglutide (32.6%).
At baseline, 82.1% and 84.6% of cohort 1 and 2, respectively, were > 50 years old. Overall, the proportions of male and female patients were similar between the dulaglutide (52.0% and 47.0%, respectively) and s.c. semaglutide users (50.9% and 48.1%, respectively). Available mean follow-up time for cohort 2 (mean [SD], 8.7 months [5.1]) was approximately twice that of cohort 1 (mean [SD], 4.1 months [3.7]).
Dose Distributions Over the Follow-Up Period
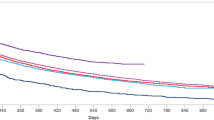
The dose distributions of dulaglutide and s.c. semaglutide, stratified by cohort 1, cohort 2, and overall, at the pre-specified time points are presented in Fig. 3 (see Supplementary Material, Table S5C and S5D).
At index, 39.0% of patients in cohort 1 and 13.2% for cohort 2 were prescribed 0.75-mg dose of dulaglutide. In cohort 2, the 1.5-mg formulation was prescribed to 86.8% of the patients, while in cohort 1 the proportion was 60.2% at index. At 3 months post-index, however, there was a substantial increase in 1.5-mg dulaglutide use among cohort 1 patients (75.1%), whereas use among cohort 2 patients was relatively stable (87.7%). At 12 months post-index, 1.5-mg dulaglutide remained the most dispensed formulation, slightly decreasing for cohort 1 (71.7%) and cohort 2 (80.9%), while the 0.75-mg dulaglutide dosage was dispensed to 12.2% and 8.9% of cohort 1 and cohort 2, respectively. Dispensation of the 3.0-mg formulation was rare at the start of the study, prescribed to 0.7% of patients in cohort 1 and no patients in cohort 2 at index. However, at 12 months post-index, 13.6% and 9.0% of patients in cohorts 1 and 2, respectively, were receiving the 3.0-mg dose. The highest dosage formulation of dulaglutide was infrequently dispensed, at index only to 0.1% of cohort 1 and to none of the patients of cohort 2. At 12 months post-index, 2.5% and 1.2% of cohort 1 and 2, respectively, received the 4.5-mg dose.
At index, 77.2% of cohort 1 and 9.3% of cohort 2 received the non-maintenance s.c. semaglutide 0.25-mg dose. In cohort 2 most patients received the 0.5-mg (41.6%) or 1.0-mg (49.1%) dose. At 3 months post-index, there was a substantial decrease in 0.25-mg s.c. semaglutide dispensation among cohort 1 patients (10.4%), with most users receiving 0.5 mg (50.7%). At the same time point, most patients in cohort 2 received 1.0 mg (56.9%). At 12 months post-index, the 0.25-, 0.5-, and 1.0-mg dosages were prescribed to 5.1%, 38.9%, and 56.0% of cohort 1, respectively, while the percentages for the same dosages in cohort 2 were 3.4%, 29.5%, and 67.1%, respectively.
Co-occurring Anti-diabetic Medications
Co-occurring anti-diabetic medications were similar between cohort 1 and cohort 2 during the index period, with biguanides (82.2% and 80.4%, respectively) and SGLT-2i (38.6% and 33.1%, respectively) the two most prescribed classes.
Cohort 1
There were no significant differences in co-occurring dispensation among dulaglutide and s.c. semaglutide users during the index period. The proportions of patients who were prescribed one, two, or three co-occurring anti-diabetic medications were similar for both treatment groups (21.9% vs. 23.5%, 38.2% vs. 37.7%, and 29.9% vs. 29.0%, respectively). Most dulaglutide and s.c. semaglutide users received biguanides (81.9% and 82.4%, respectively) and as second most common medication, SGLT-2i (39.5% and 37.8%, respectively).
Cohort 2
Co-occurring anti-diabetic medication dispensation among the dulaglutide and s.c. semaglutide users was comparable during the index period. Most dulaglutide patients received one (26.2%), two (44.2%), or three (21.6%) co-occurring anti-diabetic medications. The proportions for s.c. semaglutide users were similar: 28.9%, 40.9%, and 20.6%, respectively.
Biguanides were the most common co-occurring anti-diabetic medications (dulaglutide 81.1% and s.c. semaglutide 79.0%). The second most common co-occurring anti-diabetic medication was SGLT-2i, which was prescribed to 33.2% of dulaglutide users and 33.0% of s.c. semaglutide users.
Switching
Only 3.9% of cohort 1 and 5.1% of cohort 2 switched to another anti-diabetic medication during the follow-up period. The list of the above-mentioned medications is presented in Table 4. Among cohort 1 patients, non-index GLP1-RAs were the most recorded medications (26.1%) followed by SGLT-2i (22.8%). For cohort 2, there was a slightly higher proportion that switched to the non-index GLP1-RAs recorded (37.9%). The second and third most common anti-diabetic medications within this cohort were SGLT-2i (18.3%) and basal insulins (17.8%).
Oral Semaglutide
Baseline Characteristics
The baseline characteristics of oral semaglutide users are presented in Table 5 as a single cohort. Among oral semaglutide users, 84.3% were at least 50 years old and 52.9% were male. Follow-up time for this patient subpopulation was 2.7 months [2.7] (mean [SD]).
Dose Distributions over the Follow-Up Period
The dose distributions of oral semaglutide at all the pre-specified time points, presented as overall, is shown in Fig. 4 (see Supplementary Material, Table S5E). The majority (89.0%) of oral semaglutide users received 3.0 mg at first dispensation. However, at 3 months post-index, only 18.5% of patients received 3.0 mg while most patients (55.5%) received 7.0 mg. At 12 months post-index, just over half of the patients received the 14.0-mg formulation (56.3%), while 37.5% received the 7-mg formulation.
Co-occurring Anti-diabetic Medications
Overall, oral semaglutide users commonly received one (23.9%), two (36.6%), or three (29.1%) co-occurring anti-diabetic medications, these mainly consisting of biguanides (79.5%) and SGLT-2i (42.3%).
Switching
Only 6.0% of oral semaglutide users switched to another anti-diabetic medication during the follow-up period. The list of anti-diabetic medications is presented in Table 6. Similarly to dulaglutide and s.c. semaglutide, non-index GLP1-RAs (35.4%) and SGLT-2i (19.2%) were the most common anti-diabetic medications to switch to. Moreover, patients of both cohorts generally did not initiate new anti-diabetic medications post-index.
Discussion
To the best of our knowledge, given the lack of data on the real-world use of the recently introduced 3.0- and 4.5-mg dulaglutide dosage formulations, this is the first study evaluating real-world utilization patterns of the above-mentioned medications. Contrarily, oral semaglutide real-world use has been analyzed by Aroda et al. in the USA, using the US IBM Explorys electronic health record database, and the authors identified 782 patients between the 28 October 2019 and 15 December 2020 [18].
The aim of this study was to understand the real-world utilization patterns of dulaglutide and s.c. semaglutide in Germany and the UK, and of oral semaglutide in the UK, given that these patterns are often different from the ones implemented in clinical trials and in the guidelines. Results suggest that dosing patterns were heterogeneous over time and depended on GLP-1 RA type.
Results obtained from incident users (cohort 1) in the UK in this study showed that 60.2% of the GLP-1 RA naïve patients who received dulaglutide started on the 1.5-mg dosage formulation at first dispensation, while in German incident users (cohort 1), a similar proportion of patients received the 0.75-mg (49.3%) and the 1.5-mg (49.2%) dulaglutide formulation. These results from UK complement a multi-country study using IQVIA real-world adjudicated pharmacy claims which showed that 74.7–93.2% of dulaglutide users received the 1.5-mg formulation at first dispensation in five European countries [19]. At the 12-month time point, 70.5% of the German dulaglutide users were receiving the 1.5-mg dose, while in the UK the proportion was 79.9%.
In Germany, 88.0% of the prevalent users (cohort 2) who received dulaglutide started on the 1.5-mg formulation at index, with a similar proportion in the UK (86.8%). These percentages decreased at 12 months post-index, but dulaglutide 1.5 mg remained the most common formulation in Germany and the UK (71.2% and 80.9%, respectively).
Dose escalation to 3.0-mg dulaglutide may be implemented for additional glycemic control, and if further control should be needed, it can be increased to the 4.5-mg dose after at least 4 weeks [5, 20]. However, notably, the 3.0- and 4.5-mg dosage formulations were introduced to the market relatively recently in January 2021 in both countries. Furthermore, the launch of these dosage formulations and the entirety of the study period were during the COVID-19 pandemic. Patients may have been less likely to change treatment due to lack of access to primary care [21]. Therefore, more longitudinal data and additional information on glycemic control are required to determine whether 3.0- and 4.5-mg dulaglutide is in fact being underutilized.
In this study, 78.0% of the incident s.c. semaglutide users (cohort 1) in Germany and 77.2% of those in UK received the non-maintenance 0.25 mg dosage formulation at first dispensation. This is in line with guideline recommendations and with the SmPC, whether taken as a monotherapy or add-on medication [8, 22]. Between the 3- and 12-month time points, the proportion of s.c. semaglutide users receiving 0.25- and 0.5-mg dispensations decreased while the proportion receiving the 1.0-mg dosage formulation increased. At 12 months post-index, 29.6% and 69.1% of s.c. semaglutide users in Germany were prescribed 0.5- and 1.0-mg dispensations, respectively, while in the UK the proportions were 31.7% and 64.4%.
Among the prevalent s.c. semaglutide users (cohort 2) in Germany and the UK, only 12.1% and 9.3% received the non-maintenance 0.25-mg dosage at index, respectively. At 12 months post-index, s.c. semaglutide 1.0 mg was the most common formulation in Germany (73.3%) and the UK (67.1%).
Guidelines recommend escalation from the 0.5-mg dosage formulation only if required to achieve glycemic control [8, 22].
In relation to oral semaglutide, at index 89.0% of the cohort received the 3.0-mg starting dose, an indication that dispensation was generally in line with dosage recommendations [23]. The proportion of patients receiving 3.0-mg oral semaglutide at index was higher than that recorded in the study by Aroda et al., which showed that 51.7% of the study population received the 3.0-mg formulation alone at first prescription, while 25.7% and 5.2% received the 7.0 mg and 14.0 mg formulations, respectively [18]. The lower proportion of patients receiving the starting dosage formulation in the Aroda et al. study was probably due to the study population including patients who received more than one dosage formulation at first prescription, which was not the case in this study. Additionally, the authors of that study suggest that patients in US clinical practice may have received higher oral semaglutide dosage formulations at first prescription because they had already been prescribed a GLP-1 RA before, which is permitted in the US [18, 24], or because they received samples of higher dosage, thus inflating the proportion of patients receiving higher oral semaglutide formulations [18].
Between 3 and 12 months post-index, fewer patients received the 7.0-mg formulation (55.5 to 37.5%) in this study while dispensation of the 14.0-mg formulation increased (26.0 to 56.3%). This distribution pattern, similar to that of s.c. semaglutide users, suggests that approximately half the users remained on the maintenance dose of 7.0 mg.
These results indicate that the higher doses of dulaglutide may be underutilized and that the initial non-maintenance dose of semaglutide may be prescribed for a longer time than recommended, thus suggesting a clinical inertia in the management of T2DM, which results in patients’ glycemia not being under control. However, further investigations including clinical outcomes such as glycated hemoglobin, weight, and BMI are recommended to ascertain whether patients are appropriately titrated.
A key strength of this real-world retrospective study was the large volume of dispensation and population coverage of the German LRx and UK LRx. German LRx comprises approximately 80.0% of all dispensed medications provided through SHI policies, while UK LRx contains approximately 64.0% of all dispensations made by general practitioners. Therefore, the findings from this study can be generalized to dulaglutide, s.c. semaglutide users in both Germany and the UK, and to oral semaglutide users in the UK.
With the exception of the German GLP-1 RA utilization study undertaken by Otto et al.[25], previous real-world studies investigating GLP-1 RA utilization have examined incident use only [19, 26,27,28]. The stratification of incident (cohort 1) and prevalent (cohort 2) users in this study, along with the long follow-up time between 1 August 2020 and 31 December 2021, provides a detailed insight into the treatment patterns of patients newly initiating dulaglutide and s.c. semaglutide, as well as those who had previously received GLP-1 RAs.
Limitations
The authors acknowledge that the number of patients over follow-up gets smaller when switching to another anti-diabetic class or discontinuation occurs. Consequently, at the 12-month time point only the persistent patients are included, and the number is smaller compared to the one registered at index.
Moreover, there are some limitations due to the use of a pharmacy-based database: first, patients who purchased prescriptions outside the pharmacies included in the database were not trackable; additionally, patients included in the database may not have been fully representative of all patients in the UK and Germany. However, LRx Germany data are broadly representative of the German SHI population (~ 80.0%), and Richter et al. [29] have validated it as suitable data source for Germany. Second, the data used in this study lacked any further clinical data (e.g., medical diagnoses or procedures); therefore, there was an assumption that patients dispensed GLP-1 RA medications were patients with T2DM. However, the requirement of an oral anti-diabetic medication prescription in the lookback period was used to mitigate this. Furthermore, patient demographic characteristics were restricted to age and gender, thus limiting inferences regarding GLP-1 RA choice and patient prognosis at index.
Finally, LRx UK only captures dispensations processed by general practitioners and not by specialist providers. As LRx UK cannot track patients across pharmacies, patients that moved pharmacies may have been counted twice. However, with the requirement of 6 months of prescription activity during the lookback period and relatively short study window, this likely affected very few patients.
Conclusion
This study provides real-world evidence (RWE) of current dose distributions among dulaglutide and s.c. semaglutide users in Germany and the UK, as well for oral semaglutide users in the UK, but given that the higher dulaglutide doses and oral semaglutide were recently introduced to the market, additional RWE including clinical outcomes is needed.
References
International Diabetes Federation. IDF Diabetes Atlas 10th edition. 2021. IDF_Atlas_10th_Edition_2021.pdf. IDF Diabetes Atlas 2021 | IDF Diabetes Atlas. Accessed 26 Oct 2022.
Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20:546–51.
Landgrave R, Kellerer M, Fach E, et al. Practical recommendations DDG/DGIM: treatment of type 2 diabetes. https://www.researchgate.net/publication/285804464_Practical_recommendations_of_the_DDGDGIM_Treatment_of_type_2_diabetes. Accessed 26 Oct 2022.
National Institute for Health and Care Excellence. NICE guideline [NG28]. https://www.nice.org.uk/guidance/ng28. Accessed 26 Oct 2022.
Trulicity (dulaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/trulicity. Accessed 26 Oct 2022.
Trulicity (dulaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/trulicity#authorisation-details-section. Accessed 26 Oct 2022.
Trulicity (dulaglutide). European Medicines Agency. https://www.ema.europa.eu/en/documents/variation-report/trulicity-h-c-2825-x-0045-epar-assessment-report-variation_en.pdf. Accessed 26 Oct 2022.
Ozempic (s.c. semaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/ozempic. Accessed 26 Oct 2022.
Ozempic (s.c. semaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/ozempic#authorisation-details-section. Accessed 26 Oct 2022.
Ozempic (s.c. semaglutide). European Medicines Agency. https://www.ema.europa.eu/en/documents/variation-report/ozempic-h-c-004174-x-0021-epar-assessment-report-variation_en.pdf. Accessed 26 Oct 2022.
Rybelsus (oral semaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus. Accessed 26 Oct 2022.
Rybelsus (oral semaglutide). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus#authorisation-details-section. Accessed 26 Oct 2022.
Trulicity (dulaglutide). Trulicity bei medizinfuchs.de. 2023. https://www.medizinfuchs.de/trulicity.html?params%5Border%5D=relevance¶ms%5Bview%5D=list¶ms%5Bword_suggestions%5D%5Bpackages%5D%5B0%5D=4+ST¶ms%5Bfilter_price%5D=. Accessed 8 Mar 2023.
Ozempic (subcutaneous semaglutide). OZEMPIC bei medizinfuchs.de. 2023. https://www.medizinfuchs.de/ozempic.html. Accessed 8 Mar 2023.
Trulicity (dulaglutide). BNF content published by NICE. https://bnf.nice.org.uk/drugs/dulaglutide/medicinal-forms/. Accessed 8 Mar 2023.
Ozempic (subcutaneous semaglutide) and Rybelsus (oral semaglutide). BNF content published by NICE. https://bnf.nice.org.uk/drugs/semaglutide/medicinal-forms/. Accessed 8 Mar 2023.
IQVIA. Available IQVIA data. https://www.iqvia.com/insights/the-iqvia-institute/available-iqvia-data. Accessed 28 Nov 2022.
Aroda VR, Faurby M, Lophaven S, Noone J, Lyng Wolden M, Lingvay I. Insights into the early use of oral semaglutide in routine clinical practice: the IGNITE study. Diabetes Obes Metab. 2021;23:2177–82.
Divino V, Boye KS, Lebrec J, DeKoven M, Norrbacka K. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10:1067–88.
Trulicity (dulaglutide). Summary of product characteristics. Hampshire, UK; Eli Lilly and Company. 2021. https://www.medicines.org.uk/emc/medicine/29747#gref. Accessed 26 Oct 2022.
Carr MJ, Wright AK, Leelarathna L, et al. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK-wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. 2022;31:503–14.
Ozempic (s.c. semaglutide). Summary of product characteristics. West Sussex, UK; Novo Nordisk. 2021. https://www.medicines.org.uk/emc/product/9749/smpc. Accessed 26 Oct 2022.
Rybelsus (oral semaglutide) Summary of product characteristics. West Sussex, UK; Novo Nordisk. 2020. https://www.medicines.org.uk/emc/product/11507/smpc. Accessed 26 Oct 2022.
Rybelsus (oral semaglutide). Prescribing information. Bagsvaerd, Denmark; Novo Nordisk. 2019. https://www.novo-pi.com/rybelsus.pdf. Accessed 20 Jan 2023.
Otto T, Myland M, Jung H, Lebrec J, Richter H, Norrbacka K. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2019;35:893–901.
Norrbacka K, Sicras-Mainar A, Lebrec J, et al. Glucagon-like peptide 1 receptor agonists in type 2 diabetes mellitus: data from a real-world study in Spain. Diabetes Ther. 2021;12:1535–51.
Divino V, DeKoven M, Khan FA, Boye KS, Sapin H, Norrbacka K. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther. 2017;8:115–28.
Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9:789–801.
Richter H, Dombrowski S, Hamer H, Hadji P, Kostev K. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:Doc14.
Acknowledgements
We thank the participants of the study.
Funding
This study, the Rapid Service Fee, and the Open Access fee were funded by Eli Lilly and Company.
Medical Writing, Editorial, and Other Assistance
The authors acknowledge Valeria Maria Cortesi, MD, and Esther Artime, BSc, MSc, PhD, employees of Eli Lilly and Company, for providing project management support, medical writing, and editorial assistance in the preparation of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Annabel Barrett made substantial contributions to the conception and design of the work, interpretation of data for the work, and critical revision of the work for important intellectual content. Nele Debackere made substantial contributions to the interpretation of data for the work and critical revision of the work for important intellectual content. Anderson Ribeiro made substantial contributions to the design of the work and critical revision of the work for important intellectual content. Karabo Keapoletswe made substantial contributions to the interpretation of data for the work and critical revision of the work for important intellectual content. Rebecca Zingel made substantial contributions to the acquisition, analysis, and interpretation of data for the work, drafting of the work and critical revision of the work for important intellectual content. Briana Coles made substantial contributions to the acquisition, analysis, and interpretation of data for the work and critical revision of the work for important intellectual content.
Disclosures
Annabel Barrett, Nele Debackere, and Anderson Ribeiro are employees and shareholders of Eli Lilly and Company. Karabo Keapoletswe and Rebecca Zingel are employees of IQVIA, which was contracted by Eli Lilly and Company to conduct the study. Briana Coles is an employee of IQVIA, which was contracted by Eli Lilly and Company to conduct the study. She declares a grant from Novo Nordisk, an unpaid participation to a Data Safety Monitoring Committee for the North Bristol NHS Trust, and she is unpaid member of the Board of Directors of “Black Girls Do STEM”.
Compliance with Ethics Guidelines
The authors had permission to access and use the data from both the IQVIA LRx databases, and ethical approval was not required for secondary use of the data.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because of patients’ privacy protection. However, the authors will share programming code and aggregate statistics if requested.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barrett, A., Debackere, N., Ribeiro, A. et al. Dosing Patterns of Dulaglutide and Semaglutide in Patients with Type 2 Diabetes in the United Kingdom and Germany: A Retrospective Cohort Study. Adv Ther 40, 3446–3464 (2023). https://doi.org/10.1007/s12325-023-02540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02540-y