Abstract
Introduction
This study aimed to describe utilization patterns, persistence, resource utilization and costs in patients with type 2 diabetes mellitus initiating treatment with glucagon-like peptide 1 receptor agonists in routine clinical practice in Spain.
Methods
This retrospective study of medical records in the Big-Pac database identified adults starting treatment with once-weekly (QW) dulaglutide, exenatide-QW or once-daily liraglutide between 1 November 2015 and 30 June 2017. Patients were followed for up to 18 months from treatment initiation. Data on clinical characteristics of patients, treatment patterns, average daily dose and costs were obtained for the three cohorts. Persistence over the 18-month period was evaluated using Kaplan–Meier curves. All analyses were descriptive.
Results
A total of 1402 patients were included in this study (dulaglutide [n = 492], exenatide-QW [n = 438] or liraglutide [n = 472]); 52.8% were men, and the mean (SD) age was 62 (11) years, glycated haemoglobin (HbA1c) was 8.1% (1.2) and body mass index was 35.5 (3.2) kg/m2 at treatment initiation. Persistence at 18 months was 59.1% (95% confidence interval [CI] 54.8–63.4) for dulaglutide, 45.7% (95% CI 41.0–50.4) for exenatide-QW and 46.6% (95% CI 42.1–51.1) for liraglutide. The average (SD) dose was 1.2 (0.4) mg/week for dulaglutide, 1.9 (0.3) mg/week for exenatide-QW and 1.1 (0.3) mg/day for liraglutide. The average reduction in HbA1c levels at 1 year was − 0.68% for patients who initiated dulaglutide, − 0.54% for patients who initiated exenatide-QW and − 0.50% for patients who initiated liraglutide. The mean (SD) total annual health care costs were €4072 (1946) for dulaglutide, €4418 (2382) for exenatide-QW and €4382 (2389) for liraglutide.
Conclusion
Results suggest that patients who started treatment with dulaglutide had higher persistence over 18 months, presented lower HbA1c levels at 12 months and incurred lower annual total healthcare costs than patients who initiated exenatide-QW or liraglutide.
Plain Language Summary
Type 2 diabetes has a major impact on patients psychologically and socially, as well as on healthcare costs. The glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are drugs that help maintain blood sugar at healthy levels. They are often used as the first injectable drugs if oral treatments are no longer effective. This study aimed to analyse the use of GLP-1 RAs, and the costs involved, among patients with type 2 diabetes who started treatment with once-weekly dulaglutide, once-weekly exenatide or liraglutide in routine clinical practice in Spain. An electronic database of medical records was used to obtain data from 1402 patients who started treatment with these drugs and were followed for a 1.5-year period. Results of this study suggest that patients who were prescribed dulaglutide stayed on their treatment longer and could reduce their blood sugar levels more efficiently, and at a lower cost, than those who received once-weekly exenatide or liraglutide. These findings could be helpful to physicians prescribing these drugs when considering how to improve the management of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are often the first injectable therapy prescribed to patients with type 2 diabetes before starting basal insulin, but there is limited information on real-world use of these drugs in Spain. |
This study aimed to analyse utilization patterns, persistence, resource utilization and costs among patients with type 2 diabetes in Spain who initiated dulaglutide, exenatide-QW or liraglutide for the first time. |
What was learned from the study? |
This study suggests that patients who started treatment with dulaglutide had higher treatment persistence and presented better glycaemic control than patients who initiated exenatide-QW or liraglutide. |
In Spain, the total annual average diabetes-related costs were lowest for dulaglutide and highest for exenatide-QW. |
This comprehensive real-world analysis of dulaglutide, exenatide-QW and liraglutide provides important information that can be useful to guide therapeutic decisions. |
Digital Features
This article is published with digital features, including a summary side and a plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14077409.
Introduction
Diabetes is a chronic disease with a major clinical and economic impact worldwide. In 2019, diabetes affected 463 million people (9.3% of adults aged 20–79 years), and this value is expected to rise to 578 million by 2030 [1]. In Spain, prevalence has been estimated at 8–20% for those aged over 75 years [2, 3]. Diabetes impacts patients psychologically and socially and is therefore associated with a substantial and rising socioeconomic burden [1]. For Spain, the total direct costs of treating type 2 diabetes mellitus (T2DM) have been estimated at €10 billion yearly [4].
Currently, the initial therapeutic approach for the treatment of T2DM includes a variety of oral antidiabetic drugs (OADs) such as biguanides (metformin), sulfonylureas, glinides, glitazones, sodium-glucose cotransporter type 2 (SGLT2) inhibitors and dipeptidyl peptidase 4 (DPP4) inhibitors. However, when oral pharmacological therapy alone fails to control hyperglycaemia, and recognizing that T2DM is a progressive disease, escalation of treatment is often required. This is usually achieved through OAD combinations or by introducing injectable agents such as glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and insulin [5, 6]. GLP-1 RAs can be used as monotherapy or in combination with OADs or insulin and are currently recommended as the first injectable drug before starting basal insulin [5]. In addition to improved glycaemic control, GLP-1 RAs are associated with reductions in body weight and a low hypoglycaemia risk [7]. They are recommended in patients with T2DM with established or a high risk of atherosclerotic cardiovascular disease [5, 8].
Although GLP-1 RAs have broadly shown good efficacy and tolerability in patients with T2DM [9], there are relevant differences between the available GLP-1 RAs with respect to dosing regimens and the injection process [10]. These attributes have been demonstrated to be critical to patient satisfaction with the treatment and can affect persistence with the antidiabetic therapy [11, 12]. Persistence, generally defined as the percentage of patients still taking a prescribed treatment at the end of a predefined evaluation period [13], is a key aspect of antidiabetic therapy as it is directly related to positive clinical outcomes and a reduced risk of developing complications [14,15,16,17]. In this regard, observational studies providing real-world evidence that evaluate patient behaviour over extended time ranges are useful [18].
Although five GLP-1 RAs are registered in the Spanish market, information is limited on patient demographics, treatment practices, clinical outcomes, healthcare resource use and related costs among patients with T2DM initiating GLP-1 RA therapy in this country [19,20,21,22,23,24]. In Spain, GLP-1 RAs are usually prescribed in combination with OADs or OAD/insulin to relatively young patients with poor glycaemic control and high body mass index (BMI) [20]. Currently, reimbursement of GLP-1 RAs is limited to patients with a BMI of at least 30 kg/m2 and in combination with other antidiabetic treatments [6, 25].
The overall objective of this study was to provide real-world evidence on treatment patterns for once-weekly (QW) dulaglutide (Trulicity®, Eli Lilly and Company), exenatide-QW (Bydureon®, AstraZeneca) and once-daily liraglutide (Victoza®, Novo Nordisk) for the treatment of patients with T2DM using it for the first time. Persistence and treatment modifications were of special interest. Other objectives of the study included evaluating daily or weekly dosage, concomitant antidiabetic and non-antidiabetic medication, main clinical outcomes related to diabetes, and T2DM-associated resource use and costs.
Methods
This was a retrospective, observational, descriptive, cohort study including patients with T2DM aged at least 18 years who started antidiabetic treatment for the first time with dulaglutide, exenatide-QW or liraglutide in Spain. The protocol was approved by the Clinical Research Ethical Committee of the Consorci Sanitari de Terrassa (Barcelona, Spain). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, good pharmacoepidemiology practices (GPPs) and the applicable laws and regulations of Spain.
The overall study design and methodologies were deliberately similar to those in previous observational studies conducted in other European countries [26,27,28,29,30] to allow comparisons to be made.
Data Source
Data for the study were obtained from the Big-Pac database (Atrys Health-Real Life Data, Madrid, Spain), which collects and unifies computerized and anonymised patient medical records from primary and secondary care, records of drug dispensation and other complementary databases from seven autonomous regions of Spain. The Big-Pac database is registered with The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®; http://www.encepp.eu/encepp/viewResource.htm?id=29236), a network coordinated by the European Medicines Agency. The population assigned to the health centres from which data were extracted included 1,867,108 inhabitants at the time of data extraction and could be considered representative of the Spanish population [31].
Study Design and Patient Selection
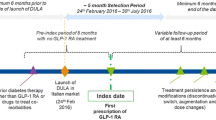
An overall view of the study design is shown in Fig. 1. The date of initiation of the GLP-1 RA treatment was termed the ‘index date’, and the GLP-1 RA therapy initiated was termed the ‘index therapy’. Patients were followed for 18 months (post-index period). All patients were required to appear in the database at least 6 months prior to the index date; this period was termed the ‘pre-index period’. During this period, patients’ clinical characteristics and patterns of antidiabetic and concomitant treatment and medication use were assessed. During the post-index period, clinical characteristics, average dose, concomitant medication at 12 months; add-on therapy; persistence at 6, 12 and 18 months; treatment modifications; and the use of resources and costs at 12 months were evaluated.
Patients included in this study were those starting a new treatment with one of three GLP-1 RAs (dulaglutide, exenatide-QW or liraglutide) within the inclusion period of 1 November 2015 to 30 June 2017 and meeting the following inclusion criteria at index date: (1) age at least 18 years; (2) diagnosis of T2DM in the database a minimum of 6 months prior to the index date; (3) had ongoing medical dispensations (with verified record of the daily dose, the time interval and duration for each treatment), and two or more dispensations for at least the 12-month follow-up and (4) feasibility of regular follow-up of the patient (one or more healthcare system records in the database). Patients were excluded if they (1) transferred to other centres, relocated or were outside the zone covered in the database; (2) were permanently institutionalized; (3) had a background of type 1 diabetes mellitus, gestational diabetes and/or secondary diabetes (Cushing’s syndrome, acromegaly, glucagonoma, pheochromocytoma or primary hyperaldosteronism, as indicated by the International Classification of Diseases [ICD] codes in the patient’s files); (4) had prior exposure to GLP-1 RAs or (5) had a terminal illness. Patients using liraglutide for the weight management indication were excluded.
The index therapy cohorts of the study were based on GLP-1 RA product initiated: (1) dulaglutide (0.75 or 1.5 mg weekly), (2) exenatide-QW (2 mg weekly) and (3) liraglutide (0.6 or 1.2 mg daily).
Measures and Analyses
In the pre-index 6-month period, data were obtained on patients’ demographic and clinical characteristics. The source of these data was the medical claims files (ICD-10-MC diagnosis codes), laboratory and pharmacy claims files (anatomical therapeutic chemical codes). Data obtained on comorbidities were used to calculate a Charlson Comorbidity Index score and to determine the number and severity of chronic comorbidities [32]. Macrovascular (ischaemic heart disease, cerebrovascular accidents) and microvascular (retinopathy, nephropathy and diabetic neuropathy) complications were quantified. The biochemical parameters of each patient were obtained throughout the study, but only the last records before the pre-index, index and post-index dates were used for each time period. These included data on clinical outcomes, glycated haemoglobin (HbA1c) levels, BMI, systolic and diastolic blood pressure and lipid profile. The mean percentage reduction of each parameter at 12 months was quantified.
Dispensations for a non-GLP-1 RA antidiabetic therapy (metformin, sulfonylureas, alpha glucosidase inhibitors, glitazone, glinide, DPP4 inhibitors, SGLT2 inhibitors and insulins and analogues) and relevant non-antidiabetic concomitant medications (antihypertensive drugs, diuretics, beta-blockers, lipid-lowering agents, angiotensin-converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists and antiplatelet agents) were evaluated during the 6-month pre-index period, at the index date and during the post-index period.
Pharmacy claims files were used to determine the index therapy cohorts and the first dispensation date for dulaglutide, exenatide-QW and liraglutide. For dulaglutide, the number of patients starting at 0.75 mg or 1.5 mg was also registered. The average daily and weekly dose (ADD and AWD, respectively) of the index therapy was assessed for all patients in the study while persistent or up to the end of the follow-up period. Daily dose was calculated by dividing the total amount of drug prescribed (calculated using package size, package count and strength) by the duration of time (days) between two consecutive dispensations. At the patient level, daily dose was averaged, whereas at the index treatment level, patient-level ADD was averaged. This was multiplied by seven to obtain the AWD for dulaglutide and exenatide-QW.
Persistence was measured as the time (days) from the index date until evidence of non-persistence, either by discontinuation or switch, over the available follow-up period up to 18 months. Discontinuation was defined as a gap in a series of successive dispensations for the patient’s initial treatment of at least 60 days’ duration. Switching was defined as the appearance in the records of a new dispensation (a non-index GLP-1 or other antidiabetic medication) within 30 days before or after discontinuation of the treatment. Patients who underwent a treatment switch of the index therapy were categorized into one of two groups: patients who switched to OAD or insulin treatment and patients who switched to other GLP-1 RAs. The number of patients who switched to each antidiabetic drug was recorded, as was the percentage of patients who received a new antidiabetic drug.
Add-on therapy was defined as the appearance of two or more dispensations of a new non-index treatment (a non-index GLP-1 or other antidiabetic medication) while the index treatment was being administered. A second dispensation of the add-on therapy was required to occur before the last days’ supply of the index therapy or the discontinuation date.
Resource use and associated costs related to T2DM for each of the study cohorts were evaluated over the 12-month post-index period. No indirect costs were considered. Direct healthcare costs included medical visits, hospitalization, emergency room visits, diagnostic tests and medications. The costs were presented as overall average cost per patient (average/unit/annual) and by cost component and were specified as relating to primary or secondary care. Unit costs of healthcare resources used are detailed in Table S1 in the electronic supplementary material (ESM), based on 2017 unit prices. Prices were obtained from the centres’ analytical accounting, except for medication. The retail price of medical dispensations was as indicated in the BOT PLUS website (http://botplusweb.portalfarma.com/) from the General Council of Pharmaceutical Associations of Spain.
Statistical Methods
Data were analysed descriptively. Categorical variables were reported using frequency and percentage distributions and 95% confidence intervals (CIs) where available. Continuous and count variables were reported using the mean and the SD. Kaplan–Meier analyses were used to estimate the proportion of patients who were persistent across the follow-up period (18 months).
For continuous and categorical variables where data were missing, missing numbers were provided. The default assumption was that subjects with missing values were excluded from analyses.
Analyses are presented overall and by index treatment. No formal statistical tests were performed to compare outcomes between index therapy cohorts.
Results
From a total of 1,867,108 patients in the database, 49,101 met the inclusion criteria of age at least 18 years at the index date, a diagnosis of T2DM at least 6 months before index date and inclusion in the dispensation program (Fig. S1 in the ESM). Of these, 1589 patients (3.2%) started treatment with dulaglutide, exenatide-QW or liraglutide during the inclusion period from 1 November 2015 to 30 June 2017. After applying the exclusion criteria, a total of 492 patients for dulaglutide, 438 patients for exenatide-QW and 472 patients for liraglutide were included in the study.
The baseline demographic and clinical characteristics of these patients in each of the index therapy cohorts are shown in Table 1. Overall, 52.8% were men, and the mean (SD) age was 62 (11) years. The mean (SD) age was 63 (11) for the dulaglutide and exenatide-QW groups and 62 (11) years for the liraglutide group. Patients had a mean (SD) disease diagnosis of 9.4 (2.6) years, a BMI of 35.5 (3.2) kg/m2 and an HbA1c of 8.1% (1.2). At baseline, the three groups were similar regarding sex distribution, clinical parameters and associated comorbidities. The mean (SD) number of antidiabetic medications in the 6-month pre-index period was 2.3 (0.9) across all index therapies. The most common antidiabetic medication taken by the patients in all groups during the pre-index period was metformin (79.7% in the dulaglutide group, 85.6% in the exenatide-QW group and 88.8% in the liraglutide group), followed by DPP4 inhibitors (46.1% in the dulaglutide group, 50.9% in the exenatide-QW group and 52.1% in the liraglutide group). The most common concomitant medications were antihypertensive and lipid-lowering drugs.
Persistence of GLP-1 RA Treatments
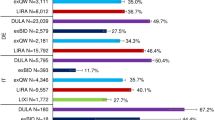
Treatment persistence and treatment discontinuation or switching at 6, 12 and 18 months are shown in Table 2. At 18 months, 59.1% (95% CI 54.8–63.4) of patients receiving dulaglutide, 45.7% (95% CI 41.0–50.4) of those receiving exenatide-QW and 46.6% (95% CI 42.1–51.1) of those receiving liraglutide were persistent (Fig. 2). Patients initiating liraglutide or exenatide-QW had a higher probability of discontinuing or switching over at 12 months than patients initiating dulaglutide (hazard ratio 1.68 [95% CI 1.28–2.20] and 1.88 [95% CI 1.43–2.46], respectively).
At 6 months, overall GLP-1 RA discontinuation was observed in 328 patients (23.4%), but at 18 months switching to other oral antidiabetics or insulin was as common as discontinuation (333 patients [23.7%] who switched vs. 343 patients [24.4%] who discontinued). Over the 18-month follow-up period, switching from the index therapy to one of the other two GLP-1 RAs was rare (2.0% of all patients). In these patients, the most frequent GLP-1 RA switch was to dulaglutide (1.2% of patients) compared with the other two groups. The patients most commonly switching to other GLP1-RAs were those initially treated with exenatide-QW (4.1%).
Add-on Therapy
Table 3 shows the therapies started after GLP-1 RA initiation (add-on therapy) at 12 months. The most common add-on therapies used were metformin (3.9% of patients), insulin and analogues (2.9%) and SGLT2 inhibitors (1.5%).
Clinical Outcomes
Regarding clinical parameters at 12 months after initiating treatment, the mean (SD) HbA1c levels were 7.4% (1.2), 7.6% (1.1) and 7.6% (1.2) for patients receiving dulaglutide, exenatide-QW and liraglutide, respectively (Table S2 in the ESM). Figure 3 shows that patients in the dulaglutide group experienced the greatest reduction in HbA1c levels from baseline compared with patients who received exenatide-QW or liraglutide. Reductions were also observed at 12 months after start of GLP-1 RA in systolic and diastolic blood pressure, BMI (Table S2 in the ESM), total cholesterol and triglycerides across all treatment groups.
Average Daily or Weekly Doses
The database records showed that the starting dose of dulaglutide was 0.75 mg in 23.6% of patients and 1.5 mg in 76.4%. During the 18-month follow-up period, the AWD was 1.2 (0.4) mg for dulaglutide and 1.9 (0.3) mg for exenatide-QW, whereas the ADD for liraglutide was 1.1 (0.3) mg.
Resource Use and Costs
The detailed list of resources used and their associated costs for each treatment at the 12-month follow-up period after GLP-1 RA initiation is shown in Table S1 in the ESM, and Fig. 4 shows an aggregated view of costs of primary and specialised care. The mean (SD) total cost at 12 months was €4072 (1946) for dulaglutide, €4418 (2382) for exenatide-QW and €4382.7 (2389) for liraglutide. Overall, 71.2–79.6% of total costs were derived from primary care items, especially the cost of GLP-1 RA medication (24.5–31.1%), and 20.5–28.8% were derived from specialised care (Fig. 4a). The overall lower cost for dulaglutide was derived mainly from costs associated with specialised care, which were €832 (1591) for dulaglutide, €1274 (1953) for exenatide-QW and €1202 (1977) for liraglutide (Table S3 in the ESM).
Resource utilization and mean costs by study cohorts over the 12-month follow-up period. a Aggregated costs for each resource category and for each study cohort are shown. Diagnostic tests include laboratory expenses, imaging and testing strips. GLP-1 RAs represent costs of medication for each of the three index therapies. Other drugs include antidiabetic and other concomitant medication. b Total costs of treatment of each of the study cohorts. Error bars represent 95% confidence intervals. GLP-1 RA glucagon-like peptide 1 receptor agonist, OAD oral antidiabetic treatments, QW once weekly
Discussion
This study analysed the Big-Pac database for demographic and clinical characteristics of patients with T2DM starting treatment with dulaglutide, exenatide-QW or liraglutide in Spain. The results suggest that patients initiating treatment with dulaglutide had a higher probability of being persistent over 18 months and incurred lower annual total healthcare costs than patients who initiated exenatide-QW or liraglutide. Descriptive data showed a greater reduction in HbA1c levels at 12 months in patients who started dulaglutide treatment than the other two drugs.
This real-world study indicated that patients starting GLP-1 RAs were on average 63 years old, with a mean diagnosis of 9.4 years, a BMI of 35.5 kg/m2 and an HbA1c of 8.1%. These results are very similar to those in a previous report of a retrospective study of real-world clinical use of GLP-1 RAs in Spain [20] and a more recent observational study from patients initiating GLP-1 RAs in Catalonia, a region that represents about 12% of the Spanish population [24]. As shown in the present study, GLP-1 RA therapy is usually used in combination with OADs or OADs and insulin in Spain [20], which is in line with current guideline recommendations from the Spanish Society for Endocrinology and Nutrition [6]. This study also showed that, overall, only 3.2% of patients with T2DM started GLP-1 RA treatment (1589 of 49,101 patients). This low utilization of GLP-1 RAs may reflect a gap between clinical practice and clinical guidelines, which recommend GLP-1 RAs as a second-line treatment after metformin for patients with established cardiovascular disease, at high cardiovascular risk or with chronic kidney disease [6].
In our study, persistence of treatment during the 18 months of follow-up was 59.1% with dulaglutide, 45.7% with exenatide-QW and 46.6% with liraglutide. These results are consistent with those of previous studies showing that dulaglutide elicited higher persistence than liraglutide, weekly exenatide or the newly available semaglutide [28, 33,34,35]. No appreciable differences were found in persistence between exenatide-QW and liraglutide, unlike a similar study in the USA [33]. However, persistence rates derived from real-world settings have revealed substantial variations among studies and countries [13, 28, 33, 34], so these data should be viewed with caution. Further, the reasons behind the higher persistence with dulaglutide than with other GLP-1 RA treatments have not been fully studied. The weekly administration of dulaglutide, along with the simplicity of the injection device and no need for titration, could be linked to the higher persistence rates [36]. Attributes such as dosing frequency, type of delivery system, perceived treatment benefit and the rate of side effects have been proposed as important drivers of patient preference for specific GLP-1 RAs and could influence persistence [12, 37]. Persistence is an important measure as it can influence therapeutic outcomes. Previous studies have correlated higher persistence with improved glycaemia control and decreased healthcare resource utilization [14, 15]. Similarly, poor persistence with antidiabetic medications has been shown to increase the risk of complications and declining health in patients with T2DM [13, 38].
The reasons for treatment non-persistence (discontinuation or switch) were not studied in detail. However, it is interesting to note that, at 6 months, non-persistence was mainly driven by discontinuation, but, by the end of the study, treatment switches had increased markedly. Early discontinuation rates could be due to gastrointestinal side effects that commonly appear at the beginning of GLP-1 RA treatment, although these effects were not reported in our study and this cannot be confirmed. In addition, it should be noted that, for some regions of Spain, such as Catalonia, current guidelines recommend discontinuation if no beneficial response is observed within 6 months of therapy initiation (defined as a combined reduction of at least 1.0% in HbA1c levels and 3% in initial weight) [24]. Similar advice is found in other European guidelines, such as those of the UK National Institute for Health and Care Excellence [39]. Therefore, it is possible that a decision for treatment switch was based on the patient’s clinical response. In this study of treatment with GLP-1 RAs, important reductions in HbA1c levels were observed after 12 months, consistent with recent real-world studies carried out in Spain, which showed average reductions of − 0.84% and − 0.93% in GLP-1 RA initiators [23, 24]. In the current study, the observed mean HbA1c reduction was − 0.68% with dulaglutide, − 0.54% with exenatide-QW and − 0.50% with liraglutide. A real-world study conducted in US patients revealed that dulaglutide initiators experienced a reduction of − 0.9% in HbA1c levels after 6 months of treatment [40], and another study found reductions of − 0.5% for both dulaglutide and exenatide-QW after a similar follow-up period [41]. A recent comparative meta-analysis of observational studies of dulaglutide, exenatide-QW and liraglutide found differences in HbA1c reduction between these three GLP-1 RA treatments, with dulaglutide presenting the highest reduction [36].
In this study, the ADD and AWD of the GLP-1 RAs were within the label-indicated ranges and were similar to those stated in previous European studies [26,27,28]. The recommended dose for dulaglutide as add-on therapy is 1.5 mg weekly, but for some potentially vulnerable populations 0.75 mg weekly is the starting add-on dose [42]. The AWD of 1.2 mg reported here is comparable to that in Canada (1.25 mg) but lower than that in France (1.43 mg) or the Netherlands (1.53 mg) [28]. For exenatide-QW, the recommended dose is 2 mg once weekly [43]. In this study, the AWD of 1.9 mg was also somewhat lower than that in other European countries, which ranges from 2.03 to 2.14 mg [28]. For liraglutide, the recommended starting dose is 0.6 mg daily, increasing to 1.2 mg after at least 1 week [44]. The ADD of liraglutide was 1.1 mg in this study and was lower than that reported in previous studies for other European countries, which ranged from 1.44 to 1.68 mg [28].
There are no recent estimates of the costs derived from GLP-1 RA treatments for diabetes in Spain at the national level. Regional studies investigating the healthcare costs associated with T2DM from a Spanish health system perspective ranged from an average cost per patient/year of €1108 to €6268 [45, 46]. In this study, the results showed that costs were mostly derived from primary care but were similar among the three treatments. In contrast, analysis of costs derived from resource use in specialised care revealed that costs for dulaglutide were lower than for the other two groups, resulting in lower annual costs overall.
The authors acknowledge some limitations in the study related to the retrospective design and the data sources, suggesting that the results should be interpreted with caution. Even though a 20-month index period was designed to maximize the number of patients included, the study was limited by the relatively low number of patients with GLP-1 RA prescriptions in Spain. Regarding database quality, there was no evaluation of the possible variability or accuracy of healthcare professionals’ classifications of disease, comorbidities or medication associated with the treatment. Also, there was no record of possible confounding variables that could influence the final results, such as the patient’s socioeconomic level or functional status. The duration of treatment for the different types of concomitant OADs was also not considered because of difficulties quantifying this during follow-up. Neither prescriptions issued outside the public healthcare system nor paper prescriptions were measured, which could have led to an underestimation of the percentage of treatment use. Other GLP-1 RA treatments were not included because of the low number of patients being treated or the lack of drug availability in Spain at the time of study initiation. Finally, since the analyses were descriptive and not powered to detect differences between the three cohorts (which were not propensity score matched), no definitive conclusions could be drawn regarding any dissimilarities between groups.
Since T2DM requires long-term care and substantial changes in lifestyle and is a progressive disease, patient involvement is essential. Patient-centred approaches that consider individual needs and preferences have been a strong focus of T2DM therapies. This view is highlighted in the recent consensus recommendations from the American Diabetes Association and European Association for the Study of Diabetes, which emphasize patient choice and shared decision-making in T2DM management [5]. For this reason, it is critical to understand patient preferences and behaviours that could improve treatment decisions, patient satisfaction and ultimately medication adherence. In this context, real-world studies can shed light on patient adherence patterns that would not be obvious in other types of studies [18]. Additionally, although this study was based on a database in Spain, the results should also be of interest to other countries, particularly since registries with outcomes data are not common.
Conclusions
This study suggests that persistence through the 18-month post-index period was highest in the dulaglutide cohort and lower in the exenatide-QW and liraglutide cohorts. Greater reduction in HbA1c levels was observed in patients who started dulaglutide treatment than in those who started exenatide-QW or liraglutide. Despite differences in prescribing and dispensing practices, the results are consistent with previously published data from other countries and with other studies in Spain. The results also indicated that, in Spain, the total annual average T2DM-related costs were lowest for dulaglutide and highest for exenatide-QW. This multicentric, national-level, real-world analysis of GLP-1 RA treatment patterns in Spain may be useful in the future design of therapeutic algorithms and economic evaluations.
References
International Diabetes Federation. IDF Diabetes Atlas, 9th edition. Brussels: International Diabetes Federation, 2019. http://www.idf.org/diabetesatlas. Accessed 2 June 2020.
Aguayo A, Urrutia I, Gonzalez-Frutos T, et al. Prevalence of diabetes mellitus and impaired glucose metabolism in the adult population of the Basque Country. Spain Diabet Med. 2017;34:662–6.
Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Diabetes Study. Diabetologia. 2012;55:88–93.
Mata-Cases M, Casajuana M, Franch-Nadal J, et al. Direct medical costs attributable to type 2 diabetes mellitus: a population-based study in Catalonia. Spain Eur J Health Econ. 2016;17:1001–10.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701.
Reyes-Garcia R, Moreno-Perez O, Tejera-Pérez C, et al. Sociedad Española de Endocrinología y Nutrición. Documento de abordaje integral de la DM2. Grupo de trabajo de diabetes de la SEEN (versión 2019.2). 2019. https://www.seen.es/docs/apartados/791/Abordaje%20Integral%20DM2_SEEN_2019_OCT_ISBN%20.pdf. Accessed 6 June 2020.
Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10:178–88.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–93.
Levin PA, Nguyen H, Wittbrodt ET, et al. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123–39.
Romera I, Cebrian-Cuenca A, Alvarez-Guisasola F, et al. A review of practical issues on the use of glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes. Diabetes Ther. 2019;10:5–19.
Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naive patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther. 2017;8:321–34.
Thieu VT, Robinson S, Kennedy-Martin T, et al. Patient preferences for glucagon-like peptide 1 receptor-agonist treatment attributes. Patient Prefer Adher. 2019;13:561–76.
Guerci B, Charbonnel B, Gourdy P, et al. Efficacy and adherence of glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes mellitus in real-life settings. Diabetes Metab. 2019;45:528–35.
Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33:74–109.
Buysman EK, Liu F, Hammer M, et al. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32:341–55.
Simpson SH, Lin M, Eurich DT. Medication adherence affects risk of new diabetes complications: a cohort study. Ann Pharmacother. 2016;50:741–6.
Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19–29.
Gerstein HC, McMurray J, Holman RR. Real-world studies no substitute for RCTs in establishing efficacy. Lancet. 2019;393:210–1.
Mezquita-Raya P, Reyes-Garcia R, Moreno-Perez O, et al. Clinical effects of liraglutide in a real-world setting in spain: eDiabetes-Monitor SEEN Diabetes Mellitus Working Group study. Diabetes Ther. 2015;6:173–85.
Conget I, Mauricio D, Ortega R, et al. Characteristics of patients with type 2 diabetes mellitus newly treated with GLP-1 receptor agonists (CHADIG Study): a cross-sectional multicentre study in Spain. BMJ Open. 2016;6:e010197.
Dilla T, Alexiou D, Chatzitheofilou I, et al. The cost-effectiveness of dulaglutide versus liraglutide for the treatment of type 2 diabetes mellitus in Spain in patients with BMI >/=30 kg/m(2). J Med Econ. 2017;20:443–52.
Gorgojo-Martinez JJ, Gargallo-Fernandez MA, Brito-Sanfiel M, et al. Real-world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: the CIBELES project. Int J Clin Pract. 2018;72:e13055.
Tofe S, Arguelles I, Mena E, et al. Real-world GLP-1 RA therapy in type 2 diabetes: a long-term effectiveness observational study. Endocrinol Diabetes Metab. 2019;2:e00051.
Franch-Nadal J, Mata-Cases M, Ortega E, et al. Glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: prescription according to reimbursement constraints and guideline recommendations in Catalonia. J Clin Med. 2019;8:1389.
Ministry of Health (Spain) BIFIMED: Buscador de la Información sobre la situación de financiación de los medicamentos. https://www.mscbs.gob.es/profesionales/medicamentos.do. Accessed 20 Aug 2020.
Divino V, DeKoven M, Hallinan S, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5:499–520.
Divino V, DeKoven M, Khan FA, et al. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther. 2017;8:115–28.
Divino V, Boye KS, Lebrec J, et al. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10:1067–88.
Orsini Federici M, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in italy: a retrospective cohort study. Diabetes Ther. 2018;9:789–801.
Otto T, Myland M, Jung H, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2019;35:893–901.
Sicras-Mainar A, Enríquez JL, Hernández I, et al. Validation and representativeness of the Spanish BIG-PAC database: integrated computerized medical records for research into epidemiology, medicines, and health resource use (real world evidence). Value Health. 2019;22(Suppl 3):S734.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Alatorre C, Fernandez Lando L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953–61.
Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes Metab. 2019;21:920–9.
Mody R, Yu M, Nepal BK, et al. Dulaglutide has higher adherence and persistence than semaglutide and exenatide QW: 6-month follow-up from US real-world data. Diabetes. 2020;69(Supp 1):928.
Morieri ML, Rigato M, Frison V, et al. Effectiveness of dulaglutide vs liraglutide and exenatide once-weekly. A real-world study and meta-analysis of observational studies. Metabolism. 2020;106:154190.
Gelhorn HL, Poon JL, Davies EW, et al. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naive type 2 diabetes patients in the UK. Patient Prefer Adher. 2015;9:1611–22.
Gatwood JD, Chisholm-Burns M, Davis R, et al. Differences in health outcomes associated with initial adherence to oral antidiabetes medications among veterans with uncomplicated type 2 diabetes: a 5-year survival analysis. Diabet Med. 2018;35:1571–9.
National Institute for Health and Care Excellence (NICE). Clinical guideline NG28. Type 2 diabetes in adults: Management. 2015. Last updated 28 August 2019. https://www.nice.org.uk/guidance/ng28. Accessed 4 July 2020.
Mody R, Grabner M, Yu M, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34:995–1003.
Unni S, Wittbrodt E, Ma J, et al. Comparative effectiveness of once-weekly glucagon-like peptide-1 receptor agonists with regard to 6-month glycaemic control and weight outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:468–73.
European Medicines Agency. Summary of product characteristics: dulaglutide (Trulicity). Last updated 8 November 2019. https://www.ema.europa.eu/documents/product-information/trulicity-epar-product-information_en.pdf. Accessed 31 May 2020.
European Medicines Agency. Summary of product characteristics: exenatide (Bydureon). Last updated 28 May 2020. https://www.ema.europa.eu/documents/product-information/bydureon-epar-product-information_en.pdf. Accessed 31 May 2020.
European Medicines Agency. Summary of product characteristics: liraglutide (Victoza). Last updated 7 November 2019. https://www.ema.europa.eu/documents/product-information/victoza-epar-product-information_en.pdf. Accessed 31 May 2020.
Sicras-Mainar A, Navarro-Artieda R, Ibanez-Nolla J. Clinical and economic characteristics associated with type 2 diabetes. Rev Clin Esp (Barc). 2014;214:121–30.
Nuño-Solinís R, Alonso-Morán E, Arteagoitia Axpe JM, et al. Healthcare costs of people with type 2 diabetes mellitus in the Basque Country (Spain). Endocrinol Nutr. 2016;63:543–50.
Acknowledgements
Funding
Sponsorship for this study and the journal's Rapid Service Fee were funded by Eli Lilly and Co., Indianapolis, IN, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing Assistance
The authors would like to acknowledge Francisco López de Saro, PhD, and Karen Goa (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Lilly and Company.
Disclosures
Kirsi Norrbacka, Esther Artime, Silvia Díaz and Irene Romera are employees of and minor shareholders in Eli Lilly and Company. Jeremie Lebrec is employed as a consultant for Eli Lilly and Company. Santiago Tofé-Povedano has received honoraria for scientific advisory, lectures, and clinical research from Eli Lilly and Company, Novo Nordisk, Astra Zeneca, Sanofi, Boehringer and GlaxoSmithKline. Antoni Sicras-Mainar and Ignacio Hernández declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study involved a retrospective cohort analysis with secondary use of anonymized data from the Big-Pac database (Atrys Health-Real Life Data, Madrid, Spain), and the analysis does not contain new studies with human or animal subjects performed by any of the authors. The protocol was approved by the Clinical Research Ethical Committee of the Consorci Sanitari de Terrassa (Barcelona, Spain). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, GPPs, and applicable laws and regulations of Spain.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Norrbacka, K., Sicras-Mainar, A., Lebrec, J. et al. Glucagon-Like Peptide 1 Receptor Agonists in Type 2 Diabetes Mellitus: Data from a Real-World Study in Spain. Diabetes Ther 12, 1535–1551 (2021). https://doi.org/10.1007/s13300-021-01039-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01039-5