Abstract
Introduction
Emerging evidence suggests psoriatic arthritis (PsA) with axial involvement (axPsA) and radiographic axial spondyloarthritis (r-axSpA) may possibly represent distinct disorders, with some differing clinical manifestations, genetic associations, and radiographic findings. Moreover, axPsA and r-axSpA may respond differently to therapies: guselkumab (interleukin [IL]-23p19 subunit inhibitor [i]) and ustekinumab (IL-12/23p40i) demonstrated improvements in axial symptoms in patients with PsA; however, neither risankizumab (IL-23p19i) nor ustekinumab demonstrated efficacy versus placebo in patients with r-axSpA. Current analyses aim to further understand potential molecular distinctions between axPsA and r-axSpA and examine the pharmacodynamic effects of guselkumab in patients with axPsA and those with PsA without axial involvement (non-axPsA).
Methods
Post hoc analyses utilized biomarker data from blood and serum samples collected from a subset of participants in phase 3 studies of ustekinumab in r-axSpA and guselkumab in PsA (DISCOVER-1 and DISCOVER-2). Participants with axPsA were identified by investigator-verified sacroiliitis (imaging-confirmed) and axial symptoms. HLA mapping, serum cytokine analysis, and whole-blood RNA sequencing were conducted.
Results
Relative to r-axSpA, patients with axPsA had a lower prevalence of HLA-B27, HLA-C01, and HLA-C02 alleles and a higher prevalence of HLA-B13, HLA-B38, HLA-B57, HLA-C06, and HLA-C12 alleles. Compared with r-axSpA, patients with axPsA had elevated baseline levels of serum IL-17A and IL-17F cytokines, enrichment of IL-17 and IL-10 pathway-associated genes, and neutrophil gene markers. Across axPsA and non-axPsA cohorts, reductions in cytokine levels and normalization of pathway-associated gene expression with guselkumab treatment were comparable.
Conclusion
The differences in HLA genetic associations, serum cytokines, and enrichment scores support the concept that axPsA and r-axSpA may be distinct disorders. The comparable pharmacodynamic effects of guselkumab on cytokine levels and pathway-associated genes observed in patients with axPsA and non-axPsA are consistent with demonstrated clinical improvements across PsA cohorts. These findings contribute to the understanding of potential genetic and molecular distinctions between axPsA and r-axSpA.
Trial Registration
ClinicalTrials.gov identifiers, NCT03162796, NCT0315828, NCT02437162, and NCT02438787.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Based on potentially differential genetic associations, clinical manifestations, and radiographic findings, it has been hypothesized that axial psoriatic arthritis (axPsA) and radiographic axial spondyloarthritis (r-axSpA) may represent distinct disorders. |
Patients with axPsA and r-axSpA may have differing responses to interleukin (IL)-23p19 inhibitor (i) and IL-12/23p40i therapies, with clinical benefit observed in axPsA but not in r-axSpA. |
These post hoc analyses sought to further understand molecular differences between axPsA and r-axSpA to further elucidate distinctions between these disorders. |
What was learned from the study? |
Differences were observed between patients with axPsA and r-axSpA with respect to HLA alleles, serum cytokine levels, and cytokine pathway genes. Treatment with guselkumab, a fully human antibody targeting the IL-23p19 subunit, significantly reduced IL-17A and IL-17F levels versus placebo and normalized PsA-associated gene expression in patients with axPsA and non-axPsA. |
Reported findings add to the growing body of evidence suggesting that axPsA and r-axSpA are distinct disorders. |
Introduction
Spondyloarthritides (SpAs) are a group of interrelated disorders that include axial spondyloarthritis (axSpA), encompassing a spectrum from nonradiographic axSpA (nr-axSpA) to radiographic axSpA (r-axSpA); psoriatic arthritis (PsA); SpA related to inflammatory bowel disease (IBD); undifferentiated SpA; and reactive arthritis [1]. PsA and r-axSpA are chronic, progressive, inflammatory diseases [1] that are considered to represent prototypical peripheral and axial SpA, respectively. PsA is characterized by involvement across several clinical domains, including peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease; PsA is also associated with IBD and anterior uveitis [2]. Patients with PsA who have axial involvement (axPsA) can present with limited range of motion and tenderness around the spine and sacroiliac joints [2]. Radiographic axSpA is characterized by the presence of inflammatory back pain and includes pain in the hips or buttocks that improves with activity and worsens with rest, pain that occurs at night, and axial morning stiffness with a duration of 30 min or more [3].
Despite overlapping clinical features, some evidence to date suggests that axPsA and r-axSpA may be distinct disorders. For example, while the presence of inflammatory back pain is considered essential for a diagnosis of r-axSpA [4], only about 50% of patients with radiographically evident axPsA report this symptom [2]. Further, a review of genetic studies found that although patients with PsA and r-axSpA commonly carry the major histocompatibility complex (HLA) class I allele HLA-B27, frequencies differ between these disorders and axPsA is more commonly associated with other HLA alleles [1]. Additionally, radiographic studies have suggested that patients with axPsA may have less severe and asymmetric sacroiliitis, as well as a greater number of coarse nonmarginal syndesmophytes and fewer classical syndesmophytes, relative to patients with r-axSpA. Patients with axPsA may also be more likely to have asymmetric involvement in other areas of the spine [1].
Axial PsA and r-axSpA may differ in response to specific immunomodulatory therapies. In a post hoc analysis of patients with PsA from the DISCOVER-1 and DISCOVER-2 studies with investigator-confirmed sacroiliitis as documented by previous imaging or pelvic radiography at screening, guselkumab, a human monoclonal antibody targeting the interleukin (IL)-23 p19 subunit, improved axial symptoms over 24 weeks compared with placebo [5]. Furthermore, greater proportions of patients in the guselkumab groups achieved meaningful improvements in axial symptoms (as measured by the Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] and Ankylosing Spondylitis Disease Activity Score using C-Reactive Protein [ASDAS]) compared with placebo. Improvements in axial symptoms were maintained in guselkumab-treated patients through 1 year in pooled DISCOVER-1 and DISCOVER-2 patients and through 2 years in DISCOVER-2 patients [5, 6].
Ustekinumab is a human monoclonal antibody targeting the IL-12/23p40 subunit that has shown clinical benefit and inhibition of the progression of radiographic damage in peripheral joints in patients with PsA in the PSUMMIT-1 and PSUMMIT-2 studies [7,8,9]. In a post hoc analysis of patients with PsA who had peripheral arthritis and physician-reported spondylitis, ustekinumab-treated patients demonstrated significantly greater improvements in axial symptoms (as measured by BASDAI and ASDAS) through week 24 compared with placebo [10]. An open-label phase 2 study of ustekinumab in patients with r-axSpA showed an association between treatment with ustekinumab and a reduction in the signs and symptoms of r-axSpA [11]. However, efficacy was not demonstrated in two randomized, placebo-controlled, phase 3 studies of ustekinumab in patients with r-axSpA [12]. Similarly, risankizumab, a humanized monoclonal antibody targeting the IL-23p19 subunit, did not achieve the primary endpoint (Assessment in SpondyloArthritis International Society 40% response at week 12) in a phase 2 study [13].
To date, comparative molecular and biomarker data are limited. This analysis sought to understand the underlying mechanisms of axPsA and r-axSpA, and potential molecular distinctions between these diseases, as well as the pharmacodynamic effects of guselkumab in patients with axPsA and r-axSpA.
Methods
Patients and Study Design
These post hoc analyses utilized biomarker data from blood and serum samples collected from a subset of patients with PsA enrolled in the phase 3 DISCOVER-1 (ClinicalTrials.gov identifier, NCT03162796) and DISCOVER-2 (NCT0315828) studies of guselkumab and in two phase 3 studies of ustekinumab conducted in patients with r-axSpA (NCT02437162 and NCT02438787). Full methods and results of these studies have been reported previously [12, 14, 15].
The DISCOVER-1 study enrolled patients with active PsA (swollen joint count ≥ 3, tender joint count ≥ 3, and C-reactive protein [CRP] level ≥ 0.3 mg/dL) despite standard therapies. The mixed patient population comprised 69% biologic-naïve patients and 31% who received prior therapy with no more than two tumor necrosis factor inhibitors (TNFi) [14]. The DISCOVER-2 study enrolled biologic-naïve patients with active PsA (swollen joint count ≥ 5, tender joint count ≥ 5, and CRP level ≥ 0.6 mg/dL) despite standard therapies [15]. In DISCOVER-1 and DISCOVER-2, patients were randomized 1:1:1 to receive guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at week 0, week 4, and then every 8 weeks (Q8W); or placebo with crossover to guselkumab Q4W at week 24 [5].
In post hoc analyses of DISCOVER-1 and DISCOVER-2, patients were categorized as having axPsA based on investigator identification of PsA with axial involvement and evidence of sacroiliitis on prior radiograph or magnetic resonance imaging (MRI) of sacroiliac joints (DISCOVER-1 and DISCOVER-2) or pelvic radiograph at screening (DISCOVER-2 only). Patients who did not meet these criteria were categorized as having non-axPsA [5].
The two phase 3 multicenter, randomized, double-blind, placebo-controlled studies of ustekinumab in r-axSpA enrolled patients with active r-axSpA despite previous therapy, radiographic sacroiliitis according to 1984 modified New York criteria for r-axSpA, BASDAI score ≥ 4 [16], visual analog scale score for back pain ≥ 4, and CRP level ≥ 0.3 mg/dL [12]. Patients had inadequate response to or were intolerant of nonsteroidal anti-inflammatory drugs and were TNFi-naïve (study 1) or had inadequate response or intolerance to one TNFi (study 2). Across studies, patients were randomized 1:1:1 to receive ustekinumab 45 mg or 90 mg at weeks 0, 4, and 16 and then every 12 weeks (Q12W) or placebo. Patients randomized to placebo were re-randomized at week 24 to receive ustekinumab 45 mg or 90 mg at weeks 24 and 28 and then Q12W [12].
The four phase 3 studies were conducted in accordance with the Declaration of Helsinki. The protocols were approved by the institutional review board or ethics committee at each site [12, 14, 15]. All patients gave written informed consent, with an additional consent provided for voluntary genetic testing (HLA). An independent data monitoring committee regularly reviewed unblinded safety data.
Whole-Blood Transcriptomic Profiling via RNA Sequencing
RNA was isolated from whole blood of patients with axPsA, non-axPsA, and r-axSpA collected in PAXgene RNA blood tubes (BD Biosciences, Franklin Lakes, NJ). PAXgene samples from demographically matched healthy individuals were procured independently of the clinical studies (BioIVT, Westbury, NY) and included as a control. Globin messenger RNA (mRNA) was removed using GlobinClear™ (Invitrogen, Waltham, MA). Whole-blood transcriptome profiling by paired-end RNA sequencing (TruSeq Stranded mRNA; Illumina, San Diego, CA) was performed using the Novaseq Sequencing System (Illumina) at a depth of 30 million reads per sample.
HLA Genotype Analyses
HLA genotype analyses were performed for participants with axPsA, non-axPsA, and r-axSpA and healthy controls who provided additional consent and had available whole-blood RNA sequencing data. The primary analyses of HLA genotype were performed in White patients to minimize potential genetic variability in HLA across races [17], because sample sizes were not sufficient to allow meaningful comparison of HLA genotypes in patients of other races. The genotypes of class I HLA alleles across all cohorts (healthy controls, axPsA, non-axPsA, and r-axSpA) were determined using RNA sequencing data as described elsewhere [18]. Patients were classified as positive for an allele if they had at least one copy of that allele [5].
Serum Cytokine Levels
Levels of serum IL-17A and IL-17F were assessed at baseline across patients with axPsA, non-axPsA, and r-axSpA with adequate samples. Serum samples from demographically matched healthy individuals were procured independently of the clinical studies and biomarker and genetic analyses (BioIVT, Westbury, NY; Biological Specialty Corporation, Colmar, PA) and were included as a control. The pharmacodynamic effects of guselkumab on serum IL-17A, IL-17F, and IL-22 levels were assessed at weeks 4 and 24 among patients with axPsA and non-axPsA. Biomarker data from guselkumab Q4W and Q8W treatment arms were pooled in the analysis. Serum IL-17A, IL-17F, and IL-22 concentrations were determined using the Singulex system, as previously described (Alameda, CA) [12].
Pathway Enrichment Score Analyses
Expression of pathway genes associated with IL-17 and IL-10, as well as neutrophil gene markers, were analyzed using RNA sequencing of whole-blood samples. IL-17 pathway-associated genes were derived from differentially expressed genes from cultured normal human bronchial epithelial cells, normal human airway smooth muscle cells, and/or keratinocytes with 50 ng/mL of human IL-17A for 24 h. The IL-17 gene set is shown in Table S1 in the supplementary material. Pathway-associated genes for IL-10 and neutrophils were as described previously [19, 20]. Gene Set Variation Analysis (GSVA) was performed in R (R Foundation for Statistical Computing, Vienna, Austria) using the GSVA package to calculate enrichment scores for the IL-10 and IL-17 pathway gene set [21]. The enrichment scores were calculated using the magnitude of difference between the largest positive and negative random walk deviations. Neutrophil and leukocyte abundance values were estimated with the MCP-counter R package [20]. Disease-related genes up- and downregulated in patients with PsA were analyzed among all patients with PsA as previously described [22]. Baseline pathway enrichment scores in patients with axPsA were further examined in patients stratified by baseline body surface area (BSA) with psoriasis (PsO) involvement (BSA < 3% and BSA ≥ 3%) and HLA-B27 status.
Statistical Analyses
The prevalence of HLA alleles was compared across cohorts using the Fisher’s exact test with p values adjusted for multiplicity of testing. Alleles included in these analyses were present in at least four carriers in all disease cohorts. A generalized linear model was performed using log2-transformed data to determine the significance of differential baseline serum cytokine levels across disease cohorts and the effect of guselkumab on serum cytokine levels versus placebo among patients with axPsA and non-axPsA. Analysis of variance testing was used to compare enrichment scores across disease cohorts (baseline), and between treatment groups (week 4 and 24), as well as changes in enrichment scores from baseline (week 4 and 24) within each treatment group. Sensitivity analyses for HLA prevalence were conducted in the overall population (all races) and for pathway enrichment scores in the White population to assess the effects of differing populations on those analyses. As analyses were post hoc (i.e., exploratory), all p values presented are nominal.
Results
Patient Characteristics
Overall, biomarker data were available from 996 patients, including 483 with non-axPsA, 190 with axPsA, and 323 with r-axSpA (Figure S1 in the supplementary material). In all three disease cohorts, baseline patient demographics, disease characteristics, and use of conventional synthetic disease-modifying antirheumatic drugs were consistent with attributes of patients with active disease (Table 1). Mean CRP levels were 2.8, 2.4, and 1.5 mg/dL in patients with axPsA, r-axSpA, and non-axPsA, respectively, suggesting potential differences in systemic inflammation across diseases. For patients included in the HLA genotype analysis, except for race, the baseline demographic and disease characteristics of the White patients were consistent with those of the overall study cohorts (Table S2 in the supplementary material). Baseline demographics and disease characteristics of patients included in the cytokine analyses were consistent with the overall study cohorts (Table S3 in the supplementary material).
HLA Genotype Analysis
The prevalence of class I HLA alleles examined in this analysis is shown in Fig. 1. Significantly lower proportions of patients with axPsA versus those with r-axSpA were carriers of HLA-B27 (31% vs 92%), HLA-C01 (6% vs 32%), and HLA-C02 (28% vs 62%) alleles. Significantly higher proportions of patients with axPsA than with r-axSpA were carriers of HLA-B13 (15% vs 5%), HLA-B38 (18% vs 4%), HLA-B57 (14% vs 2%), HLA-C06 (36% vs 9%), and HLA-C12 (30% vs 10%) alleles. The prevalence of HLA alleles was comparable between the axPsA and non-axPsA cohorts. In a sensitivity analysis in the overall cohort, the prevalence of these HLA alleles, with the exception of HLA-C12, remained significantly different between patients with axPsA and r-axSpA (data not shown).
Prevalence of class I HLA alleles in White HCs and participants with axPsA, non-axPsA, and r-axSpA. *p < 0.01; †p < 0.001. ‡HC group was included as a descriptive reference. Alleles included in this analysis were present in at least four carriers in all disease groups. axPsA axial psoriatic arthritis, HC healthy control, HLA human leukocyte antigen, r-axSpA radiographic axial spondyloarthritis
The prevalence of other class I HLA alleles among HLA-B27-positive patients is shown in Table S4 in the supplementary material. Among HLA-B27-positive patients, greater proportions of those with axPsA versus those with r-axSpA were carriers of HLA-C02 (79% vs 51%) and HLA-C06 (21% vs 5%), while lower proportions of patients with axPsA versus r-axSpA were carriers of HLA-A11 (5% vs 28%), HLA-B40 (0% vs 14%), and HLA-C03 (5% vs 19%). Differences were also observed among axPsA patients, with lower prevalence of HLA-B40 (0% vs 14%), HLA-C03 (5% vs 24%), and HLA-C06 alleles in HLA-B27-positive compared with HLA-B27-negative patients. Conversely, a higher prevalence of HLA-C02 was observed for HLA-B27-positive compared with HLA-B27-negative patients (79% vs 5%) with axPsA.
Baseline Serum Cytokine Levels
Serum cytokine levels were determined in a subset of patients with available samples, including 229 with non-axPsA, 71 with axPsA, and 58 with r-axSpA. Participants of any race were included in this analysis. At baseline, serum IL-17A levels were higher in both patients with non-axPsA and axPsA than in healthy controls (nominal p < 0.05), with comparable levels between patients with non-axPsA and axPsA (Fig. 2). IL-17A levels were higher in patients with axPsA than patients with r-axSpA (nominal p < 0.05). Similar results were observed for IL-17F levels at baseline.
Serum cytokine levels were assessed according to HLA-B27 carrier status. In patients with axPsA, IL-17A and IL-17F levels were comparable between HLA-B27-positive and HLA-B27-negative patients (Fig. S2 in the supplementary material). When disease cohorts were compared, HLA-B27-positive patients with r-axSpA had significantly lower levels of IL-17A and IL-17F than HLA-B27-positive or HLA-B27-negative patients with axPsA.
Baseline Pathway Enrichment Scores
Whole-blood transcriptome profiling was conducted at baseline for genes related to the IL-17 and IL-10 pathways, which are involved in T helper (Th)17 development [23], and neutrophil gene markers. At baseline, enrichment scores for genes related to IL-17 and IL-10 pathways and for neutrophil markers were higher in patients with axPsA, non-axPsA, and r-axSpA than in healthy controls (Fig. 3). Moreover, patients with axPsA had higher IL-17- and IL-10-related pathway and neutrophil marker enrichment scores compared with patients with r-axSpA. Patients with axPsA also had higher IL-10-related pathway and neutrophil marker enrichment scores compared with patients with non-axPsA (Fig. 3). In a sensitivity analysis of White patients only, differences in these enrichment scores at baseline were also observed across disease cohorts (nominal p < 0.05; data not shown).
Baseline enrichment scores of a IL-17 pathway, b IL-10 pathway, and c neutrophil-associated genes in HCs and participants with axPsA, non-axPsA, and r-axSpA. Error bars represent standard error. axPsA axial psoriatic arthritis, HC healthy control, IL interleukin, r-axSpA radiographic axial spondyloarthritis
When baseline pathway scores for genes related to the IL-17 and IL-10 pathways and neutrophil gene markers were stratified by patient baseline PsO BSA involvement (< 3% and ≥ 3%), enrichment scores were comparable for both BSA subgroups in patients with axPsA. Enrichment scores were also similar between patients with axPsA and those with r-axSpA regardless of baseline PsO BSA involvement (Fig. S3 in the supplementary material).
No differences in enrichment scores were observed between patients with axPsA who were HLA-B27-positive and -negative (Fig. S4 in the supplementary material). Consistent with the overall cohorts, HLA-B27-positive patients with axPsA had higher enrichment scores for genes related to IL-17 and IL-10 pathways and for neutrophil markers than HLA-B27-positive patients with r-axSpA.
Pharmacodynamic Response to Guselkumab Treatment in Patients with PsA
Guselkumab significantly reduced serum IL-17A, IL-17F, and IL-22 levels versus placebo at week 4 and also at week 24 in both patients with axPsA and non-axPsA (nominal p < 0.05; Fig. 4).
Changes in serum a IL-17A, b IL-17F, and c IL-22 levels through week 24 in participants with axPsA and non-axPsA treated with guselkumab or placebo. *p < 0.05 guselkumab vs placebo in each subgroup. Error bars represent standard error. axPsA axial psoriatic arthritis, BL baseline, GUS guselkumab, IL interleukin, PBO placebo
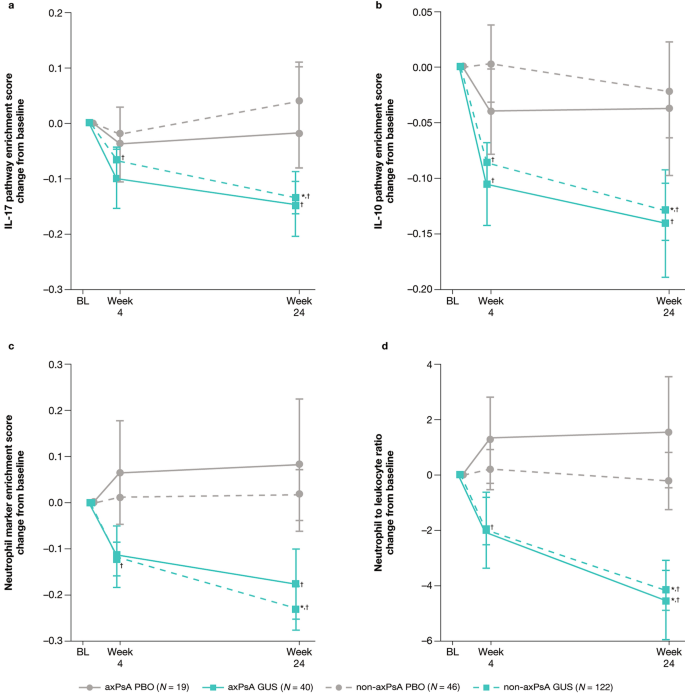
At week 24 in patients treated with guselkumab, both patients with axPsA and non-axPsA had comparable decreases in IL-17 and IL-10 pathway enrichment scores from baseline (nominal p < 0.05; Fig. 5). The same pattern was also observed for both neutrophil enrichment score and neutrophil to leukocyte ratios (NLRs). No significant reductions were observed in placebo-treated patients for any enrichment scores post baseline. Patients with non-axPsA treated with guselkumab had lower enrichment scores at week 24 compared with those who received placebo (nominal p < 0.05). A numerical difference was also observed in patients with axPsA, with a nominal p value > 0.05 as a likely artifact of a smaller sample size.
Changes from baseline through week 24 in a IL-17 pathway enrichment scores, b IL-10 pathway enrichment scores, c neutrophil marker enrichment score, and d neutrophil to leukocyte ratio in patients with axPsA and non-axPsA treated with guselkumab or placebo. *p < 0.05 guselkumab vs placebo in each subgroup; †p < 0.05 post-treatment versus baseline in each subgroup. Error bars represent standard error. axPsA axial psoriatic arthritis, BL baseline, GUS guselkumab, IL interleukin, PBO placebo
For PsA disease-related genes from expression profiling that were elevated at baseline relative to healthy controls, both patients with axPsA and non-axPsA treated with guselkumab had significant and comparable decreases in the enrichment score from baseline at week 24 (Fig. 6). Similarly, among PsA-related genes with lower expression at baseline compared with healthy controls, both patients with axPsA and non-axPsA treated with guselkumab had significant and comparable increases in the enrichment scores from baseline at week 24. A significant treatment effect in genes both upregulated and downregulated at baseline was observed as early as week 4 in patients with non-axPsA; a similar nonsignificant trend was observed in patients with axPsA, though the sample size was smaller. No changes in PsA-related gene expression were observed in patients who received placebo.
Effect of guselkumab on PsA disease-related genes that are a upregulated or b downregulated in patients with PsA through week 24. *p < 0.05 guselkumab vs placebo in each subgroup; †p < 0.05 post treatment versus baseline in each subgroup. Error bars represent standard error. axPsA axial psoriatic arthritis, BL baseline, GUS guselkumab, PBO placebo, PsA psoriatic arthritis
Discussion
The results of these analyses indicate that patients with axPsA and r-axSpA show differences in HLA genetic associations, serum IL-17A and IL-17F levels, enrichment of IL-17 and IL-10 gene expression pathways, and neutrophil gene markers. These results contribute to evidence suggesting that axPsA and r-axSpA may be distinct diseases with overlapping clinical features.
The differences observed in class I HLA alleles between axPsA and r-axSpA were consistent with previous reports demonstrating that axPsA and r-axSpA overlap with respect to the contribution of HLA-B27 but also have distinct genetic risk factors [1, 24]. Previous studies have reported that approximately 80% of patients with r-axSpA are HLA-B27-positive [25, 26], whereas approximately 20% of patients with axPsA are HLA-B27-positive [26, 27]. Results of this analysis, which found rates of HLA-B27 alleles of 92% for patients with r-axSpA versus 31% for those with axPsA, are thus generally consistent with previous reports. A moderated role of HLA-B27 status in predicting response to treatment in patients with axPsA was also reflected in a previous analysis of the DISCOVER-1 and DISCOVER-2 studies that showed consistent guselkumab efficacy across HLA-B27-positive and HLA-B27-negative participants [5]. The current analysis also found higher prevalence of the HLA-C01 and HLA-C02 alleles in patients with r-axSpA compared with axPsA. The HLA-C01 finding is consistent with a previous report [28]. To our knowledge, this is the first comparison of prevalence of the HLA-C02 allele between patients with axPsA and r-axSpA. In patients with axPsA, higher prevalences of HLA-B13, HLA-B38, HLA-B57, HLA-C06, and HLA-C12 alleles were found compared with r-axSpA. With the exception of the HLA-B13 allele, these have been previously reported to be more common in patients with PsA, and the HLA-B38 allele has been reported to be more common in patients with axPsA compared with patients with r-axSpA [1]. When patients were examined by HLA-B27 carrier status, the majority of those with r-axSpA or axPsA who were HLA-B27 carriers were also HLA-C02 carriers (51% and 79%, respectively). The increased frequency of HLA-C06 in patients with axPsA compared with r-axSpA remained consistent between the overall cohort and patients with HLA-B27. Other than a higher proportion of HLA-C02 carriers among HLA-B27-positive versus HLA-B27-negative patients with axPsA, no clear patterns were observed by HLA-B27 status. These results, however, should be interpreted with caution due to the limited number of patients with axPsA who were HLA-B27 carriers (n = 57).
The IL-23 pathway is associated with the pathogenesis of PsO, IBD, and SpA. IL-23 promotes activation of the Th17 pathway, which induces expression of IL-17. The clinical benefit of IL-17 inhibition in patients with axPsA and r-axSpA supports a role for IL-17 in the pathogenesis of both diseases [29, 30]. While post hoc analyses of data collected in the DISCOVER-1 and DISCOVER-2 studies showed that guselkumab provided significant and sustained improvement in signs and symptoms of axial disease in patients with PsA [5], the negative results in studies of ustekinumab and risankizumab in patients with r-axSpA [12, 13] suggest that biologic consequences of alterations in the IL-17/IL-23 axis may differ between diseases. However, an alternative explanation for the discrepant clinical results noted for ustekinumab in axPsA as compared with r-axSpA is that the outcome measures used (e.g., BASDAI) may capture improvements in domains not specific to axial involvement in patients with PsA. Although the role of the IL-17/IL-23 axis in the pathogenesis of axPsA and r-axSpA is unclear, IL-17 secretion can occur independently of IL-23 in skeletal inflammation in patients with r-axSpA, possibly driven by γδ T cells and mucosal-associated invariant T cells [31]. In addition, in rat models of HLA-B27-associated SpA, IL-23 blockade prevented the development of SpA when given prophylactically, but did not affect spondylitis and arthritis severity when given therapeutically [32]. These results also suggest that although IL-23 may be involved in the initial stages of r-axSpA, it may perhaps not be a predominant factor influencing later stages of disease. Further research is necessary to better understand the role of these cells and cytokines in axPsA and r-axSpA, including the association between disease activity and circulating levels of the IL-17 cytokines.
Findings from previous investigations of the role of IL-17 cytokines in r-axSpA have varied. Serum IL-17A levels were similar between patients with r-axSpA and control participants in one study [33], whereas other studies reported higher serum IL-17 levels in patients with r-axSpA than in healthy controls [34, 35]. Another study found that serum levels of IL-17 and IL-23 were not associated with disease activity in patients with r-axSpA [36]. Results of our analyses showed distinct differences in baseline serum IL-17A and IL-17F levels, with patients with axPsA having higher levels than those with r-axSpA and healthy controls, and patients with r-axSpA exhibiting IL-17A and IL-17F levels similar to healthy controls. These findings remained consistent in the subgroup of HLA-B27-positive patients, with higher levels of IL-17A and IL-17F observed in axPsA versus r-axSpA. IL-17A and IL-17F levels were similar between HLA-B27-positive and HLA-B27-negative patients; this is in line with the HLA-B27 status-independent improvements in patients with axPsA treated with guselkumab [5].
Consistent observations were seen when comparing the IL-17 pathway enrichment scores from whole-blood transcriptome in these diseases. Thus, although the IL-17/IL-23 pathway has been implicated in the pathogenesis of both r-axSpA and PsA, the results of the cytokine analyses presented here, together with clinical evidence from IL-23p19-subunit inhibitor-treated patients with PsA, suggest that the IL-17/IL-23 pathway might be involved in different aspects of pathogenesis in each disease.
Further examination of the whole-blood transcriptome of patients with axPsA and r-axSpA also showed distinct differences in gene enrichment scores for neutrophil markers and the IL-10 pathway. Activation and accumulation of neutrophils in synovium and psoriatic lesions is one of the distinguishing features of PsA [37], and IL-17 can stimulate the activation and migration of neutrophils primarily through the production of other factors such as granulocyte macrophage colony-stimulating factor and IL-8 [38, 39]. IL-10 is generally considered an anti-inflammatory cytokine that functions as a negative regulator of inflammation. It can be produced in response to pro-inflammatory signals by virtually all immune cells, including Th17 cells [40, 41]. In this analysis, baseline levels of neutrophil markers were higher across patients with non-axPsA, axPsA, and r-axSpA compared with healthy controls, with higher levels observed in those with axPsA than r-axSpA. This is consistent with the observed elevated levels of IL-17A and IL-17F and the known role of IL-17 in promoting activation and recruitment of neutrophils to the skin and joints, and subsequent inflammation [42, 43]. The similar pattern observed for the IL-10 pathway enrichment score suggests a relatively heightened inflammatory state and perhaps greater stimulation of the negative regulator in axPsA than in r-axSpA in response to greater systemic inflammation. To our knowledge, this is the first comparison of these markers between axPsA and r-axSpA. Previous studies have reported both higher [44] and similar [45] NLRs in patients with r-axSpA versus controls. Although the NLR has been suggested to be a poor marker of severe disease activity in patients with r-axSpA [44, 45], it has shown significant association with the development of PsA in patients with PsO [46]. In our analyses, significant increases in enrichment scores for IL-17 and IL-10 pathway genes and neutrophil markers in axPsA versus r-axSpA were observed regardless of the degree of BSA with PsO involvement in patients with axPsA, suggesting that these differences were not driven solely by skin inflammation. These findings support further investigation of the utility of neutrophil marker scores, NLRs, and IL-10 pathway activity in further differentiating axPsA from r-axSpA.
Although not the focus of the present study, differences in IL-10 and neutrophil marker scores were also observed between patients with axPsA and non-axPsA. The significance of this finding is unclear, although it may represent a heightened inflammatory state in patients with axPsA compared with non-axPsA. As a result of the post hoc nature of the present analysis, further studies are required to confirm these findings.
In patients with axPsA and non-axPsA, guselkumab significantly reduced levels of serum IL-17A, IL-17F, and IL-22 versus placebo as early as week 4 and through week 24. This is consistent with the known clinical effect of guselkumab in patients with axPsA and non-axPsA. Guselkumab-treated patients had greater improvements in axial symptoms, as assessed by the BASDAI, modified BASDAI, and ASDAS instruments, from baseline at week 24 compared with placebo regardless of HLA-B27 status [5]. Improvements in axial symptoms were maintained through 1 year in DISCOVER-1 and DISCOVER-2 (pooled) and 2 years in DISCOVER-2 among guselkumab-treated patients [5, 6]. The changes in IL-17A, IL-17F, and IL-22 levels observed in the current analysis were similar between axPsA and non-axPsA cohorts, as were IL-17 and IL-10 pathway-associated genes and neutrophil markers, demonstrating that guselkumab has suppressive effects on these immune pathways across distinct groups of patients with PsA. The NLR was decreased with guselkumab treatment at week 24 to similar levels in patients with axPsA and non-axPsA. Finally, guselkumab normalized PsA-associated gene expression, which could lead to long-term disease control. These changes were observed in whole-blood samples. However, tissue-specific variation exists between IL-17 and IL-23 (such as those observed between the gut and skin) due to the presence of different local and resident T cell populations. Such differences can be further compounded between diseases; additional studies are necessary to determine tissue-specific changes and any differences between axPsA and r-axSpA.
The current analyses were limited by their post hoc nature and lack of prespecified endpoints. Numbers of available samples were limited for some analyses, particularly for patients with axPsA, thus reducing the likelihood of detecting significant between-cohort differences. Data on the effect of guselkumab in patients with r-axSpA were not available, precluding evaluation of pharmacologic effects in this cohort, and the small number of non-White patients included in these studies prevented analysis of HLA genotypes in more diverse patient populations. Additionally, the axPsA cohort in DISCOVER-1 and DISCOVER-2 was identified by locally read, imaging-confirmed sacroiliitis, which excluded assessment of spinal involvement in axPsA; as a result of the inclusion criteria used in the DISCOVER-1 and DISCOVER-2 studies, the axPsA group may have included some patients with non-axPsA. These clinical trials also did not collect data on the duration of axial symptoms, which would aid differentiation of axPsA from early axSpA and potentially explain differences in response to IL-23 inhibitors. Results from an ongoing randomized placebo-controlled clinical trial dedicated to evaluating the effects of guselkumab in patients with MRI-confirmed axPsA (≥ 3 swollen and ≥ 3 tender joints, CRP level ≥ 0.3 mg/dL despite prior therapy, MRI Spondyloarthritis Research Consortium of Canada score ≥ 3 and BASDAI score ≥ 4; NCT04929210) [47] will aid in further characterizing the role of IL-23p19-subunit inhibitors in treating patients with axPsA.
Conclusions
Patients with axPsA and r-axSpA exhibited differences in genetic markers and serum cytokine levels, supporting the concept that axPsA and r-axSpA may be distinct disorders. Guselkumab demonstrated significant pharmacodynamic effects in patients with axPsA similar to patients with non-axPsA, consistent with observed clinical improvement. These findings may contribute to our understanding of the potential genetic and molecular distinctions between axPsA and r-axSpA and will be further evaluated in the context of anticipated clinical trial data that could inform treatment paradigms in the future.
References
Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14:363–71.
Gottlieb AB, Merola JF. Axial psoriatic arthritis: an update for dermatologists. J Am Acad Dermatol. 2021;84:92–101.
McVeigh CM, Cairns AP. Diagnosis and management of ankylosing spondylitis. BMJ. 2006;333:581–5.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8.
Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021;3:E715–23.
Mease P, Helliwell PS, Gladman D, et al. Effect of guselkumab (TREMFYA®), a selective IL-23p19 inhibitor, on axial-related endpoints in patients with active PsA: results from a phase 3, randomized, double-blind, placebo-controlled study through 2 years [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-guselkumab-tremfya-a-selective-il-23p19-inhibitor-on-axial-related-endpoints-in-patients-with-active-psa-results-from-a-phase-3-randomized-double-blind-placebo-controlled-study/.
Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000–6.
Kavanaugh A, Puig L, Gottlieb AB, et al. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res (Hoboken). 2015;67:1739–49.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9.
Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75:1984–8.
Poddubnyy D, Hermann KG, Callhoff J, Listing J, Sieper J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis. 2014;73:817–23.
Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019;71:258–70.
Baeten D, Ostergaard M, Wei JC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77:1295–302.
Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–25.
Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–36.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91.
Huang YW, Tsai TF. HLA-Cw1 and psoriasis. Am J Clin Dermatol. 2021;22:339–47.
Buchkovich ML, Brown CC, Robasky K, et al. HLAProfiler utilizes k-mer profiles to improve HLA calling accuracy for rare and common alleles in RNA-seq data. Genome Med. 2017;9:86.
Verma R, Balakrishnan L, Sharma K, et al. A network map of interleukin-10 signaling pathway. J Cell Commun Signal. 2016;10:61–7.
Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218.
Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14:7.
Siebert S, Sweet KM, Ritchlin CT, et al. Guselkumab treatment modulates core psoriatic arthritis gene expression in two phase 3 clinical trials (DISCOVER-1 and -2). Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-eular.479.
Guo B. IL-10 modulates Th17 pathogenicity during autoimmune diseases. J Clin Cell Immunol. 2016;7:400.
Chimenti MS, Perricone C, D’Antonio A, et al. Genetics, epigenetics, and gender impact in axial-spondyloarthritis susceptibility: an update on genetic polymorphisms and their sex related associations. Front Genet. 2021;12: 671976.
Machado P, Landewe R, Braun J, et al. Ankylosing spondylitis patients with and without psoriasis do not differ in disease phenotype. Ann Rheum Dis. 2013;72:1104–7.
Feld J, Ye JY, Chandran V, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford). 2020;59:1340–6.
Eder L, Chandran V, Gladman DD. What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol. 2015;27:91–8.
Reveille JD, Zhou X, Lee M, et al. HLA class I and II alleles in susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2019;78:66–73.
Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–48.
Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80:582–90.
McGonagle D, Watad A, Sharif K, Bridgewood C. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front Immunol. 2021;12: 614255.
van Tok MN, Satumtira N, Dorris M, et al. Innate immune activation can trigger experimental spondyloarthritis in HLA-B27/Hubeta2m transgenic rats. Front Immunol. 2017;8:920.
Sveaas SH, Berg IJ, Provan SA, et al. Circulating levels of inflammatory cytokines and cytokine receptors in patients with ankylosing spondylitis: a cross-sectional comparative study. Scand J Rheumatol. 2015;44:118–24.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56.
Chen WS, Chang YS, Lin KC, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc. 2012;75:303–8.
Deveci H, Turk AC, Ozmen ZC, Demir AK, Say Coskun SU. Biological and genetic evaluation of IL-23/IL-17 pathway in ankylosing spondylitis patients. Cent Eur J Immunol. 2019;44:433–9.
Kruithof E, Baeten D, De Rycke L, et al. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther. 2005;7:R569–80.
von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–8.
Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496–502.
Rutz S, Ouyang W. Regulation of interleukin-10 expression. Adv Exp Med Biol. 2016;941:89–116.
Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217:e20190418.
Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27.
Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55:379–90.
Gokmen F, Akbal A, Resorlu H, et al. Neutrophil-lymphocyte ratio connected to treatment options and inflammation markers of ankylosing spondylitis. J Clin Lab Anal. 2015;29:294–8.
Al-Osami MH, Awadh NI, Khalid KB, Awadh AI. Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with ankylosing spondylitis: a case-control study. Adv Rheumatol. 2020;60:13.
Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43:305–10.
Gladman DD, Mease PJ, Bird P, et al. Efficacy and safety of guselkumab in biologic-naïve patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial. Trials. 2022;23:743.
Acknowledgements
The authors wish to acknowledge Michelle Miron, PhD, of Janssen Research & Development for her contributions to these analyses.
Funding
The studies providing data for these analyses were funded by Janssen Research & Development, LLC, Spring House, PA, USA. The journal's Rapid Service and Open Access Fees for this manuscript were funded by Janssen Scientific Affairs, LLC, Horsham, PA, USA.
Medical Writing Assistance
Medical writing support was provided by Miranda Tradewell, PhD, of Lumanity Communications Inc,. under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med, 2022;175:1298-304) and was funded by Janssen Scientific Affairs, LLC, Horsham, PA, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception and design of analyses, commented on versions of the manuscript, and read and approved the final manuscript.
Disclosures
Arthur Kavanaugh discloses consulting fees from AbbVie, Amgen, BMS, Eli Lilly, Genentech, Janssen, Merck, Novartis, Pfizer, and UCB. Xenofon Baraliakos discloses consulting fees, grant/research support, and speakers bureau support from AbbVie, Biocad, Chugai, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche, and UCB. Sheng Gao, Warner Chen, Kristen Sweet, and Qingxuan Song are employees of Janssen Research & Development, LLC, and own stock or stock options in Johnson & Johnson, of which Janssen Research & Development, LLC is a wholly owned subsidiary. Soumya D. Chakravarty is an employee of Janssen Scientific Affairs, LLC, and owns stock or stock options in Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary. May Shawi is an employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies, a wholly owned subsidiary of Johnson & Johnson, and owns stock in Johnson & Johnson. Proton Rahman discloses consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB; travel support from Janssen; and grant/research support from Janssen and Novartis.
Compliance with Ethics Guidelines
The four phase 3 studies were conducted in accordance with the Declaration of Helsinki. The protocols were approved by the institutional review board or ethics committee at each site [12, 14, 15]. All patients gave written informed consent, with an additional consent provided for voluntary genetic testing (HLA). An independent data monitoring committee regularly reviewed unblinded safety data.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted in this site, requests for access to the study data can be submitted through the Yale Open Data Access project site at http://yoda.yale.edu.
Prior Presentation
These data were previously presented in part at the Maui Derm 2022 Conference, January 24–28, 2022, Grand Waileu, Maui, Hawaii.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kavanaugh, A., Baraliakos, X., Gao, S. et al. Genetic and Molecular Distinctions Between Axial Psoriatic Arthritis and Radiographic Axial Spondyloarthritis: Post Hoc Analyses from Four Phase 3 Clinical Trials. Adv Ther 40, 2439–2456 (2023). https://doi.org/10.1007/s12325-023-02475-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02475-4