Abstract
Background
Axial involvement constitutes a specific domain of psoriatic arthritis (PsA). Interleukin (IL)-23 inhibitors have demonstrated improvement in axial PsA (axPsA) symptoms, but have not shown efficacy in treating ankylosing spondylitis (AS), suggesting differences in axPsA processes and treatments. In a post hoc, pooled analysis of patients with investigator- and imaging-confirmed sacroiliitis in two phase 3, randomized, placebo-controlled studies (DISCOVER-1 and DISCOVER-2), patients treated with guselkumab, an IL-23p19 inhibitor, had greater axial symptom improvements compared with placebo. Confirmatory imaging at baseline was restricted to the sacroiliac (SI) joints, occurred prior to/at screening, and was locally read.
Methods
The STAR study will prospectively assess efficacy outcomes in PsA patients with magnetic resonance imaging (MRI)-confirmed axial inflammation. Eligible, biologic-naïve patients with PsA (N = 405) for ≥ 6 months and active disease (≥ 3 swollen and ≥ 3 tender joints, C-reactive protein [CRP] ≥ 0.3 mg/dL) despite prior non-biologic disease-modifying antirheumatic drugs, apremilast, and/or nonsteroidal anti-inflammatory drugs will be randomized (1:1:1) to guselkumab every 4 weeks (Q4W); guselkumab at week (W) 0, W4, then every 8 weeks (Q8W); or placebo with crossover to guselkumab at W24, W28, then Q8W. Patients will have Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score ≥ 4, spinal pain component score (0–10 visual analog scale) ≥ 4, and screening MRI-confirmed axial involvement (positive spine and/or SI joints according to centrally read Spondyloarthritis Research Consortium of Canada [SPARCC] score ≥ 3 in ≥ 1 region). The primary endpoint is mean change from baseline in BASDAI at W24; multiplicity controlled secondary endpoints at W24 include AS Disease Activity Score employing CRP (ASDAS), Disease Activity Index for PsA (DAPSA), Health Assessment Questionnaire – Disability Index (HAQ-DI), Investigator’s Global Assessment of skin disease (IGA), and mean changes from baseline in MRI SI joint SPARCC scores. Centrally read MRIs of spine and SI joints (scored using SPARCC) will be obtained at W0, W24, and W52, with readers blinded to treatment group and timepoint. Treatment group comparisons will be performed using a Cochran-Mantel-Haenszel or chi-square test for binary endpoints and analysis of covariance, mixed model for repeated measures, or constrained longitudinal data analysis for continuous endpoints.
Discussion
This study will evaluate the ability of guselkumab to reduce both axial symptoms and inflammation in patients with active PsA.
Trial registration
This trial was registered at ClinicalTrials.gov, NCT04929210, on 18 June 2021.
Protocol version: Version 1.0 dated 14 April 2021.
Similar content being viewed by others
Background
Substantial proportions of patients with psoriatic arthritis (PsA) develop inflammation of the sacroiliac (SI) joints and/or spine, both early (5–28%) and particularly later (25–70%) in the disease process; thus, axial PsA (axPsA) constitutes an important disease domain in PsA [1,2,3,4,5,6,7,8,9]. While axial involvement is considered part of the spectrum of axial spondyloarthritis (axSpA), where ankylosing spondylitis (AS) is the prototypical presentation, a large body of literature suggests that axial involvement in patients with PsA may be a distinct presentation from AS or axSpA, with differing clinical manifestations, genetic markers, and radiographic findings [8, 10]. About 20% of patients with PsA have the major histocompatibility class one surface antigen human leukocyte antigen (HLA)-B27, which has been associated with axial involvement and more severe disease [11]. Axial PsA has also been associated with HLA-B08, B38, and B39 [12]. Axial involvement has been associated with significantly worse disease across multiple clinical measures, including more severe skin manifestations, worse nail psoriasis, higher likelihood of enthesitis, higher tender joint counts, and lower likelihood of achieving minimal disease activity (MDA) [13].
To date, only one dedicated prospective randomized clinical trial has evaluated axPsA using imaging assessments in a subset of patients. The phase 3b study evaluated the efficacy and safety of secukinumab, an inhibitor of interleukin (IL)-17A, a downstream effector cytokine in the IL-23/Th17 pathway implicated in the pathogenesis of PsA [14]. A separate analysis (post-hoc) from two phase 3 multicenter, double-blind, placebo-controlled studies demonstrated that ustekinumab, an anti-IL-12/IL-23 monoclonal antibody, showed significant improvements in axial signs and symptoms of PsA [9]. However, these findings have not been replicated among patients with AS, which further suggests differential disease processes for axial involvement [15].
Guselkumab, an IL-23 inhibitor that specifically binds the p19 subunit, has been approved for the treatment of adults with active PsA worldwide, based on two phase 3 studies, DISCOVER-1 (NCT03162796) and DISCOVER-2 (NCT03158285), conducted in patients with active PsA [16, 17]. A post hoc analysis of a DISCOVER-1 and DISCOVER-2 study subset of PsA patients with investigator-confirmed sacroiliitis (radiograph or magnetic resonance imaging [MRI]) showed significant and robust improvement in axial symptoms of PsA with both guselkumab 100 mg every 4 weeks (Q4W) and every 8 weeks (Q8W) treatment, as assessed by mean changes from baseline in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and AS Disease Activity Score employing C-reactive protein (CRP) (ASDAS) and by achievement of ≥50% improvement in BASDAI (BASDAI50) and ASDAS responses [18]. In these studies, confirmatory imaging at baseline was restricted to the SI joints, occurred prior to or at screening as confirmed by the investigator, and was locally read.
MRI of the SI joints and spine are the only feasible instrument to objectively measure treatment efficacy on the target organ in axPsA patients. Thus, the STAR study will prospectively evaluate the efficacy and safety of both guselkumab Q4W and Q8W in biologic-naive PsA patients with axial involvement confirmed by centrally read imaging (Additional file 1). Improvements in clinical axial symptoms and objective reduction in axial inflammation of the spine and the SI joints using MRI will be assessed. Although previous studies have demonstrated the efficacy of guselkumab in adults with active PsA, a placebo control was selected for STAR to establish the effects of guselkumab in this subpopulation of PsA patients with axial disease. The placebo selected for this study is identical in appearance to guselkumab.
Methods
This is a randomized, double-blind, placebo-controlled, parallel, multicenter, interventional study in biologic-naive patients with axPsA (Fig. 1; Standard protocol items: recommendation for interventional trials (SPIRIT) checklist is provided as Additional file 2 ). Patients will be recruited at private clinics and hospitals across global regions including Asia, Australia, Europe, North America, and South America. A listing of study sites can be found at https://clinicaltrials.gov/ct2/show/NCT04929210?term=CNTO1959PSA4002&draw=2&rank=1.
This study protocol follows the SPIRIT reporting guidelines [19].
Objectives
An overview of objectives and endpoints is provided in Table 1. The primary study objective is to evaluate the efficacy of guselkumab treatment in patients with active axPsA in reducing axial symptoms assessed through the primary endpoint of change from baseline in BASDAI at week 24 (Table 1). The major secondary objectives are to evaluate the efficacy of guselkumab in treating axial symptoms utilizing additional outcome measures, including reduction in axial inflammation as assessed by MRI of the spine and/or SI joints, other signs and symptoms of PsA including skin psoriasis, and patient-reported outcomes. Among the major secondary endpoints are the changes from baseline in Spondyloarthritis Research Consortium of Canada (SPARCC) score for MRI SI joints and MRI spine at week 24 (for patients with positive MRI of the SI joints and spine, respectively, at baseline; Table 1). Efficacy will be evaluated through 1 year. Additional objectives include evaluating the safety of guselkumab in patients with axPsA through week 60, as well as the pharmacokinetics (PK) and immunogenicity of guselkumab in these patients. Patients will be monitored for adverse events (AEs) throughout the study.
The statistical analysis plan includes database locks at weeks 24 and 60. Primary and major secondary outcomes will be evaluated using data from the week 24 database lock. Post-week 24 data will subsequently be analyzed to examine the maintenance and trajectory of response through 1 year.
Serum samples will be collected at regular intervals for PK and immunogenicity assessments. Some HLA-B alleles have been shown to confer higher risk of developing axial involvement in patients with PsA; therefore, the HLA-B allele status will be determined for all patients. HLA-B27 status, associated with increased risk for developing axial PsA early in the disease course, will be used for stratification purposes in statistical analyses.
Biomarker assessments will be made to examine the biologic response to treatment and to identify biomarkers that are relevant to guselkumab treatment and/or PsA, where local regulations permit. Assessments (detailed below) will include the evaluation of relevant biomarkers in serum, plasma, and whole blood collected.
Assessments
Disease activity will be assessed using the BASDAI. The BASDAI, scored from 0–10, is a patient-reported instrument evaluating the following six symptoms on a visual analog scale (VAS) (0–10 cm, 0 = none, 10 = very severe): fatigue, spinal pain, peripheral joint pain, pain at entheseal sites, severity of morning stiffness, and duration of morning stiffness [20, 21].
The ASDAS is a composite instrument, originally developed for patients with AS, that includes measures of back pain, duration of morning stiffness, patient global assessment, peripheral pain and swelling, and CRP [22]. The results of the post-baseline CRP measurements performed by the central laboratory will be blinded to the investigative sites. PsA disease activity will be assessed using the Disease Activity Index for Psoriatic Arthritis (DAPSA) [23], and the Investigator’s Global Assessment (IGA) [24] documents the investigator’s assessment of the patient’s psoriasis at a given timepoint. The functional status of the patient will be assessed by the Health Assessment Questionnaire-Disability Index (HAQ-DI) [25].
MRIs will be utilized to objectively assess reduction in axial inflammation. Centrally read MRIs of the spine and SI joints will be obtained at week 0, week 24, and week 52, with readers blinded to treatment group and timepoint. MRI scoring methods are summarized in Table 2. The SPARCC scoring system for MRI spine will be applied to the discovertebral unit, which is defined as the region between 2 imaginary lines drawn through the middle of adjacent vertebrae and including adjacent vertebral end plates with the intervening disc. All 23 discovertebral units are scored to yield the Spine Total score. Each MRI lesion is assessed on 3 consecutive sagittal slices, with additional points for “depth” and high “intensity” of the lesion [26, 27]. For the SI joint (among the patients with a positive MRI of the SI joint at baseline), the SPARCC method focuses on the cartilaginous portion of the SI joint, and documents presence (score of 1) versus absence (score of 0) of bone marrow edema in each SI joint quadrant (defined according to a vertical axis through the joint cavity and a horizontal axis bisecting this line at its midpoint) in each of 6 consecutive semicoronal slices and adds points for depth and intensity [26].
As exploratory assessments, MRIs will also be evaluated using the Canada-Denmark (CAN-DEN) score for spine MRI and the Outcome Measures in Rheumatology (OMERACT) Psoriatic Arthritis MRI Scoring System (PsAMRIS) score for MRI of the hands [30] and feet [31]. The CAN-DEN MRI spine scoring system evaluates inflammation, fat, bone erosion, and new bone formation of the spine in patients with spondyloarthritis. This system permits a detailed description of the involvement of different spinal structures, various topographic parts of the vertebral bodies, the facet joints, the spinous processes, the transverse processes, and the ribs and soft tissue. The system is designed for assessment of the individual types of MRI lesions and for acquiring total scores for the different types of lesions (inflammation, fat, erosion, and new bone formation). The OMERACT PsAMRIS score assesses MRI features (synovitis, tenosynovitis, periarticular inflammation, bone edema, bone erosion, and bone proliferation) in the hands and feet of PsA patients [29,30,31]. For this study, the investigator will select the most inflamed hand and the most inflamed foot; the selected hand and foot will be assessed by MRI at baseline, week 24, and week 52. MRI review for CAN-DEN and PsAMRIS will be undertaken by trained readers who have undergone prior calibration and will be blinded to the clinical data. Two readers are planned, with the utilization of a blinded adjudication reader for results that show a discrepancy between the two independent central readers during the screening and treatment phases.
Blood samples for genetic testing will be obtained from patients who provide additional consent. Samples will be collected before study intervention administration at visits when a study intervention administration is scheduled and will be used for pharmacogenomic analysis.
Guselkumab safety will be assessed through the frequency and type of AEs, serious adverse events (SAEs), AEs leading to discontinuation of study intervention, infections, and injection-site reactions. Malignancies and major adverse cardiovascular events will also be summarized. Adverse events will be reported by the patient (or, when appropriate, by a caregiver, surrogate, or the patient's legally acceptable representative) for the duration of the study.
Study population
The target study population is patients with active, MRI-confirmed axPsA of the spine and/or SI joints. The planned enrolment is 135 patients per intervention group, for a total of 405 patients. To detect differences between each guselkumab group and placebo for the primary endpoint of change from baseline in BASDAI score at week 24, assuming a 2-sided alpha level of 0.05 and a power of > 99%, a sample size of 135 patients per treatment group was determined. Power calculations were performed utilizing a 2-sample T-test assuming equal variance for BASDAI change with mean (standard deviation [SD]) of − 1.28 (2.24), − 2.61 (2.47), and − 2.51 (2.00) for placebo, guselkumab 100 mg Q8W, and guselkumab 100 Q4W, respectively, based on observed mean changes from baseline in the DISCOVER 1 and 2 studies (data on file). The larger of the standard deviations between the guselkumab 100mg Q8W/Q4W and placebo groups was used, and the estimated effect sizes are 1.23 and 1.33 for guselkumab 100 mg Q4W and Q8W, respectively. No adjustments were made for multiplicity in the sample size calculations. Methods of patient recruitment will include referral networks, site patient databases, posters in hospitals and waiting rooms, and advertising.
Patient screening will be performed by the study investigator. Patients will be eligible for STAR if they have a diagnosis of PsA for ≥ 6 months, meet ClASsification criteria for Psoriatic ARthritis (CASPAR), and have active disease (≥ 3 swollen joints, ≥ 3 tender joints, and CRP ≥ 0.3 mg/dL), despite standard therapies (i.e., conventional synthetic disease-modifying anti-rheumatic drugs [csDMARDs], non-steroidal anti-inflammatory drugs [NSAIDs], or apremilast). A BASDAI score of ≥ 4, spinal pain component score ≥ 4, and MRI-confirmed axPsA (positive MRI spine and/or SI joints, defined as a SPARCC score ≥ 3 in either the spine and/or SI joints) are also required for study entry. Because there is currently no consensus defining MRI-confirmed axPsA, a SPARCC cutoff of ≥ 3 was selected by Steering Committee consensus. This was based on the established use of positive MRI to confirm axSpA [32], where the standard SPARCC cutoff is between ≥ 2 and ≥ 5 for the SI joints and ≥ 4 for the spine [33, 34]. A cutoff of ≥ 3 in either the spine and/or SI joints was judged to be adequately sensitive to the early signs of inflammation that distinguish axPsA.
Patients must also have current (plaque ≥ 2 cm) or a documented history of psoriasis. Patients with other inflammatory diseases and patients with any prior biologic DMARD or Janus kinase (JAK) inhibitor therapy, as well as patients who have received apremilast within 4 weeks of study intervention, are not eligible. Concomitant use of stable doses of NSAIDs (≥ 2 weeks prior to first study agent administration); oral corticosteroids (equivalent to ≤ 10mg of prednisone/day for ≥ 2 weeks prior to first study agent administration); and one csDMARD, limited to methotrexate (MTX) (≤ 25 mg/week), sulfasalazine (≤ 3g/day), hydroxychloroquine (≤ 400 mg/day), and leflunomide (≤ 20 mg/day), will be permitted. Other key inclusion and exclusion criteria are provided in Table 3.
This study will be conducted in accordance with principles that originated in the Declaration of Helsinki, current International Conference on Harmonisation and Good Clinical Practice (GCP) guidelines, applicable regulatory requirements, and sponsor policy. The protocol and any modifications will be approved by the Institutional Review Board or Ethics Committee at each site and by local Health Authorities for each participating country. Investigators at each study site will collect written informed consent from all patients, with additional consent provided for voluntary genetic testing, prior to the conduct of any study-related procedures.
Randomization and blinding
Central randomization will be implemented in this study to minimize bias in the assignment of patients to intervention groups, to increase the likelihood that known and unknown patient attributes (e.g., demographic and baseline characteristics) are evenly balanced across intervention groups, and to enhance the validity of statistical comparisons across intervention groups. Patients will be randomly assigned 1:1:1 to 1 of 3 intervention groups (guselkumab Q4W, guselkumab Q8W, or placebo with crossover to guselkumab Q8W at week 24) utilizing a computer-generated randomization schedule prepared before the study by or under the supervision of the sponsor. The interactive web response system will assign a unique intervention code, which will dictate the intervention assignment and matching intervention kit for the patient, who will be enrolled by the investigator at each study site. The randomization will be balanced by using randomly permuted blocks and will be stratified by csDMARD use (yes/no) and MRI peripheral (hands/feet) sub-study consent (yes/no). Of note, the MRI peripheral (hands/feet) sub-study will not be used in statistical analyses as a stratification factor; its sole purpose will be to balance the number of patients being assessed for MRI peripheral (hands/feet) among the 3 treatment groups.
Patients will receive a subcutaneous (SC) injection of guselkumab or placebo from the investigator at the study site at weeks 0 and 4. Beginning at week 8, the patient may administer study intervention at the study site under supervision. At week 32 and thereafter, patients may administer study intervention at home and on-site. The investigator will maintain a record of all study intervention dispensed to and returned by patients for home administration. Blinded intervention will be used to reduce potential bias during data collection and evaluation of clinical endpoints. To maintain the study blind, study intervention containers will be labeled only with the study name, intervention number, reference number, and storage instructions, and will not identify the study intervention itself. Data that may potentially unblind the intervention assignment (i.e., study intervention serum concentrations, anti-guselkumab antibody levels) will be handled with special care to ensure that the integrity of the blind is maintained and the potential for bias is minimized. The investigator will not be provided with patient randomization codes.
An independent joint assessor (IJA) will be designated at each study site to perform joint assessments (swollen and tender joint counts), as well as evaluations of enthesitis and dactylitis, and will be blinded to patient data. The IJA will have no other contact (other than joint assessments) with the patient once the patient is randomized, will not be the treating physician, will not discuss the patient’s clinical status with the patient or other site personnel during the joint assessment, and will not be permitted to review the patients’ medical records or the electronic case report form (eCRF) or any of the previous joint assessments.
At the week 24 database lock, the data will be unblinded to a limited number of sponsor personnel for analysis of the primary and major secondary endpoints (see Table 1) while patients are still participating in the study. Identification of sponsor personnel who will have access to the unblinded patient-level data will be documented prior to unblinding. Steering committee members, including Janssen employees, will not be unblinded prior to final database lock. No interim analyses are planned. Investigative study sites and patients will remain blinded to initial treatment assignment until after the final database is locked. Under normal circumstances, the blind should not be broken until all patients have completed the study and the database is finalized. The investigator, may in a medical emergency, determine the identity of the intervention by contacting the interactive web response system.
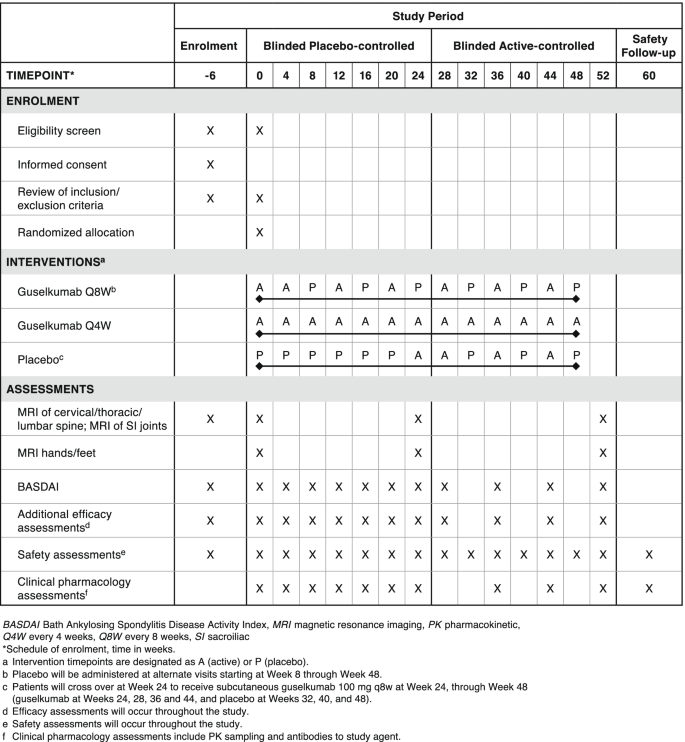
Study design
A target of 405 patients will be randomly assigned (1:1:1) in this study, with 135 patients planned per intervention group: SC guselkumab 100 mg Q4W, SC guselkumab 100 mg at week 0. week 4 and Q8W, or SC placebo with crossover at week 24 to SC guselkumab 100 mg Q8W (Fig. 2). Patients who meet early escape criteria (< 10% improvement from baseline in total back pain, for the purpose of this study assessed using BASDAI Question #2 and in morning stiffness measures as assessed by BASDAI Questions #5 and 6) at both week 12 and week 16 will be allowed to initiate or increase the dose of one permitted concomitant medication, up to the maximum allowed dose, at the investigator’s discretion.
STAR study schema. Refer to Fig. 1 for study agent administration and dosing details. The asterisk (*) symbol indicates the following: 12-week safety follow-up (F/U) period begins at W48 after final study drug administration. EE, early escape; F/U, follow-up; GUS, guselkumab; MRI, magnetic resonance imaging; PBO, placebo; PE, primary endpoint; Q4W, every 4 weeks; Q8W, every 8 weeks; R, randomization; SC, subcutaneous; SI, sacroiliac; W, week
The study comprises a screening phase of up to 6 weeks, a treatment phase of approximately 1 year that will include a placebo-controlled period from week 0 to week 24 and an active-controlled treatment period from week 24 to week 52 (last administration at week 48), and safety follow-up at week 60 (approximately 12 weeks after the last intended dose at week 48 per protocol).
Intervention
Overall, the two guselkumab dose regimens demonstrated clinically meaningful efficacy and were well-tolerated with an acceptable safety profile in patients with active PsA in DISCOVER-1 and DISCOVER-2. This study is expected to provide additional clinical safety and efficacy data in patients with axPsA. Inclusion of both the 100 mg Q4W and Q8W dosing intervals will allow a relative benefit-risk assessment of both dose regimens.
The placebo control will be used to establish the frequency and magnitude of changes in clinical and imaging endpoints that may occur in the absence of active intervention. While guselkumab has been approved for patients with active PsA in several countries, the use of a placebo control is still necessary in the context of this study because the primary objective is to establish the efficacy of guselkumab for the treatment of axPsA for which there are limited data.
Guselkumab 100 mg and matching liquid placebo for guselkumab will be provided in single-use prefilled syringes assembled with the Ultrasafe PLUSTM Passive Needle Guard. Study intervention should be administered under the supervision of the investigator or a qualified study site personnel. Patients will have the option to self-administer the last 3 doses of the study medication at home. Therefore, drug accountability will be important for adherence.
Patients who discontinue study intervention for any reason will be encouraged to continue in the study by returning for all remaining study visits. For patients who discontinue study intervention prior to week 24, MRIs of the SI joint, spine, and hands and feet (if consented separately) should be performed at the time of study intervention discontinuation. In addition, MRIs should be repeated at week 24 if discontinuation occurs prior to or at week 16. If a patient discontinues study intervention at or after week 24, but prior to the week 52 visit, the final efficacy visit should occur at the time of discontinuation or as soon as possible thereafter and all assessments under the week 52/final efficacy visit should be performed with the exception of study intervention administration. In either scenario, the patients will be instructed to return for a final safety visit to perform assessments under the week 60/final safety visit approximately 12 weeks after the last study intervention administration.
Statistical methods
Descriptive statistics, such as mean, SD, median, interquartile range, minimum, and maximum for continuous variables and counts and percentages for discrete variables, will be used to summarize most efficacy data. For binary response endpoints, treatment comparisons (difference versus placebo with 95% CI) will generally be performed using a chi-square test or a Cochran-Mantel-Haenszel test. For continuous endpoints, treatment comparisons will be performed using an analysis of covariance, a mixed model for repeated measures (MMRM) or a constrained longitudinal data analysis model. In general, statistical testing will be performed using 2-sided tests. The overall type I error of the treatment comparisons of both dose regimens versus placebo for the primary and the 5 selected major secondary endpoints (Table 1) will be controlled at a significance level of ≤ 0.05. The power calculations for the key study endpoints with N = 135 per treatment group and a 1:1:1 randomization ratio are based on a 2-sided significance level of 0.05 using a 2-sample T-test, assuming equal variances for continuous variables and testing 2 proportions using the Z-test with pooled variance for binary variables. Sensitivity analyses will be performed for some endpoints. Treatment group comparisons will not be performed after week 24 when patients in the placebo group will cross over to guselkumab.
The primary endpoint, change from baseline in BASDAI score at week 24, will be analyzed based on the adjusted composite estimand defined by 5 components: population, treatment, variable (endpoints), intercurrent events, and population level summary. In addition to the total BASDAI composite score, change from baseline in spinal pain (Question #2) by visit over time through week 52 will be evaluated independently. The change from baseline in the BASDAI score will be compared between each guselkumab group and the placebo group for all patients and will be carried out on the full analysis set defined by all randomized patients who received at least one partial or full administration of study intervention. The MMRM, which relies on the missing at random (MAR) assumption for the missing data, will be used to test the difference between each guselkumab group and the placebo group. Under the assumption of MAR, missing data will be accounted for through correlation of repeated measures in the model. Explanatory variables of the MMRM model will include treatment group, visit, baseline BASDAI score, csDMARD use (Yes/No), baseline HLA-B27 status (positive/negative), an interaction term of visit with treatment group, and an interaction term of visit with baseline BASDAI score. Treatment difference of change from baseline in BASDAI score at week 24 between each guselkumab group and the placebo group will be provided by the difference in the least squares means (LSmeans). The 95% CI for the differences in LSmeans and p-values will be calculated based on the MMRM. An unstructured covariance matrix for repeated measures within a patient will be used. The F-test will use Kenward-Roger’s approximation for degrees of freedom.
Patients meeting treatment failure criteria, i.e., patients who discontinue study intervention due to any reason except due to study conduct affected by COVID-19, who initiated or increased the dose of csDMARDs or oral corticosteroids from baseline for treatment of PsA, or who initiated protocol prohibited medications/therapies for PsA prior to week 24, will be considered nonresponders for binary endpoints or will be assumed to be MAR for continuous endpoints in the MMRM (except for the MRI endpoint, which will utilize multiple imputation). Through week 24, observed data from patients who discontinue study intervention due to study conduct affected by COVID-19, or who exhibit substantial treatment non-compliance due to study conduct affected by COVID-19, will be assumed to be MAR.
In addition to the primary endpoint, five major secondary endpoints have been identified as important to assess different attributes of the disease, and only these variables will be multiplicity controlled (Table 1). The overall type I error of the treatment comparisons of both guselkumab dosing regimens versus placebo for the primary and the 5 selected major secondary endpoints will be controlled at a significance level of ≤ 0.05. For these pre-specified primary and secondary endpoints, both adjusted and nominal (unadjusted) p-values will be provided. In the instance that an adjusted p-value is not significant, the nominal (unadjusted) p-value must only be interpreted as supportive. All other secondary endpoints will be summarized over time by treatment groups, with treatment comparisons performed by visit through week 24 as detailed in the statistical analysis plan.
Subgroup analyses will be performed to evaluate consistency in the primary efficacy endpoint by demographic characteristics, baseline disease characteristics, and baseline medications. Interaction testing between the subgroups and treatment group will also be provided if appropriate.
For the major secondary endpoints of change from baseline at week 24 in SPARCC score for MRI SI joints and for MRI spine, change from baseline for the outcomes assessed will be calculated among patients with a positive MRI of SI joints and spine, respectively, at baseline. Analyses of other major secondary endpoints will include calculating the proportion of patients achieving a BASDAI50 response, as well as the proportion of patients with IGA 0/1 response at week 24 among the patients with ≥ 3% body surface area psoriatic involvement and an IGA score of ≥ 2 (at least mild) at baseline. The proportions of patients achieving clinically important improvement in ASDAS (change of ≥ 1.1), major improvement in ASDAS (change of ≥ 2.0), ASDAS inactive disease (score < 1.3) [35], and ASDAS low disease activity (score < 2.1) will be calculated [36]. Change from baseline in CAN-DEN score for MRI spine will be assessed among patients with baseline CAN-DEN score ≥ 3.
All safety analyses will be performed using the Safety Population, i.e., all patients who receive ≥ 1 study agent administration. Analyses of AEs used to assess the safety of guselkumab will include the incidence and type of AEs, SAEs, infections, and injection site reactions. Laboratory data will be summarized by type of laboratory test; descriptive statistics will be calculated for selected laboratory analytes at baseline and for observed values and changes from baseline at each scheduled timepoint. Vital signs including pulse/heart rate and blood pressure (systolic and diastolic) will be summarized over time, using descriptive statistics and/or graphically. The proportion of patients with values beyond clinically important limits will be summarized.
Oversight and monitoring
A Trial Steering Committee of independent members has been created for study consultation purposes. Steering committee objectives are to (1) provide practical advice on strategy and direction of the trial; (2) provide clinical expertise and advice on best clinical study parameters (program design, population, endpoints, etc.); (3) participate in data review, analysis, and interpretation of the result from the trial; and (4) guide/suggest important analyses to inform clinical practice. As part of study oversight, sponsor personnel will monitor study site conduct to ensure the protocol and GCP are followed.
Frequency and procedures for auditing trial conduct
To ensure accuracy and reliability of data, qualified investigators and appropriate study sites have been selected for this study. Protocol procedures and eCRF guidelines have been reviewed with investigators and study site personnel, and clinical laboratory data will be transmitted directly to the sponsor’s database and verified for accuracy and consistency. Representatives of the sponsor’s clinical quality assurance department may visit the study site at any time during or after completion of the study to conduct an audit of the study in compliance with regulatory guidelines and company policy to review study records. The sponsor will also review the eCRF for accuracy and completeness, and discrepancies will be resolved with the appropriate investigator.
Patient privacy guidelines and applicable laws will be adhered to. Similar auditing procedures may also be conducted by agents of any regulatory body, either as part of a national GCP compliance program or to review the results of this study in support of a regulatory submission.
Discussion
A post hoc analysis of pooled data from the Phase 3 DISCOVER-1 and DISCOVER-2 studies indicated that treatment with guselkumab improved axial symptoms in patients with PsA who had investigator and imaging-confirmed sacroiliitis. STAR, a phase 4, prospective, multicenter, randomized clinical trial, will now allow for an in-depth evaluation of the efficacy and safety of selectively inhibiting the IL-23p19 subunit with guselkumab in patients with MRI-confirmed axPsA. MRIs of the SI joints and spine in STAR will be centrally read, with readers blinded to treatment group and timepoint, using methods specifically designed to assess axial inflammation.
AxPsA as a unique presentation is supported by differing responses among patients with axSpA to biological agents that target the IL-23/IL-17 axis. Despite the efficacy of IL-12/23 and IL-23 inhibitors in PsA [9, 16,17,18] and the efficacy of IL-17 inhibitors in PsA and axSpA [37, 38], a trial of an IL-23 inhibitor, risankizumab, in AS demonstrated negative results [39]. Additionally, cumulative evidence from three phase 3 placebo-controlled trials of patients with axSpA showed that patients treated with the anti-IL-12/23p40 monoclonal antibody ustekinumab did not achieve clinically meaningful improvement across key efficacy endpoints when compared with placebo [15]. In contrast, significant improvements in axial signs and symptoms of PsA were demonstrated in two phase 3, multicenter, double-blind, placebo-controlled studies that evaluated the efficacy and safety of ustekinumab [9]. A subsequent analysis from these same PsA studies, which focused on spondylitis-related endpoints in tumor necrosis factor inhibitor (TNFi)-naïve patients with peripheral arthritis and physician reported-spondylitis, found that ustekinumab demonstrated clinically meaningful changes across BASDAI measures of neck/back/hip pain, as well as the modified BASDAI (omission of Question #3, peripheral joint pain or swelling), when compared with placebo. From this, it was concluded that ustekinumab has the potential to improve disease activity in TNFi-naïve PsA patients with axial involvement [40]. The pathophysiology of AS and axPsA might indicate two different diseases. There has, for example, been discussion of biologic mechanisms in the spine that differ from peripheral joints and entheses, such as IL-23-independent production of IL-17 [41, 42]. Additionally, a recent analysis of AS patients receiving ustekinumab and axPsA patients receiving guselkumab showed that AS and axPsA patients have different genetic risk factors and serum IL-17 levels [43]; differential expression of bone biomarkers between patients with AS and those with axPsA has also been reported in a cohort of cases of PsA without axial arthritis, psoriatic spondyloarthritis, and AS [44].
The lack of a consensus definition for classifying axPsA, as well as validated instruments for assessing response to treatment, represent a significant unmet need. Ongoing initiatives with Assessment of SpondyloArthritis international Society (ASAS) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), including a study of patients with PsA that focuses on inflammatory changes in the axial skeleton as assessed by imaging (radiograph and MRI), may inform efforts to prospectively develop such criteria [45, 46]. Currently, assessments of axial symptoms in patients with PsA rely primarily upon instruments designed originally for patients with AS. For example, in the only existing dedicated prospective randomized clinical trial that has evaluated axPsA using imaging assessments, biologic-naïve adults with PsA were eligible if they showed symptoms of active spinal disease, which were defined as a BASDAI score ≥ 4 and spinal pain score ≥ 40 (0–100 mm VAS) despite NSAID therapy. In part, efficacy was assessed by MRI of the spine and SI joint using Berlin scoring methodology. However, imaging-confirmed axPsA, whether by radiograph or MRI, was not among the study inclusion criteria. While results indicated significant improvement across imaging endpoints, lack of agreement surrounding the imaging criteria used to define axPsA, as well as the limited translatability of criteria used for AS in assessing axPsA, were noted as study limitations. The absence of a definition for axPsA, including imaging criteria, was also noted as a limitation in another study that evaluated the efficacy of secukinumab, a monoclonal antibody that directly inhibits IL-17A, in patients with PsA, where exploratory analyses of ASAS and BASDAI responses were not significantly influenced by MRI status at baseline [14]. Thus, a better understanding of axPsA is needed, as are effective therapies to improve axial symptoms and reduce axial inflammation as objectively assessed by imaging.
Notably, the present study introduces new methods of MRI assessment that can advance the understanding of how imaging is used to evaluate axPsA. The imaging needs of the present study required using estimations based on prior axSpA studies [32,33,34] to develop a SPARCC scoring method for MRI that would best capture the inflammation levels specific to axial involvement. Additionally, this study will be the first time that CAN-DEN, traditionally used for patients with spondyloarthritis, will be used to assess axPsA. With the unique application of these scoring methods, the results of the present study will offer valuable information about the classification and outcome measures of axPsA.
Efforts to define disease and outcomes in axPsA, and to advance treatment recommendations, are ongoing [8, 10, 40, 46,47,48]. Differences in the etiology of axial inflammation between axSpA and PsA might require a different treatment approach; thus, an increased understanding of axPsA has the potential to improve the treatment options available for patients. Research supports the necessity of SI and spinal imaging in detecting axPsA; however, data surrounding differences in axial involvement using MRI are limited [10]. The STAR study will provide an opportunity to demonstrate that the selective IL-23 inhibitor guselkumab not only significantly relieves the symptoms of axPsA but also decreases axial inflammation as shown by MRI.
Trial status
The first patient was screened on 30 August 2021, and the last patient out is expected on 10 February 2024. Protocol version 1.0, 14 April 2021.
Availability of data and materials
Data will be available according to the data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson (https://www.janssen.com/clinical-trials/transparency). Trial results will be posted online as required by ClinicalTrials.gov, and results will be shared with patients as required by local regulations.
Abbreviations
- AE:
-
Adverse event
- AS:
-
Ankylosing spondylitis
- ASAS:
-
Assessment of SpondyloArthritis international Society
- ASAS40:
-
≥40% improvement in Assessment of SpondyloArthritis international Society response criteria
- ASDAS:
-
AS Disease Activity Score utilizing C-reactive protein
- axPsA:
-
Axial psoriatic arthritis
- axSpA:
-
Axial spondyloarthritides
- BASDAI:
-
Bath Ankylosing Spondylitis Disease Activity Index
- BASDAI50:
-
≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index score
- CAN-DEN:
-
Canada-Denmark
- CASPAR:
-
Classification criteria for Psoriatic ARthritis
- CRP:
-
C-reactive protein
- csDMARD:
-
Conventional synthetic disease-modifying anti-rheumatic drug
- CTCAE 5.0:
-
Common Terminology Criteria for Adverse Events
- DAPSA:
-
Disease Activity Index for Psoriatic Arthritis
- eCRF:
-
Electronic case report form
- EE:
-
Early escape
- F/U:
-
Follow-up
- GCP:
-
Good Clinical Practice
- GRAPPA:
-
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis
- HAQ-DI:
-
Health Assessment Questionnaire – Disability Index
- HLA:
-
Human leukocyte antigen
- ICH:
-
International Conference on Harmonisation
- IGA:
-
Investigator’s global assessment
- IJA:
-
Independent joint assessor
- IL:
-
Interleukin
- IV:
-
Intravenous
- JAK:
-
Janus kinase
- LS:
-
Least squares
- MAR:
-
Missing at random
- MDA:
-
Minimal disease activity
- MMRM:
-
Mixed model for repeated measures
- MRI:
-
Magnetic resonance imaging
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- OMERACT :
-
Outcome Measures in Rheumatology
- PK:
-
Pharmacokinetics
- PsA:
-
Psoriatic arthritis
- PsAMRIS:
-
Psoriatic Arthritis MRI Scoring System
- Q4W:
-
Every 4 weeks
- Q8W:
-
Every 8 weeks
- R:
-
Randomization
- RA:
-
Rheumatoid arthritis
- SAE :
-
Serious adverse event
- SC:
-
Subcutaneous
- SD:
-
Standard deviation
- SI:
-
Sacroiliac
- SPARCC:
-
Spondyloarthritis Research Consortium of Canada
- TNFi:
-
Tumor necrosis factor inhibitor
- VAS:
-
Visual analog scale
References
Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep. 2007;9(6):455–60.
Baraliakos X, Coates LC, Braun J. The involvement of the spine in psoriatic arthritis. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S31–5.
Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–14.
Moghaddassi M, Shahram F, Chams-Davatchi C, Najafizadeh SR, Davatchi F. Different aspects of psoriasis: analysis of 150 Iranian patients. Arch Iran Med. 2009;12(3):279–83.
Coates LC, Conaghan PG, Emery P, Green MJ, Ibrahim G, MacIver H, et al. Sensitivity and specificity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2012;64(10):3150–5.
Niccoli L, Nannini C, Cassara E, Kaloudi O, Susini M, Lenzetti I, et al. Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis. 2012;15(4):414–8.
Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol. 2009;38(4):251–5.
Feld J, Chandran V, Gladman DD. What Is axial psoriatic arthritis? J Rheumatol. 2018;45(12):1611–3.
Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, You Y, Li S, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984–8.
Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford). 2020;59(6):1340–6.
Queiro R, Sarasqueta C, Belzunegui J, Gonzalez C, Figueroa M, Torre-Alonso JC. Psoriatic spondyloarthropathy: a comparative study between HLA-B27 positive and HLA-B27 negative disease. Semin Arthritis Rheum. 2002;31(6):413–8.
Eder L, Chandran V, Pellet F, Shanmugarajah S, Rosen CF, Bull SB, et al. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann Rheum Dis. 2012;71(1):50–5.
Mease PJ, Palmer JB, Liu M, Kavanaugh A, Pandurengan R, Ritchlin CT, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2018;45(10):1389–96.
Baraliakos X, Gossec L, Pournara E, Jeka S, Mera-Varela A, D’Angelo S, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582–90.
Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019;71(2):258–70.
Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–25.
Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–36.
Mease PJ, Helliwell PS, Gladman DD, Poddubnyy D, Baraliakos X, Chakravarty SD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021;3:e715–23.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Eder L, Chandran V, Shen H, Cook RJ, Gladman DD. Is ASDAS better than BASDAI as a measure of disease activity in axial psoriatic arthritis? Ann Rheum Dis. 2010;69(12):2160–4.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–91.
Lukas C, Landewe R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18–24.
Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–8.
Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator's Global Assessment (IGA) Scale: A modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26(1):23–31.
Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45.
Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53(5):703–9.
Maksymowych WP, Dhillon SS, Park R, Salonen D, Inman RD, Lambert RG. Validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging spinal inflammation index: is it necessary to score the entire spine? Arthritis Rheum. 2007;57(3):501–7.
Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53(4):502–9.
Krabbe S, Ostergaard M, Pedersen SJ, Weber U, Krober G, Makysmowych W, et al. Canada-Denmark MRI scoring system of the spine in patients with axial spondyloarthritis: updated definitions, scoring rules and inter-reader reliability in a multiple reader setting. RMD Open. 2019;5(2):e001057.
Ostergaard M, McQueen F, Wiell C, Bird P, Boyesen P, Ejbjerg B, et al. The OMERACT psoriatic arthritis magnetic resonance imaging scoring system (PsAMRIS): definitions of key pathologies, suggested MRI sequences, and preliminary scoring system for PsA Hands. J Rheumatol. 2009;36(8):1816.
Glinatsi D, Bird P, Gandjbakhch F, Mease PJ, Boyesen P, Peterfy CG, et al. Validation of the OMERACT Psoriatic Arthritis Magnetic Resonance Imaging Score (PsAMRIS) for the hand and foot in a randomized placebo-controlled trial. J Rheumatol. 2015;42(12):2473–9.
Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–7.
de Winter J, de Hooge M, van de Sande M, de Jong H, van Hoeven L, de Koning A, et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the assessment of spondyloarthritis international society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 2018;70(7):1042–8.
Maksymowych WP, Lambert RG, Baraliakos X, Weber U, Machado PM, Pedersen SJ, et al. Data-driven definitions for active and structural MRI lesions in the sacroiliac joint in spondyloarthritis and their predictive utility. Rheumatology (Oxford). 2021;60(10):4778–89.
Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47–53.
Machado PM, Landewe R, van der Heijde D, ISAoS. Ankylosing Spondylitis Disease Activity Score (ASDAS): 2018 update of the nomenclature for disease activity states. Ann Rheum Dis. 2018;77(10):1539–40.
Wasilewska A, Winiarska M, Olszewska M, Rudnicka L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol. 2016;33(4):247–52.
Atzeni F, Carriero A, Boccassini L, D’Angelo S. Anti-IL-17 agents in the treatment of axial spondyloarthritis. Immunotargets Ther. 2021;10:141–53.
Baeten D, Østergaard M, Wei JC, Sieper J, Järvinen P, Tam LS, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77(9):1295–302.
Helliwell PS, Gladman DD, Chakravarty SD, Kafka S, Karyekar CS, You Y, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-naive active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020;6(1):e001149.
Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. 2019;78(8):1015–8.
Mease P. Ustekinumab fails to show efficacy in a phase III axial spondyloarthritis program: the importance of negative results. Arthritis Rheumatol. 2019;71(2):179–81.
Kavanaugh A, Baraliakos X, Gao S, Chen W, Sweet K, Chakravarty SD, et al. Genetic and molecular distinctions between axial psoriatic arthritis and ankylosing spondylitis. Maui: Maui Derm for Dermatologists; 2022 Jan 24-28; Maui, HI.
Jadon DR, Nightingale AL, McHugh NJ, Lindsay MA, Korendowych E, Sengupta R. Serum soluble bone turnover biomarkers in psoriatic arthritis and psoriatic spondyloarthropathy. J Rheumatol. 2015;42(1):21–30.
Gladman DD, Helliwell PS, Poddubnyy D, Mease PJ. Updates on axial psoriatic arthritis from the 2020 GRAPPA annual meeting. J Rheumatol Suppl. 2021;97:30–3.
Poddubnyy D, Baraliakos X, Van den Bosch F, Braun J, Coates LC, Chandran V, et al. Axial Involvement in Psoriatic Arthritis cohort (AXIS): the protocol of a joint project of the Assessment of SpondyloArthritis international Society (ASAS) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Ther Adv Musculoskelet Dis. 2021;13:1759720x211057975.
Coates LC. How should we define disease and outcomes in axial psoriatic arthritiis? Lancet Rheumatol. 2021;3(10):e677–e8.
Kavanaugh A, Coates LC, van der Windt DA, Corp N, Soriano ER. GRAPPA Treatment Recommendations: updates and methods. J Rheumatol Suppl. 2020;96:41–5.
Acknowledgements
The authors thank Alexandra Guffey, MS, and Rebecca Clemente, PhD, Janssen Scientific Affairs, LLC, for writing support.
Name and contact information for the trial sponsor
Janssen Scientific Affairs, LLC
Study responsible physician
Evan Leibowitz (eleibowi@its.jnj.com)
Role of sponsor
Authors who were employees of the study sponsor (SDC, MS, SX, STQ, CG, and EL) participated in the study design and protocol development. A medical writer employed by the study sponsor provided writing and editorial support. Employees of the study sponsor, along with members of the study steering committee, will participate in analysis and interpretation of data resulting from this study, and will serve as authors on future publications based on results of this study. All authors will satisfy ICMJE authorship criteria (https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html) and will participate in the decision to approve and submit future reports for publication.
Funding
This study is supported by Janssen Scientific Affairs, LLC.
Author information
Authors and Affiliations
Contributions
Study/protocol design: DDG, PJM, PB, ERS, SDC, MS, SX, STQ, CG, EL, DP, LT, PSH, AK, AD, MØ, XB. Data collection: DDG, PJM, PB, ERS, DP, LT, PSH, AK, AD, MØ, XB. Data analysis: SX. Data interpretation: DDG, PJM, PB, ERS, SDC, MS, SX, STQ, CG, EL, DP, LT, PSH, AK, AD, MØ, XB. Drafting/revising manuscript: DDG, PJM, PB, ERS, SDC, MS, SX, STQ, CG, EL, DP, LT, PSH, AK, AD, MØ, XB. Approval: all authors. Study oversight: CG, EL, SDC.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is being conducted in compliance with the Declaration of Helsinki and International Council for Harmonization Guidelines for Good Clinical Practice. The protocol will be approved by each site’s governing ethical body. Each study patient is required to have provided written informed consent prior to the conduct on any study-related procedures.
Consent for publication
Not applicable.
Competing interests
Dafna D. Gladman received grant support from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB and consulting fees from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Philip J. Mease has received research support from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun Pharma, and UCB; consultant fees from AbbVie, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma, and UCB; speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB. Paul Bird received speaker honoraria from AbbVie, Eli Lilly, Gilead, Janssen, MSD, Pfizer, and UCB; served as advisor for Eli Lilly, Gilead, Janssen, Novartis, and Pfizer. Enrique R. Soriano has served as advisor for AbbVie, Janssen, Novartis, and Roche; has received grant/research support from AbbVie, Janssen, Novartis, Pfizer, Roche, and UCB; has served as speaker/received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB. Soumya D. Chakravarty, Sean T. Quinn, Cinty Gong, and Evan Leibowitz are employees of Janssen Scientific Affairs, LLC, and own stock or stock options in Johnson & Johnson, of which Janssen Scientific Affairs is a wholly owned subsidiary. May Shawi is an employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson and owns stock or stock options in Johnson & Johnson. Stephen Xu is an employee of Janssen Research & Development and owns stock or stock options in Johnson & Johnson, of which Janssen Scientific Affairs is a wholly owned subsidiary. Denis Poddubnyy has received consulting fees from AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Novartis, Pfizer, Roche, and UCB and grants from AbbVie, Eli Lilly, MSD, Novartis, and Pfizer. Lai-Shan Tam has received grant/research support from Amgen, Boehringer Ingelheim, Janssen, GlaxoSmithKline, Novartis, and Pfizer, and has acted as a consultant for AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, Pfizer, and Sanofi. Philip S Helliwell has received speaker payment from AbbVie, Janssen, and Novartis; consulting fees from Eli Lilly, Galapagos, Janssen, and Pfizer. Arthur Kavanaugh has received consulting fees from AbbVie, Amgen, BMS, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer and UCB. Atul Deodhar received consulting fees for participation in Advisory Boards from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; Research Grant funding from AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB; and Speaker fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Mikkel Østergaard received research grants from AbbVie, Bristol Myers Squibb, Celgene, and Novartis, and speaker and/or consultancy fees from AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, Eli-Lilly, Hospira, Janssen, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB. Xenofon Baraliakos has received consulting fees, grant/research support/speaker support from AbbVie, Biocad, Chugai, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche, and UCB.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
STAR Trial Registration Data.
Additional file 2.
STAR Trial SPIRIT Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gladman, D.D., Mease, P.J., Bird, P. et al. Efficacy and safety of guselkumab in biologic-naïve patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial. Trials 23, 743 (2022). https://doi.org/10.1186/s13063-022-06589-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06589-y