Abstract
Introduction
Plaque psoriasis is a chronic skin disease characterised by periods of remission and relapse and associated with considerable burden to patients and healthcare systems. For most patients, standard-of-care is reactive management (RM) with topical therapies, but, more recently, the benefits of proactive management (PAM) have been recognised. This study aimed to gain consensus on real-world use and consumption in RM versus PAM regimens, based on fixed-dose combination calcipotriol and betamethasone dipropionate (Cal/BD) foam which, following a recent update, is currently the only topical therapy for psoriasis with a long-term maintenance regimen in its label.
Methods
The modified-Delphi approach was used to gain insights and consensus on real-world views, use and consumption in RM versus PAM from a panel of dermatologists with experience prescribing Cal/BD foam as PAM. The panel included 16 dermatologists, 4 each from France, Germany, Italy, and Spain, and included two questionnaire rounds and a meeting to obtain final consensus.
Results
The panel agreed that topicals are burdensome to apply in clinical practice and that poor patient adherence, particularly long-term, is a barrier to effective psoriasis management. The panel advised that, as they prescribe a similar number of cans for RM and PAM over a given period, consumption is not a key driver influencing future decisions to prescribe PAM, even in instances where prescribing differences could be observed. Instead, the panel agreed that patient- and disease-related factors better determine patient suitability for PAM.
Conclusion
This modified-Delphi study confirms that prescription of RM or PAM, with Cal/BD foam, is largely driven by patient-related factors and patient involvement is key to optimise outcomes. Real-world experiences captured in this study suggest that a PAM regimen does not increase overall consumption, and thus costs per patient for payers and prescribers, in comparison to RM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In October 2020, the European Medicines Agency (EMA) label for topical calcipotriol and betamethasone dipropionate (Cal/BD) foam was updated to include a long-term maintenance (LTM) treatment regimen for patients with psoriasis who respond favourably to the initial four-week flare treatment (FT) regimen |
A modified-Delphi panel was conducted to explore dermatologists' real-world experiences with, and opinions on consumption of, Cal/BD foam. Views on FT [reactive management (RM)] and LTM [proactive management (PAM)] were explored to provide insights that can be used to inform payer and prescriber discussions regarding the potential impact on consumption-related costs if a dermatologist prescribes PAM rather than RM |
What was learned from the study? |
In real-world clinical practice, dermatologists considered that they prescribe patients with mild-to-moderate psoriasis a similar number of Cal/BD foam cans for RM and PAM over a given period. In instances where there could be a difference (+ or −) in consumption, it was not considered to influence decisions to prescribe RM or PAM |
Treatment decisions in psoriasis, including prescription of RM and PAM with Cal/BD foam, are largely driven by patient-related factors |
These findings support the engagement of patients in treatment decision-making and highlight the importance of assessing patient suitability for PAM in order to improve adherence and optimise the success of long-term treatment in psoriasis |
Introduction
Plaque psoriasis is a chronic skin disease characterised by fluctuating patterns of remission and relapse [1]. Symptoms can include itching, redness, flaking/scaling, pain, burning, bleeding and fatigue, all of which are exacerbated during relapse and with increasing disease severity [2]. Disease severity can be described using the Body Surface Area (BSA) tool, measuring body surface involvement, and the Physicians Global Assessment (PGA) tool, measuring the severity of psoriasis lesions on a 5- or 6-point scale [3,4,5]. Mild disease is defined as BSA < 3% and a PGA score of 2, while moderate disease is defined as BSA 3–10% and a PGA score of 3 [3,4,5]. In Europe, plaque psoriasis affects approximately 1.7–7.9% of the population, whereby 19–65% have mild-to-moderate disease [6, 7]. In addition to the high disease burden, psoriasis is associated with substantial healthcare and indirect costs [8,9,10,11]. In 2016, total costs ranged from US$2077 to $13,132 per patient per year, across France, Germany, Italy, Spain, and the United Kingdom, with direct costs the largest component of total expenditure [8]. Direct costs are also the main driver of the higher costs associated with increased disease severity [8].
For most patients with psoriasis, standard-of-care is reactive management (RM) with topical therapies, which is effective for the short-term control of flares [1, 3]. However, patient adherence to topical treatments is low (40–70%), due to the need for regular application, corticosteroid-phobia, psychological burden of chronic treatment, and topical fatigue [12, 13]. As such, patients often experience relapses and a poor quality of life [10, 14]. Patients who cannot achieve symptomatic control with topicals, or demonstrate continued poor adherence, can be progressed to systemic treatments, including ultraviolet phototherapy, biologics or traditional systemics [3]. However, these more potent therapies are often not indicated for mild disease, due to their associated tolerability issues and increased indirect and direct costs, driven in part by frequent monitoring and testing [8, 15,16,17].
As psoriasis is a burdensome, chronic disease, the rationale and need for long-term control over symptoms is well recognised [13]. Expert consensus’ in Asia and Europe support long-term topical maintenance regimens for the control of disease and prevention of relapse; however, there is limited guidance for their long-term use and adherence remains a key challenge [5, 13, 18, 19]. Moreover, although recent literature has emphasised the need for proactive management (PAM) of psoriasis (also known as long-term maintenance [LTM] treatment), few studies have investigated long-term topical use and there is limited evidence regarding real-world use and costs of PAM regimens [20]. The PSO-LONG trial investigated the efficacy and safety of the fixed-dose combination calcipotriol and betamethasone dipropionate (Cal/BD) foam (Enstilar®, LEO Pharma) as a PAM regimen [4]. Patients who responded well to the initial 4-week flare treatment with once-daily application (RM) were randomised to receive PAM or placebo twice-weekly (including 4 weeks of once-daily rescue treatment with Cal/BD foam) for up to 52 weeks [4]. Patients on PAM had longer time to first relapse, more days in remission, and fewer relapses [4]. In post hoc analyses, these favourable efficacy outcomes were most pertinent in patients with moderate psoriasis [21]. These data support the use of PAM regimens, which in the broader context of psoriasis treatment, may be beneficial in reducing or delaying the need for systemic treatments. In October 2020, the European Medicines Agency (EMA) label for Cal/BD foam was updated to include an LTM regimen of twice-weekly application on 2 non-consecutive days to areas previously affected by psoriasis in patients who respond favourably to flare treatment, assuming no signs of relapse [22]. Following this update, Cal/BD foam is the first topical therapy for psoriasis with LTM (PAM) in its label [20].
Given its approval for both RM and PAM regimens, Cal/BD foam was used as the basis for our discussions, which aimed to gain consensus from dermatologists regarding their experiences prescribing RM versus PAM regimens.
Methods
This study used the Delphi methodology, whereby appropriate panellists complete a series of questionnaires with controlled feedback in order to reach consensus in an area where little evidence is available [23, 24]. A modified-Delphi technique was used with a consensus meeting that allowed group discussion on topics where agreement was not reached during the questionnaire rounds. The approach included two questionnaire rounds and an online consensus meeting and was conducted between October 2021 and March 2022 (Fig. 1).
The outputs of the first questionnaire informed development of the second; in instances where consensus was reached, the relevant questions were omitted from the second round. Where consensus was not reached, questions were reiterated in the second round alongside the median group responses. The consensus meeting provided an opportunity to discuss topics where consensus had not been reached and additional relevant topics raised during the meeting.
Panellists
Dermatologists were recruited via a third-party fieldwork agency using a screening questionnaire (see Table S1 in the supplementary material) to ensure the panel were able to appropriately contribute to discussions regarding their use of PAM. Inclusion required dermatologists to have the following experience: practiced dermatology for > 5 years; currently be treating patients with mild-moderate plaque psoriasis with Cal/BD foam as monotherapy (and be a medium–high prescriber of Cal/BD foam, defined as 31 to > 50 patients every 3 months); treat > 200 patients with mild-moderate plaque psoriasis using topicals every 3 months; currently treating > 5 patients with PAM.
Sixteen dermatologists were recruited to participate in the panel, including 4 dermatologists from each of France, Germany, Italy, and Spain. All 16 completed the questionnaires, and 14/16 attended the consensus meeting (1 panellist from each of France and Spain was unavailable).
Once recruited, panellists were issued a study brief and definitions list (see Tables S2 and S3 in the supplementary material) which outlined the study rationale, aims and methods, their role and responsibilities, and key concepts for consideration throughout the study. Panellist recruitment was conducted by a third-party agency and each panel member was assigned a unique identification number to maintain anonymity throughout the study. All panellists provided informed consent to participate in the study and identifying information was not disclosed to the investigators or sponsor.
Panellist Experience and Patient Caseload
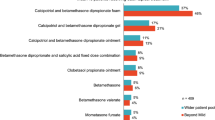
Panellist characteristics and patient caseloads are detailed in Table 1 (market-specific details are in Table S4). Panellists had approximately 380 patients with plaque psoriasis in 3 markets, although in Spain panellists reported a caseload of approximately 600. Approximately 40% of panellists’ patients with plaque psoriasis had moderate psoriasis, while 30% each had mild and severe, across all markets. Panellists’ prescribing patterns by psoriasis disease severity are detailed in Table 2 (market-specific details are in Table S5). For mild psoriasis, topical therapies were usually prescribed, while for moderate psoriasis, several classes were prescribed. Panellists prescribing topical steroid monotherapy suggested approximately half their patients were receiving Cal/BD foam. Panellists reported similar proportions of patients receiving Cal/BD foam as RM and PAM treatment. This confirmed that panellists had suitable experience to discuss real-world use and prescribing habits of RM versus PAM regimens.
Data Collection
Both questionnaires were developed in Microsoft Word, programmed online using SoGoSurvey, and distributed via an email link. Questions were related to panellist characteristics, their patients’ disease characteristics, factors impacting prescribing decisions, advantages/disadvantages of Cal/BD foam, experiences with RM and PAM regimens, and opinions on consumption patterns based on experience in clinical practice (see Tables S6 and S7 in the supplementary material for the questionnaires).
Open questions were used to gain qualitative insights regarding their views, experiences and use of the foam formulation. Closed questions were used to confirm areas of agreement and to identify topics close to agreement or considered unlikely to reach agreement. If two or more panellists reported a new topic in the first round which had not previously been considered, it was incorporated into the second round to understand whether all panellists deemed it important. Panellists were able to change their answers in the second round.
The first and second questionnaires included 38 and 23 questions, respectively, and took approximately 45 min to complete. Reminders were sent to panellists 48 and 24 h ahead of the completion deadline.
The consensus meeting was held on March 23, 2022, and lasted approximately 2 h. This meeting allowed discussion of responses from the surveys and further opportunity to reach group consensus, with the discussion focussing on positive and negative experiences with topicals, and prescribing decisions and consumption of RM versus PAM. The sponsor was not present in the consensus meeting and did not influence discussions or outcomes of the final consensus. The investigator remained blinded to the identities of the panellists.
Data Analysis
The definition of consensus used in the study was: ≥ 80% of panellists rated their “disagreement” between 1 and 3 or their “agreement” between 7 and 9 (on a 9-point scale). A threshold of ≥ 80% was considered suitable for consensus where small sample sizes are used [25]. Whenever no panellist voiced an objection in the consensus meeting, consensus in agreement was established.
Responses to the questionnaires were analysed using the consensus definition and statements that reached consensus were excluded from the subsequent stage(s). For questions with multiple answer options, only options where consensus was not reached were explored in the subsequent stage(s). For open-ended questions, thematic analysis of qualitative responses collected in questionnaires was conducted and key themes were carried forward to subsequent rounds and presented as closed questions. At this stage, ≥ 80% of panellists were required to report the same answer for consensus to be achieved. If panellists could not reach consensus, the statement was either adjusted until consensus was reached, or the panel could agree that no consensus could be achieved.
Compliance with Ethics Guidelines
This article is based solely on the opinions of a group of experts on a specific topic as part of a market research activity. Therefore, for the purpose of this study, no patient data or information were collected and there was no requirement to obtain informed consent. As market research falls outside the remit of the governance arrangements for research ethics committees, in accordance with existing guidelines from the British Healthcare Business Intelligence Association and European Pharmaceutical Market Research Association, ethical committee approval was not required for this study.
Results
Real-World Use and Experience with Topical Treatments
Panel’s Views of Topicals and the Foam Formulation
The panel considered the foam formulation to be a safe and effective psoriasis treatment. The foam was considered to contribute to efficacy due to improved penetration of the ingredients into the skin compared to gel or ointment formulations.
The panel agreed that topical treatments are typically burdensome due to their greasy and messy nature, impractical application, and impact on daily living (e.g. stained clothing and increased frequency of laundry compared with alternative treatments). In line with other topicals, the foam was considered oily/greasy and messy/sticky, and, as with other topical steroids, was associated with a potential risk of irritation.
Drivers of RM and PAM Treatment Decisions
Key drivers for prescribing Cal/BD foam as RM and PAM are listed in Table 3. Particular emphasis was placed on the importance of flare location when determining suitability of RM; patients with flares located in sensitive areas (i.e. face) were deemed less suitable candidates for topical steroids, while those with flares that commonly reappear in the same place were better candidates.
All factors influencing RM prescription, except for ‘flare severity’, ‘flare frequency’ and ‘disease severity’, were considered key determinants of whether to prescribe Cal/BD foam as PAM. The possibility of prolonging patient time on topical treatment was noted as particularly important in driving PAM prescription, especially in patients with milder psoriasis. However, with an evolving treatment landscape, it is harder to define a group of patients unsuitable for systemic treatments, which have historically been viewed as having inferior safety profiles. Given the heterogeneity in clinical presentations across patients with mild-to-moderate plaque psoriasis, characteristics such as flare and disease severity were not classed as key drivers for PAM prescription, but rather factors to be considered as part of a more holistic review of individual patients by which the patient’s overall suitability and motivation for PAM should be assessed. Flare location and frequency should be similarly considered. For example, a patient with regular flare-ups, but of smaller plaques in a less visible location, may not be as motivated to engage with PAM as a patient who has flare-ups less regularly but involving large and visible plaques. Similarly, those with flares in sensitive areas may be less suited to long-term treatment with a topical steroid such as Cal/BD foam due to potential irritation. There was consensus on the importance to take a patient-centred approach to consider each factor during initial prescription, and future re-prescription, for either a RM or PAM regimen.
The panel did not agree or disagree that cost was an independent driver for prescription of RM or PAM, as cost impact is dependent on costs of alternative treatments and whether patients pay for their treatment.
Patient Suitability for PAM
The panel did not consider that there was a particular type of patient usually prescribed PAM vs RM in terms of disease characteristics. When prescribing PAM, disease characteristics are considered alongside a patient’s motivation, understanding of the disease and treatment regimen, and socioeconomic background. The panel agreed that suitability for PAM might be indicated by ‘BSA of ≤ 25%’, ‘Psoriasis Area Severity Index (PASI) score < 5’ and ‘some lesions on elbows/knees’ Footnote 1. However, none are a pre-requisite for PAM prescription and should be considered in relation to other characteristics; for example, if a patient’s BSA of ≤ 25% relates to small and widely distributed plaques, then PAM with the foam formulation may be appropriate. Conversely, those with large, localised lesions may be less suitable.
Patient adherence was agreed as a key determinant of patient suitability for PAM, with patients’ understanding of their disease and the treatment regimen being two key factors. As PAM is intended to manage flares that are already under control following an initial RM regimen, panellists highlighted that patients often feel they no longer need to apply the foam once flares are not visible. Similarly, if flares are less frequent, patients may be less adherent. Patients with mild disease, infrequent flares or complete clearance of skin lesions are therefore typically less suitable for PAM. Patients with plaques on their scalp or hair-bearing areas often struggle with topicals, which were described by panellists as ‘oily’ and ‘messy’; therefore, these patients may also be less suitable for PAM with the foam formulation. The ‘messy’ nature of topicals was reported to further exacerbate poor adherence in patients. Patients who experience improvement in their flares often stop applying topicals due to the burdensome application. Patients’ concerns about long-term steroid use and potential irritation were also reported to challenge adherence to a PAM regimen.
Patients’ experiences with their disease and their treatment were recognised as key considerations when selecting prescription of a PAM regimen. Those with clear skin were considered more likely to adhere to PAM if they had previously experienced flare-ups during non-compliant periods. Importantly, the panel reported that patients’ motivations are prone to fluctuate, thereby highlighting the need for ongoing discussions between dermatologists and patients regarding the most suitable treatment regimen. Moreover, environmental, and sociological factors and stress can contribute to relapse, even in compliant patients, and should be considered when determining treatment regimens.
It was agreed that adherence to PAM is difficult to assess outside a controlled setting and likely varies significantly both within and between markets based on patient-related factors. Nonetheless, the panel agreed that patient adherence and the factors contributing to this are key determinants of patient suitability for PAM.
Real-World Experience with PAM
The panel agreed that, to optimise the therapeutic benefit of PAM, patients should spend 6–12 months on the regimen. However, a more realistic time frame of up to 2–3 months for PAM was agreed, given the poor adherence to topicals in clinical practice. While non-adherence to PAM may be suspected, in clinical practice, panellists would continue to re-prescribe in the hope that patients are appropriately adhering to PAM.
The panel agreed that, when properly adhered to, PAM increases the time between flare-ups, reduces the number of flares observed in each flare period, and increases the total time a patient spends in remission, all of which subsequently improve a patient’s quality of life. The panel therefore reported that these outcomes, and the success of similar regimens in other therapy areas, suggest there is great value in PAM for psoriasis.
Considering stopping rules, a PASI score of < 1 was agreed as a clinical indicator that PAM should be ceased. While a complete clearance of flares and/or a reduction of flares to fewer than four a year might prompt the decision to stop PAM, the panel reiterated that the decision to stop treatment is ultimately a patient’s choice, regardless of clinical outcomes observed. Lack of adherence, patient request, disease remission/control, poor efficacy/lack of flare control, and loss of efficacy over time were all agreed as potential reasons for ceasing use of PAM. The panel agreed that systemic treatment, restarting RM and waiting to assess flares should all be considered for patients following cessation of PAM.
Real-World Consumption Differences Between RM and PAM
Based on their experience in clinical practice, the panel agreed that the number of cans of foam they prescribe for RM and PAM in a given period is within a similar range, and that PAM does not increase consumption. The panel also agreed that, even if the number of cans they prescribe for each regimen could differ (+ or −), it does not influence their decision as to whether to prescribe RM or PAM.
Notably, the panel highlighted that, while dermatologists can check the number of cans that they prescribe each patient as a proxy for consumption, this is not to say patients use all the product they are prescribed or use it correctly for their specified regimen (i.e. using too much or too little foam with the wrong frequency of application), and therefore neither their adherence nor product usage can be confidently determined. The patient and disease characteristics discussed were agreed to impact upon consumption, given their influence over patient adherence and real-world use. The number of cans prescribed is therefore heavily dependent on the patient, and decisions regarding whether to opt for RM or PAM are largely driven by patient-related factors and preference.
Discussion
This modified-Delphi panel explored use, experience and consumption of Cal/BD foam based on insights from dermatologists across France, Germany, Italy, and Spain with experience using the foam formulation. Evidence relating to the effectiveness of PAM is primarily available from controlled/clinical trial settings. Given the recent approval of the PAM regimen with Cal/BD foam, there is limited real-world evidence regarding the suitable patient profile or regarding the use and consumption as a PAM versus RM regimen. This study provided valuable insights into the real-world use of PAM and RM regimens for psoriasis in clinical practice.
The panel considered the foam formulation to be a safe and effective psoriasis treatment. The panel agreed that topical therapies, including the foam formulation, are generally more burdensome than non-topical therapies (systemics and biologics), describing their application as ‘greasy’ and ‘messy’. Topical steroids were also recognised to be associated with a risk of irritation. Topicals, including the foam formulation, were generally considered less suitable for patients with lesions on hair-bearing areas and sensitive skin.
When considering key drivers for prescribing RM or PAM, the panel were unable to reach consensus on whether cost influences their decision-making, as country-specific reimbursement determines patient out-of-pocket costs. It was agreed that patients’ disease history, treatment experience, socioeconomic background, disease and treatment understanding, and motivations for long-term treatment all have a substantial impact on dermatologists’ decisions to start and continue prescribing the PAM regimen. These decisions are largely driven by patient suitability for PAM and their preference of treatment regimen, which mirrors recent literature recognising the need for a patient-centred approach to psoriasis treatment, particularly long-term [12].
Engagement of patients in treatment decision-making with active discussions around available regimens, and their concerns and preferences, is key to optimising the success of both RM and PAM. Such discussions should continue throughout the course of the disease to consider changes in disease characteristics and patient attitudes and experiences. Moreover, greater involvement of patients helps drive improved adherence to topicals, which is generally considered to be poor [12, 13, 26]. Likewise, this study highlighted that adherence to topicals, including the foam formulation, is poor and can impact patient suitability for PAM. Specifically, the burdensome nature of topicals, irritation risk, fear of long-term steroid use and patient understanding of the need for long-term adherence during remission were all reported as barriers to PAM adherence.
Given challenges with long-term adherence to topical steroids, 2–3 months was considered a realistic timeframe for patients to remain on PAM. However, it is generally unclear how well patients apply the foam as directed at home in an uncontrolled environment. Nevertheless, panellists confirmed they would continue to prescribe Cal/BD foam as long as the patient was happy with their treatment. Despite limitations of topical therapies, the need for proactive, long-term management of psoriasis was agreed, with reference to success seen with long-term proactive treatment with topical calcineurin inhibitors for atopic dermatitis [12, 27].
In the panel’s opinion, the number of cans of foam prescribed for RM and PAM in a given period is usually similar. These findings provide clarity on the real-world consumption across the two regimens, suggesting that PAM does not increase consumption and associated costs for payers or patients. Moreover, potential differences (+ or −) in consumption and costs between the regimens does not influence decision-making nor prevent the use of PAM treatment in clinical practice. Instead, consumption is largely influenced by the patient, who should be fully involved in treatment decisions. This study did not specifically investigate the impact of disease severity on the number of cans of foam prescribed for RM and PAM, which may be an area for further research.
The findings of this study provide clarity on the real-world use and views of PAM in psoriasis following the EMA label update to include an LTM regimen for Cal/BD foam, and suggest that consumption-related costs do not differ between PAM and RM regimens with the foam formulation.
Limitations
One limitation of this study was that panellists were required to have experience using Cal/BD foam as PAM; therefore, results are reflective of dermatologists who were already opting to prescribe the PAM regimen in clinical practice. As such, findings may not be generalisable to the experiences that all dermatologists across these markets have with Cal/BD foam and other plaque psoriasis treatments. However, the decision to include panellists with PAM experience allowed for insights into its real-world use from dermatologists familiar with Cal/BD foam. This modified-Delphi panel therefore provides robust findings into the current experiences and use of RM and PAM in clinical practice. Secondly, the questionnaires were completed in an uncontrolled environment, and therefore assumed that dermatologists took the appropriate time to consider and accurately answer questions. To mitigate this, responses from panellists who took a significantly shorter time than allocated were reviewed to ensure the same level of detail was provided by those who took more time. There was potential for the biassing effects of different personalities in the consensus meeting, in that less extroverted personalities may have felt unable to provide their input. However, the use of a moderator and chat box function throughout likely minimised this limitation. Finally, this study may be limited by the inclusion of only English-language-speaking dermatologists. However, this requirement ensured that participants were able to fully contribute to consensus meeting discussions.
Conclusions
This modified-Delphi study confirms that treatment decisions in psoriasis, including prescription of either RM or PAM, are largely driven by patient-related factors. Engagement of the patient in decision-making, and assessment of their suitability for PAM, is key to improving adherence and optimising success of proactive long-term treatment in psoriasis. In real-world clinical practice, dermatologists prescribe a similar number of Cal/BD foam cans for RM and PAM, suggesting similar costs per patient between regimens. Any potential differences in consumption (− or +) were not considered to influence decisions to prescribe RM or PAM. These real-world findings can inform payer and prescriber conversations regarding what to expect in terms of the real-world use of PAM, patient suitability for treatment regimens, and consumption associated with the RM and PAM regimens.
Notes
PASI measures lesion severity and area affected on a 0–72 scale: the average redness, thickness and scaliness of the lesions are weighted by the area of involvement (head, upper extremities, trunk and lower extremities). BSA is measured in terms of the percentage of the body that is covered with lesions: 1% coverage equates to approximately the size of one hand with outstretched fingers.
References
Brandon A, Mufti A, Sibbald RG. Diagnosis and management of cutaneous psoriasis: a review. Adv Skin Wound Care. 2019;32(2):58–69.
Korman NJ, Zhao Y, Li Y, Liao M, Tran MH. Clinical symptoms and self-reported disease severity among patients with psoriasis–implications for psoriasis management. J Dermatol Treat. 2015;26(6):514–9.
Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–85.
Lebwohl M, Kircik L, Lacour J-P, Liljedahl M, Lynde C, Mørch MH, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84(5):1269–77.
Imafuku S, Zheng M, Tada Y, Zhang X, Theng C, Thevarajah S, et al. Asian consensus on assessment and management of mild to moderate plaque psoriasis with topical therapy. J Dermatol. 2018;45(7):805–11.
Egeberg A, Andersen YM, Thyssen JP. Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort. BMJ Open. 2019;9(3): e028116.
Fernández-Armenteros J, Gómez-Arbonés X, Buti-Solé M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M, et al. Epidemiology of psoriasis. A population-based study. Actas Dermo-Sifiliogr (English Edn). 2019;110(5):385–92.
Burgos-Pol R, Martínez-Sesmero J, Ventura-Cerdá J, Elías I, Caloto M, Casado MÁ. The cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic review. Actas Dermo-Sifiliogr. 2016;107(7):577–90.
Svedbom A, Dalén J, Mamolo C, Cappelleri JC, Mallbris L, Petersson IF, et al. Economic burden of psoriasis and potential cost offsets with biologic treatment: a Swedish register analysis. Acta Derm Venereol. 2016;96(5):651–7.
Thomsen SF, Skov L, Dodge R, Hedegaard MS, Kjellberg J. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235(5):372–9.
Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(5):685–705.
Segaert S, Calzavara-Pinton P, de la Cueva P, Jalili A, LonsDanic D, Pink AE, et al. Long-term topical management of psoriasis: the road ahead. J Dermatol Treatment. 2020;33:111–20.
Carrascosa JM, Theng C, Thaçi D. Spotlight on topical long-term management of plaque psoriasis. Clin Cosmet Investig Dermatol. 2020;13:495.
World Health Organization (2022) Global report on psoriasis. 2016. Available from https://apps.who.int/iris/handle/10665/204417. Accessed August 20, 2022
Evans C. Managed care aspects of psoriasis and psoriatic arthritis. Am J Manage Care. 2016;22(8 Suppl):s238–43.
Girolomoni G, Calzavara Pinton P, Cristaudo A, Cicchetti A. Back to the future: a new topical approach for mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153(3):375–82.
Martin G, Young M, Aldredge L. Recommendations for initiating systemic therapy in patients with psoriasis. J Clin Aesthet Dermatol. 2019;12(4):13–26.
Paul C, Gallini A, Archier E, Castela E, Devaux S, Aractingi S, et al. Evidence-based recommendations on topical treatment and phototherapy of psoriasis: systematic review and expert opinion of a panel of dermatologists. J Eur Acad Dermatol Venereol. 2012;26:1–10.
Koerber A, Wilsmann-Theis D, Augustin M, von Kiedrowski R, Mrowietz U, Rosenbach T, et al. Topical therapy of psoriasis vulgaris—a treatment pathway. J Dtsch Dermatol Ges. 2019;17:3–14.
Bark C, Brown C, Svangren P. Systematic literature review of long-term efficacy data for topical psoriasis treatments. J Dermatol Treat. 2022;33(4):2118–28.
Lebwohl MG, Papp KA, Mørch MH, Bernasconi MYJ, Warren RB. Long-term proactive treatment of plaque psoriasis with calcipotriene/betamethasone dipropionate foam prolongs remission and reduces relapses irrespective of patient baseline characteristics. Dermatol Ther. 2021;11(5):1657–65.
European Medicines Agency (EMA) (2022) Summary of product characteristics (SmPC): Enstilar 50 micrograms/g + 0.5 mg/g cutaneous foam. Published 2021. Available from https://www.medicines.org.uk/emc/product/2139/smpc#gref. Accessed February 22, 2022
Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–82.
Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5(1):1–12.
Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–9.
Belinchón I, Rivera R, Blanch C, Comellas M, Lizán L. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adher. 2016;10:2357–67.
Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16:75.
Acknowledgements
The authors acknowledge Adept Field Solutions for completing the recruitment and management of panellists included in this study. The authors thank the panel for their participation in this study.
Funding
This study was funded by LEO Pharma, Ballerup, Denmark. Adelphi Values PROVE received payment from LEO Pharma to conduct this study. Fieldwork support was provided by Adept Field Solutions and was funded by LEO Pharma. LEO Pharma funded the Rapid Service and Open Access Fees associated with journal publication.
Medical Writing and Editorial Support
Medical writing and editing were performed by Amy Sears and Richard Perry of Adelphi Values PROVE.
Author Contributions
Conceptualisation: Elisabeth Reimer Rasmussen, Richard Perry, Abigail Beveridge; Methodology: Elisabeth Reimer Rasmussen, Richard Perry, Abigail Beveridge; Formal analysis and investigation: Richard Perry, Abigail Beveridge, Amy Sears; Writing—original draft preparation: Richard Perry, Abigail Beveridge, Amy Sears; Writing—review and editing: Elisabeth Reimer Rasmussen, Richard Perry, Amy Sears. All authors read and approved the final manuscript.
Disclosures
Elisabeth Reimer Rasmussen is an employee of LEO Pharma, who funded this study. Richard Perry, Abigail Beveridge, and Amy Sears are employees of Adelphi Values PROVE; Adelphi Values PROVE received payment from LEO Pharma to conduct this study.
Compliance with Ethics Guidelines
This article is based solely on the opinions of a group of experts on a specific topic as part of a market research activity. Therefore, for the purpose of this study, no patient data or information were collected and there was no requirement to obtain patient informed consent. As market research falls outside the remit of the governance arrangements for research ethics committees, in accordance with existing guidelines from the British Healthcare Business Intelligence Association (BHBIA) and European Pharmaceutical Market Research Association (EphMRA), ethical committee approval was not required for this study. All panellists provided informed consent to participate in the study and identifying information was not disclosed to the investigators or sponsor.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perry, R., Beveridge, A.J., Sears, A.J. et al. Expert Consensus on Real-World Use and Consumption Patterns of a Fixed-Dose Combination Foam for Psoriasis as a Reactive Management (RM) and Proactive Management (PAM) Regimen. Adv Ther 40, 1062–1073 (2023). https://doi.org/10.1007/s12325-022-02417-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02417-6