Abstract
Introduction
The phase 3 PSO LONG study (NCT02899962) demonstrated superior efficacy of proactive (PM) versus reactive management (RM) using calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam in adults with psoriasis. Here, we evaluated whether certain baseline parameters had an effect on time to first relapse (TTFR), number of relapses, and assessed interactions between treatment effect.
Methods
PSO LONG included an initial 4-week open-label phase (once-daily Cal/BD foam) and a 52-week maintenance phase where patients were randomized to twice-weekly Cal/BD (PM) or vehicle foam (RM), with a 4-week once-daily Cal/BD foam rescue treatment for relapse. Baseline parameters analyzed using a stepwise variable selection procedure included body surface area, modified Psoriasis Area Severity Index (mPASI), Physician Global Assessment (PGA), body mass index, age, sex, Dermatology Life Quality Index, and duration of psoriasis. Continuous variables were divided into groups based on standard criteria.
Results
Overall, the effect of treatment on TTFR did not vary across any baseline parameters. Variables with a statistically significant effect on TTFR were: treatment group (PM vs. RM hazard ratio [HR]: 0.56; p < 0.001); PGA (moderate vs. mild HR: 1.42; severe vs. mild HR: 2.32; overall p = 0.009); mPASI (moderate vs. mild HR: 1.19; severe vs. mild HR: 1.77; overall p = 0.009); and sex (women vs. men HR: 1.26; p = 0.030). Variables with a significant effect on the number of relapses were: treatment group (PM vs. RM, rate ratio [RR] 0.52; p < 0.001); PGA at baseline (moderate vs. mild, RR 1.38; severe vs. mild, RR 2.22; overall p < 0.001); and mPASI (moderate vs. mild, RR 1.25; severe vs. mild, HR 1.70; overall p = 0.002).
Conclusion
All patients benefitted from long-term PM versus RM with Cal/BD foam regardless of baseline characteristics, and the benefit of treatment increased with greater disease severity.
Trial Registration
ClinicalTrials.gov identifier, NCT02899962.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The phase 3 clinical trial PSO LONG assessed the long-term efficacy and safety of proactive psoriasis management with twice-weekly calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam |
In this post hoc analysis, we examined whether patient baseline parameters, such as modified Psoriasis Area Severity Index, Physician Global Assessment, body mass index, age, sex, Dermatology Life Quality Index, and duration of psoriasis, have a significant predictive effect on the time to first relapse and number of relapses |
What was learned from this study? |
We show that all patients benefitted from long-term proactive management versus reactive management with Cal/BD foam regardless of baseline characteristics |
Patients with more severe disease at baseline showed greater benefit with the proactive management approach than those with milder disease |
Introduction
Psoriasis vulgaris (plaque psoriasis) is a chronic inflammatory skin disease with a relapsing course that requires a long-term management strategy [1]. Topical therapies are a mainstay in the treatment of psoriasis either as monotherapy or to complement systemic treatments. The influence of patient characteristics on treatment outcome was studied in the moderate-to-severe patient population treated with systemic therapies (either orals or biologics). Thus far, long-term results from the use of topical therapies are lacking [2, 3].
Conventional long-term management with topical treatments uses a reactive approach where the treatment is used after relapse has occurred versus a proactive approach to maintain remission. Fixed-dose combination calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) aerosol foam (Enstilar®; LEO Pharma, Ballerup, Denmark) is indicated for the topical treatment of plaque psoriasis [4, 5].
The phase 3 PSO-LONG clinical trial assessed the long-term efficacy and safety of proactive psoriasis management with twice-weekly Cal/BD foam [6]. The trial comprised a 4-week open-label treatment phase, followed by a randomized 52-week maintenance phase and an 8-week follow-up phase. During the maintenance phase of the trial, median time to first relapse (TTFR), the primary endpoint, estimated using the Cox proportional hazards model was 56 days for patients in the proactive treatment group versus 30 days for the reactive treatment group with a prolongation by 26 days for the proactive group. Patients in the proactive group had an additional 41 days in remission versus the reactive group (p < 0.001) over the 1-year period. Number of relapses per year of exposure was 3.1 for the proactive group versus 4.8 for the reactive group. Assessment of the 4-week open-label lead-in phase of PSO-LONG showed that Cal/BD foam achieved 80% treatment success in 4 weeks [7]. Here, we examined whether patient baseline parameters have a significant predictive effect on the primary and secondary endpoints.
Methods
Study Design
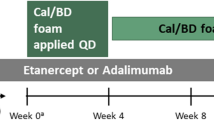
This was a post hoc analysis of the PSO LONG phase 3 trial (NCT02899962). A statement of ethics was provided for the primary study upon which this post hoc analysis is based [6]. Approval for the clinical trial protocol was obtained from the relevant institutional review boards or independent ethics committees and regulatory authorities for each participating site before patient enrollment. All patients provided written informed consent. The trial was conducted in accordance with Good Clinical Practice and Ethical Principles for Medical Research Involving Human Subjects [6]. The trial design has been described previously and is shown in Fig. 1 [6]. Patients who achieved treatment success, defined as a Physician Global Assessment (PGA) score of clear/almost clear (PGA < 2) with a ≥ 2-grade improvement from baseline, entered the maintenance phase. During the long-term maintenance phase, patients were randomly assigned to apply either Cal/BD foam or vehicle twice weekly to the disease site that was previously cleared (or almost cleared) during the preceding open-label phase. Interventions and patient eligibility for PSO LONG have been described previously [6]. For eligibility, patients had to be ≥ 18 years with truncal or limb psoriasis, or both, involving 2% to 30% of the body surface area (BSA), PGA disease severity score of mild or higher (PGA ≥ 2), and modified Psoriasis Area and Severity Index (mPASI) score ≥ 2 at baseline of the open-label lead-in phase (referred to as baseline) [6].
PSO LONG trial design. aPatients with treatment success at end of open-label lead-in phase (PGA score ‘clear’/‘almost clear’ [PGA < 2] with ≥ 2-grade improvement from baseline) were randomized 1:1 in the maintenance phase. bFollowing 4 weeks of once-daily rescue treatment, patients who regained PGA < 2 (‘clear’/‘almost clear’) re-started the twice-weekly maintenance treatment according to the original randomization scheme. cPatients who did not regain a PGA score < 2 (‘clear’/‘almost clear’) following 4 weeks of once-daily rescue treatment were withdrawn from the trial. Cal/BD calcipotriene 0.005%/betamethasone dipropionate 0.064%, FU follow-up, PGA Physician Global Assessment. Reprinted from J Am Acad Dermatol. 2021;84(5). Lebwohl M, Kircik L, Lacour JP, Liljedahl M, Lynde C, Mørch MH, Papp KA, Perrot JL, Gold LS, Takhar A, Thaçi D, Warren RB, Wollenberg A. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). Pages 1269–1277, Copyright (2020), with permission from Elsevier
Subgroup Statistical Analysis
The subgroup analysis was used to investigate whether certain baseline parameters have a significant predictive effect on the primary and secondary endpoints. The goal was to examine potential interaction effects between the treatment effect and the baseline parameters (i.e., examine whether the treatment effects vary across patient subgroups). TTFR was measured in days since randomization and was analyzed by means of a Cox proportional hazard model with treatment group, pooled sites, and baseline parameters tested for significance of the variables. Number of relapses was analyzed by means of a Poisson regression model with treatment group, pooled sites, and baseline parameters as factors and time at risk as an offset variable. Random subject effect was also included.
The stepwise variable selection included the following baseline parameters:
-
BSA: mild ≤ 3, moderate 4–10, severe > 10.
-
mPASI: mild ≤ 5, moderate 6–12, severe > 12.
-
PGA: clear (0), almost clear (1), mild (2), moderate (3), severe (4).
-
Body mass index: normal ≤ 25 kg/m2, overweight 26–30 kg/m2, obese > 30 kg/m2.
-
Age: 18–64 years, ≥ 65 years.
-
Sex: men, women.
-
Dermatology Life Quality Index: 0–1, 2–5, 6–10, 11–20, > 20.
-
Duration of psoriasis: ≤ 5 years, 6–10 years, 11–20 years, 21–30 years, 31–40 years, > 40 years.
There was no adjustment for multiplicity. The trial was not powered for subgroup analyses, and the results may be affected by the grouping of parameters.
Results
The distribution of baseline parameters in the analysis is shown in Table 1. The analysis included 521 patients; most patients were men (67.37%), were aged 18–64 years (78.12%), and had moderate psoriasis as assessed by PGA (85.22%) and BSA (54.51%) measures. Overall, the effect of treatment on TTFR did not vary across any baseline parameters. The TTFR was associated with severity of disease at baseline and sex of the patients (Table 2). Variables with a significant effect on TTFR were: treatment group (proactive management vs. reactive management, hazard ratio [HR] 0.56, p < 0.001); PGA at baseline (overall p = 0.009; moderate vs. mild, HR 1.42, p = 0.072; severe vs. mild, HR 2.32, p = 0.003); mPASI (overall p = 0.009; moderate vs. mild, HR 1.19, p = 0.160; severe vs. mild, HR 1.77, p = 0.002); and sex (women vs. men, HR 1.26, p = 0.027).
Number of relapses was dependent on baseline disease severity, and the effect of treatment on number of relapses did not vary across baseline parameters. Variables with a significant effect on the number of relapses were: treatment group (proactive management vs. reactive management, rate ratio [RR] 0.52, p < 0.001); PGA at baseline (overall p < 0.001; moderate vs. mild, RR 1.38, p = 0.043; severe vs. mild, RR 2.22, p < 0.001); and mPASI (overall p = 0.002; moderate vs. mild, RR 1.25, p = 0.035; severe vs. mild, RR 1.70, p < 0.001). Overall, patients with more severe disease at baseline had a greater benefit from proactive management with Cal/BD foam than those with milder disease.
Discussion
Data on the proactive management of psoriasis are lacking. The findings herein add to the report by Lebwohl et al. that long-term proactive management with Cal/BD foam demonstrated superior efficacy compared with reactive management [6]. Patients with psoriasis often present with comorbid conditions and have a variety of detrimental characteristics (e.g., obesity, severity of disease, long duration of disease) that can negatively affect treatment outcomes [8]. Analysis of baseline characteristics related to treatment outcome was reported for psoriasis systemic treatments (oral and biologic) [2, 3]. However, such data have not yet been produced for topical treatments as Cal/BD foam is the first drug receiving a label for long-term treatment. Our findings show that the benefits of long-term proactive management of psoriasis with Cal/BD foam are not dependent on patients’ baseline parameters; in fact, patients with more severe disease at baseline showed greater benefit with the proactive management approach than those with milder disease.
A recent study investigated the possible correlations between baseline clinical/dermoscopic features of psoriatic plaques, specifically on lesions targeted for treatment, and therapeutic response to Cal/BD foam after 4 weeks of therapy in patients presenting with moderate-to-severe plaque psoriasis [9]. The purpose was to better optimize the use of Cal/BD foam. The investigators confirmed that Cal/BD foam is effective for the treatment of plaque psoriasis. The study found that degree of infiltration of lesions at baseline and localization on the legs adversely affected treatment response, but no association was observed between treatment response and lesion duration, sex of the patient, baseline Local Psoriasis Severity Index, or baseline erythema/scaling.
Although this post hoc analysis did not address the safety of Cal/BD foam for proactive management of psoriasis, the incidence of adverse events in PSO LONG was similar between treatment groups, and Cal/BD foam was well tolerated [6]. The risk of hypothalamic-pituitary–adrenal (HPA) axis suppression with topical corticosteroid use in patients with psoriasis is well documented with no evidence of clinically significant HPA axis suppression due to absorption of topical steroids [10]. A subgroup of patients in the PSO LONG trial were monitored for HPA axis suppression during the course of the primary study. No clinically relevant effects on the HPA axis were observed in this subgroup of patients [6]. Additional investigations are underway on the long-term effects of proactive management of psoriasis with Cal/BD foam on the HPA axis.
Limitations
This post hoc analysis was not powered for comparison with the original study by Lebwohl et al. [4]; thus, any inference of statistical comparisons between this baseline analysis and primary findings in Lebwohl et al. should be done with caution.
Conclusion
Our findings show that all patients benefited from long-term proactive management versus reactive management with Cal/BD foam regardless of baseline characteristics, and the benefit of treatment increased with greater disease severity.
References
Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427.
Driessen RJ, Boezeman JB, van de Kerkhof PC, de Jong EM. Three-year registry data on biological treatment for psoriasis: the influence of patient characteristics on treatment outcome. Br J Dermatol. 2009;160(3):670–5.
Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2021;35(1):123–34.
ENSTILAR® (calcipotriene and betamethasone dipropionate) foam [US prescribing information]. Ballerup, Denmark. 2020. Revised 10 2020
Enstilar® 50 micrograms/g + 0.5 mg/g cutaneous foam [UK summary of product characteristics]. 2021. Updated 8 Mar 2021
Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2020;84(5):1269–77.
Warren RB, Gold M, Gooderham M, et al. Four-week daily calcipotriene/betamethasone dipropionate foam is highly efficacious in patients with psoriasis (PSO-LONG lead-in phase). J Drugs Dermatol. 2021;20(4):436–41.
Kavanaugh A, Papp K, Gottlieb AB, et al. Demography, baseline disease characteristics, and treatment history of psoriasis patients with self-reported psoriatic arthritis enrolled in the PSOLAR registry. BMC Rheumatol. 2018;2:29.
Errichetti E, Croatto M, Arnoldo L, Stinco G. Plaque-type psoriasis treated with calcipotriene plus betamethasone dipropionate aerosol foam: a prospective study on clinical and dermoscopic predictor factors in response achievement and retention. Dermatol Ther. 2020;10(4):757–67.
Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47–51.
Duffin KC, Papp KA, Bagel J, Levi E, Chen R, Gottlieb AB. Evaluation of the physician global assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16(2):147–53.
University of Cardiff. Dermatology Life Quality Index. 2021. https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index. Accessed 21 Apr 2021
Acknowledgements
Funding
This study and the journal's Rapid Service Fee were funded by LEO Pharma, Ballerup, Denmark. Richard B. Warren is supported by the Manchester NIHR Biomedical Research Centre.
Medical Writing, Editorial, and Other Assistance
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Gautam Bijur, PhD, and editing support by Dena McWain, both of Ashfield MedComms, an Ashfield Health company, and funded by LEO Pharma, Ballerup, Denmark.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Mark G. Lebwohl, Kim A. Papp, Marie Holst Mørch, Marie Y. Jablonski Bernasconi, and Richard B. Warren contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Marie Holst Mørch. The first draft of the manuscript was written by Mark G. Lebwohl and Marie Holst Mørch, and Mark G. Lebwohl, Kim A. Papp, Marie Holst Mørch, Marie Y. Jablonski Bernasconi, and Richard B. Warren commented on previous versions of the manuscript. Mark G. Lebwohl, Kim A. Papp, Marie Holst Mørch, Marie Y. Jablonski Bernasconi, and Richard B. Warren read and approved the final manuscript.
Disclosures
Mark G. Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie Inc., Amgen, Arcutis Biotherapeutics, Boehringer Ingelheim, Dermavant Sciences Ltd., Eli Lilly and Company, Incyte, Janssen Research & Development, LLC, LEO Pharma, Ortho Dermatologics, Pfizer, and UCB, Inc., and is a consultant for Aditum Bio, Allergan, Almirall, Arcutis Biotherapeutics, Avotres Therapeutics, Birch-BioMed Inc., BMD Skincare, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, CorEvitas, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica. Kim A. Papp has received honoraria for advisory board, speaker, and consultant services from AbbVie Inc, Actelion, Allergan, Amgen, Anacor Pharmaceuticals Inc, Astellas Pharma, Avillion, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly and Company, EMD Serono, Galderma, Gilead, GlaxoSmithKline, Incyte Corporation, Janssen Pharmaceuticals, Kyowa Kirin, LEO Pharma, Meiji Seika, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi Genzyme, Sun Pharmaceutical Industries Ltd, Takeda Pharmaceutical Company, UCB Pharma, and Vertex Pharmaceuticals and has received research grants for investigator services from AbbVie Inc., Actelion, Allergan, Amgen, Anacor Pharmaceuticals Inc., Astellas Pharma, Avillion, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly and Company, EMD Serono, Galderma, Gilead, GlaxoSmithKline, Incyte Corp., Janssen Pharmaceuticals, Kyowa Kirin, LEO Pharma, Meiji Seika, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi Genzyme, Sun Pharmaceutical Industries Ltd, Takeda Pharmaceutical Company, and UCB Pharma. Marie Holst Mørch and Marie Y. Jablonski Bernasconi are employees of LEO Pharma. Richard B. Warren receives research grants from AbbVie, Almirall, Amgen, Celgene, Janssen, Eli Lilly and Company, LEO Pharma, Medac, Novartis, Pfizer, and UCB and consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, DiCE, GlaxoSmithKline, Janssen, Eli Lilly and Company, LEO Pharma, Medac, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and UNION.
Compliance with Ethics Guidelines
This was a post hoc analysis of the PSO LONG study (NCT02899962). A statement of ethics was provided for the primary study upon which this post hoc analysis is based. Approval for the clinical trial protocol was obtained from the relevant institutional review boards or independent ethics committees and regulatory authorities for each participating site before patient enrollment. All patients provided written informed consent. The trial was conducted in accordance with Good Clinical Practice and Ethical Principles for Medical Research Involving Human Subjects.
Data Availability
Because this was a post hoc analysis, data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lebwohl, M.G., Papp, K.A., Mørch, M.H. et al. Long-Term Proactive Treatment of Plaque Psoriasis with Calcipotriene/Betamethasone Dipropionate Foam Prolongs Remission and Reduces Relapses Irrespective of Patient Baseline Characteristics. Dermatol Ther (Heidelb) 11, 1657–1665 (2021). https://doi.org/10.1007/s13555-021-00585-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00585-x