Abstract
Introduction
The objectives of this study were to (1) report long-term health-related quality of life (HRQoL) outcomes among patients using rimegepant preventatively in BHV3000-305 (NCT03732638) open-label extension (OLE) and (2) map Migraine-Specific Quality of Life questionnaire version 2.1 (MSQv2) to EQ-5D-3L utility values over the double-blind treatment (DBT; 0–12 weeks) and the OLE (13–64 weeks) to assess the influence of treatment on these values.
Methods
This was a post hoc analysis using data from a rimegepant study for the prevention of migraine (BHV3000-305). Adult patients with migraine took either rimegepant 75 mg or placebo every other day (EOD) during the DBT phase. All patients received rimegepant during the OLE. MSQv2 was measured at baseline, weeks 12, 24, and 64. A validated algorithm was used to map MSQv2 scores to EQ-5D utilities.
Results
Baseline data were available for 347 patients treated with placebo and 348 treated with rimegepant in the DBT period, who continued to the OLE. Baseline EQ-5D utilities were similar between trial arms: 0.598 for placebo and 0.614 for rimegepant. EQ-5D improved from baseline to week 12 and utilities increased by + 0.09 for placebo and + 0.10 for rimegepant (p value = 0.011). By 24 weeks, at which point patients who were originally randomized to placebo had received rimegepant 75 mg EOD for 12 weeks, HRQoL measures (MSQv2 and EQ-5D) were similar across groups, demonstrating rapid onset of treatment effect. This HRQoL improvement was durable out to 64 weeks.
Conclusion
Compared to placebo, treatment with rimegepant 75 mg was associated with greater improvement in EQ-5D utilities during the 12-week DBT phase. Patients originally randomized to placebo experienced a similar improvement in EQ-5D utilities after switching to rimegepant during the OLE, demonstrating that benefits are realized within 12 weeks of active treatment. This preventive effect was durable out to 64 weeks and was associated with an additional increase in HRQoL over time.
Trial Registration
NCT03732638.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study used a validated algorithm to map EQ-5D utilities from Migraine-Specific Quality of Life questionnaire version 2.1 (MSQv2) scores collected during 64 weeks of follow-up in study BHV3000-305 of rimegepant as a preventive migraine treatment in adult patients with 4–18 monthly migraine days (MMDs). |
Rimegepant 75 mg every other day was associated with greater improvement in MMDs and EQ-5D utilities compared to placebo during the 12-week double-blind treatment (DBT) phase. The MSQv2 and mapped EQ-5D measures of participants originally randomized to the placebo arm caught up to the rimegepant arm during the first 12 weeks of the open-label extension study (OLE), demonstrating rapid onset of effect. |
There were further improvements in MSQv2 and mapped health state utility values in both trial arms over the 52-week OLE phase, where all patients were asked to take rimegepant 75 mg every other day (EOD). |
These findings can inform economic evaluations of rimegepant, where health state utility measures are required to compare treatment efficacy against standard of care and other novel therapies. |
Introduction
Migraine is a prevalent neurological disorder characterized by recurrent, unilateral, throbbing headaches and associated symptoms including photophobia, nausea, and vomiting [1, 2]. Preventive migraine treatments are indicated in patients who experience four or more attacks per month to reduce attack frequency and severity and to improve function and health-related quality of life (HRQoL) [1, 3]. Calcitonin gene-related peptide (CGRP) inhibitors (both monoclonal antibodies [mAbs] and small molecule receptor blockers [gepants]) are a novel class of migraine medications. Both mAbs and gepants are migraine-specific whereas most of the older preventive treatments are not [1, 3]. In migraine prevention, there are four CGRP mAbs, namely fremanezumab, galcanezumab, eptinezumab, and erenumab (injectable and intravenous administration), and two small molecule gepants, atogepant and rimegepant (oral administration) [1, 3].
Rimegepant is a CGRP receptor antagonist indicated for the acute treatment of migraine with or without aura and for the preventive treatment of episodic migraine in adults [4]. One 75 mg oral dose of rimegepant is taken orally on an as-needed basis for the acute treatment of migraine and on a regular every-other-day (EOD) basis for preventive treatment [4]. The efficacy and safety of rimegepant have been demonstrated in randomized, double-blind, placebo-controlled trials [5,6,7] for the acute treatment of migraine where the percentage of patients who achieved freedom from pain and freedom from most bothersome symptom (MBS) 2 h post-dose was statistically significantly greater with rimegepant compared with placebo [4, 6, 7]. Benefits in prevention have been demonstrated in another randomized, double-blind, placebo-controlled trial (BHV3000-305; NCT03732638) [8, 9]; changes from baseline in the mean number of monthly migraine days (MMDs) during weeks 9 through 12 and the percentage of patients who achieved ≥ 50% reduction from baseline in moderate to severe MMDs during weeks 9 through 12 were statistically significantly greater with rimegepant EOD for migraine prevention compared with placebo [4, 9]. Rimegepant and placebo have similar tolerability profiles [9], and rimegepant does not cause vasoconstriction, which makes it especially useful in patients with contraindications to medications which cause vasoconstriction [1]. It also has a shorter half-life than the injectable CGRP mAbs [4, 10,11,12,13], which may be beneficial when treatment has to be stopped suddenly because of adverse events, planning pregnancies, or other circumstances. Rimegepant’s oral administration may be preferable to some patients over injectables or infusions [14], and its approval for both acute and preventive treatment of migraine may simplify the treatment regimen.
Assessing response to treatment with preventive migraine medications should focus on reduction in MMDs and improvement in migraine-related functional impairment and disability [1]. Therefore, change from baseline in MMDs is recommended by the International Headache Society as a primary endpoint in prevention trials, and measuring change in HRQoL with a disease-specific instrument is recommended as a secondary endpoint, among others [15]. The Migraine-Specific Quality of Life questionnaire version 2.1 (MSQv2) is one of the recommended, widely used, and validated instruments for measuring change in HRQoL in clinical trials of preventive therapies [15, 16]. Another recommended instrument to measure health status is the EQ-5D, a generic health state utility measure with particular value for calculating quality-adjusted life years (QALYs) [15, 17]. While there is no single gold standard HRQoL measure in migraine, disease-specific measures are more precise in capturing changes in HRQoL as a result of treatment and are preferred for use in migraine trials, while generic utility measures like the EQ-5D make it possible to directly compare disease burden and interventions across conditions and are required for economic evaluations [17, 18].
In the rimegepant clinical trial program, EQ-5D was not assessed, which presents a challenge for calculating QALYs in economic models [17]. When studies do not directly measure generic preference-based utilities such as EQ-5D, results from disease-specific measures such as MSQv2 can be used to estimate EQ-5D health-state utilities via mapping algorithms [17, 19]. Therefore, to support future economic analyses of rimegepant in migraine prevention, the objectives of this post hoc analysis were to (1) describe long-term MSQv2 outcomes among patients using rimegepant preventatively in BHV3000-305 double-blind treatment (DBT) and open-label extension (OLE) phases and (2) map MSQv2 outcomes to EQ-5D-3L health state utilities over the DBT (0–12 weeks) and OLE phases (13–64 weeks) of BHV3000-305 using a validated mapping algorithm.
Methods
Methods and results are described according to the CONSORT Checklist [20].
Summary of BHV3000-305
This is a secondary analysis of study BHV3000-305 [8, 9]. Study BHV3000-305 was a randomized, double-blind, placebo-controlled efficacy and safety trial of rimegepant for migraine prevention, conducted at 92 centers in the USA [9]. A detailed description of the study design, population, efficacy, and safety results of the DBT phase of BHV3000-305 is provided in Croop et al., [9]. Briefly, the study consisted of a screening/observation period of 4 weeks and a DBT phase of 12 weeks during which patients were randomized (1:1) to rimegepant 75 mg EOD or placebo EOD. At the completion of the 12-week DBT phase, patients were invited to enter the 52-week OLE phase, if they continued to meet study entry criteria and had acceptable laboratory test results. During the OLE phase, patients were asked to take rimegepant 75 mg EOD. During this phase, if patients experienced a migraine on a day that they were not scheduled to dose with rimegepant, they could take an additional 75 mg tablet on that day as an acute migraine treatment. Therefore, during the OLE phase, subjects could take a maximum of one rimegepant 75 mg tablet per calendar day for this 52-week period.
Study Participants
Inclusion criteria were adults (≥ 18 years) of both sexes in whom migraine developed before patients were 50 years old, with a history of migraine with or without aura or chronic migraine according to the International Classification of Headache Disorders, 3rd Edition definition [2], for a minimum of 1 year, who had experienced 4–18 moderate to severe migraine attacks per month during the last 3 months before screening, with attacks lasting for 4–72 h on average if not treated, and who were able to to distinguish between migraine and tension/cluster headaches [8, 9]. Patients were allowed to take one preventive migraine medication (other than rimegepant, CGRP mAbs, or CGRP receptor antagonists) during the DBT phase if they were using a stable dose for ≥ 3 months before the screening/observation period and if the dose did not change during the study [8, 9]. Patients were excluded if they did not respond to more than two preventive medication categories or if they had an unstable medical condition that presented an unjustifiable risk of suffering a significant adverse event or interfere with the efficacy or safety assessments, a history of HIV or cardiovascular disease, uncontrolled diabetes or hypertension, other pain or neurological disorders, psychiatric conditions, a history of certain gastrointestinal disorders, cholecystectomy, or gallstones, a history of drug or alcohol abuse, a history of allergies to medication, body mass index ≥ 33 kg/m2, or laboratory or other findings that caused concerns about interference, safety or tolerability [8, 9].
Outcomes and Data Collection
The primary endpoint of study BHV3000-305 was change in mean number of MMDs from baseline to 12 weeks of the DBT phase, as described in Croop et al. [8, 9]. This post hoc analysis is focused on a secondary patient-reported endpoint, the MSQv2, which measured migraine-related quality of life among participants throughout the trial. Specifically, MSQv2 was captured via paper survey at the following scheduled site visits: randomization baseline and week 12 of the DBT phase and week 24 and week 64 of the OLE phase.
Study BHV3000-305 was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulations [9]. The protocol was approved by Advarra Central Institutional Review Board (IRB) in Columbia (USA) and MedStar (a study site IRB at McLean USA) [9]. The study protocol is available in the supplementary information of Croop et al. [9]. Written consent was obtained from all participants prior to screening [9].
Description of MSQv2
MSQv2 is a frequently used, validated, migraine-specific measure of the impact of the disease on daily functioning (activities) and HRQoL [16, 18, 21, 22]. It consists of 14 items across 3 dimensions: 7 items assess the extent to which migraine restricts normal daily social and work function—this dimension is called role function restrictive (RFR); 4 items assess the extent to which migraine prevents the ability to function normally—this dimension is called role function preventive (RFP); 3 items assess the effects of migraine on emotional function (EF) [18, 21]. Patients report the impact of migraine on these items during the last 4-week period according to 6 options scored on a Likert scale, where 1 = none of the time; 2 = a little bit of the time; 3 = some of the time; 4 = a good bit of the time; 5 = most of the time; 6 = all of the time [18]. All the item scores are added together and rescaled on a scale ranging from 0 to 100, where 100 represents the best HRQoL [17, 18]. To assess significant differences in change on the MSQv2 at group and individual level (within group), the minimal important difference is 5 points for RFR, 5.0–7.9 for RFP, and 8.0–10.6 for EF [23].
Description of EQ-5D
In this study, a statistical mapping algorithm was used to estimate EQ-5D-3L scores based on the MSQv2 scores collected in the rimegepant prevention trial. Although EQ-5D-3L was not directly collected in the trial, a brief description of the measure is included here for context.
EQ-5D is a patient-reported outcome instrument which describes, measures, and values health to provide a generic, standardized value of health status across various diseases [24]. This is useful for clinical and economic evaluations (i.e., calculating QALYs) and for population health surveys [24]. EQ-5D is a widely used measure of HRQoL that has three versions, is available in numerous languages, and has various value sets that are representative of the societal perspective of the general population of specific countries or regions [24]. The value set and version used in this publication is the UK value set and the EQ-5D-3L version, which has five dimensions and three levels and consists of a descriptive system and a visual analogue scale (VAS) [24]. The five dimensions describe anxiety/depression, mobility, pain/discomfort, self-care, and usual activities according to three levels, where 1 = no problems; 2 = some problems; 3 = extreme problems [24]. Scores are assigned based on a specific value set, and higher scores indicate better health (e.g., 1 = perfect health; 0 is equivalent to death; negative values indicate a health state worse than death) [24]. EQ-5D has previously been used in migraine populations and captures a patient’s current state of health [25,26,27].
Utility Mapping
Similar to a previous study [19], this study uses a validated mapping algorithm developed by Gillard et al. [17] and patient-level data from study BHV3000-305 to map MSQv2 values to EQ-5D-3L utilities using separate validated algorithms for episodic and chronic migraine [9]. In study BHV3000-305, 23% of participants had chronic migraine and 77% had episodic migraine [9]. Gillard et al. developed their algorithm by using data from the International Burden of Migraine Study and using regression models to find the preferred algorithm for estimating EQ-5D-3L utilities based on the UK value set from MSQv2 scores [17]. There is an adequate relationship between MSQv2 and EQ-5D to estimate EQ-5D utilities via regression equations [17]. There is conceptual overlap between the EF dimension in MSQv2 and anxiety/depression dimension of EQ-5D as well as between the RFP and RFR dimensions of MSQv2 and the usual activities dimension of the EQ-5D [17]. The correlation coefficients between MSQv2 scores and EQ-5D-3L utility scores produced by the algorithm was statistically significant [17]. The regression models used to map MSQv2 scores to EQ-5D utility values are presented in Table 1. Mapped EQ-5D values for episodic or chronic migraine disease status at baseline were combined and reported together in the results. The models used to map the MSQv2 scores were validated linear regression models published by Gillard et al. [17].
EQ-5D utilities were mapped from MSQv2 measurements at baseline, week 12, week 24, and week 64. The Wilcoxon rank-sum test was used to test for differences between treatment groups at week 12. EQ-5D mean values are provided with standard error (SE).
Variables that were extracted from study BHV3000-305 include age (continuous), gender (categorical), history of chronic migraine (categorical), primary migraine type (categorical), and MMD (count) [9]. During study BHV3000-305, there was high compliance with treatment regimens and few missing data [28]. Therefore, no adjustments were made for missing data.
A mixed-effects regression model was fit to understand the effect of rimegepant, baseline MMD, and time on mapped EQ-5D utility. Treatment was treated as a time-varying covariate such that patients were only assigned to their respective randomized treatments up to week 12; then, everyone was assigned rimegepant. A random effect was added per subject to account for repeated measures.
Results
Baseline Characteristics
At baseline, data were available for 695 participants in the evaluable modified ITT population, 347 treated with placebo and 348 treated with rimegepant in the DBT phase (Table 2). Overall, the mean (SD) age of participants was 41.3 (13.1) years, and 83% were female. Just less than a quarter of patients reported history of chronic migraine (23%), and 46% reported their primary migraine type was migraine with aura vs. 54% without aura (Table 2).
After the DBT phase, 602/695 (87%) of these patients continued to the OLE phase of the study, and most of these subjects (430/602, 71%) completed the OLE. At week 64 (12-week DBT plus 52-week OLE), data were available for 208 participants in the rimegepant/rimegepant OLE group and for 222 participants in the placebo/rimegepant OLE group.
MSQv2 Values
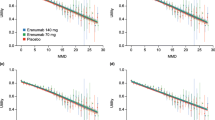
In Fig. 1, change in MSQv2 domain scores are presented for the rimegepant/rimegepant OLE group and the placebo/rimegepant OLE groups over the 12 week DBT phase and 52 week OLE phase. At baseline the mean MSQv2 values by domain were similar for rimegepant/rimegepant OLE (RFR: 51.2, RFP: 65.6, and EF: 58.5) and placebo/rimegepant OLE study groups (RFR: 50.0, RFP: 64.6, and EF: 56.2; Fig. 1A–C). At 12 weeks there were greater improvements (higher scores) from baseline in the rimegepant group across all three domains (RFR: + 18.6, RFP: + 14.4, EF: + 18.9) compared to the placebo group (RFR: + 15.1, RFP: + 11.4, EF: + 15.0; Fig. 1A–C). At 24 weeks, after the placebo/rimegepant OLE arm had been receiving rimegepant for 12 weeks, the MSQv2 values converged again (Fig. 1A–C), demonstrating rapid onset of HRQoL improvement after initiating rimegepant.
The HRQoL impact was maintained over the OLE period and saw additional improvements from baseline. At 64 weeks, mean change from baseline in MSQv2 was similar for rimegepant/rimegepant OLE patients (RFR: + 32.6, RFP: + 24.7, and EF: + 29.3) and rimegepant/placebo OLE patients (RFR: + 33.2, RFP: + 25.5, and EF: + 31.0; Fig. 1A–C). This change is three to six times greater than the minimum clinically important difference for within-group analyses, depending on domain [23].
Mapped Utility Values
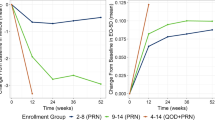
The utility values that were mapped from MSQv2 are summarized in Table 3 and presented in Fig. 2. Baseline EQ-5D utilities were similar between trial arms: 0.60 for placebo and 0.61 for rimegepant (Fig. 2; Table 3). Over the 12-week DBT, EQ-5D utilities increased by + 0.09 in the placebo arm and + 0.10 for rimegepant (p value = 0.011). By 24 weeks, EQ-5D values for the placebo/rimegepant OLE arm caught up with values from the rimegepant/rimegepant OLE arm (0.77 and 0.76 respectively; Fig. 2; Table 3). Additional improvements in health state utility values were observed throughout the OLE phase from week 24 to 64. By the end of the OLE phase, mean EQ-5D values were 0.79 (0.01) for participants who started on placebo and for participants who started on rimegepant (Table 3).
The regression model for EQ-5D utility based on the mapped data over the BHV3000-305 OLE study is presented in Table 4. Baseline MMD, treatment arm, and time were all significant predictors of mapped EQ-5D utility value in the model (Table 4). EQ-5D decreased with higher baseline MMD and increased over time, and when patients received rimegepant over placebo (Table 4). The rimegepant coefficient (0.0254) represents the additional utility associated with receiving rimegepant at any time point—which is the randomized subset of rimegepant patients from 0 to 12 weeks, and all patients at subsequent time points (Table 4). The value of + 0.0948 at 12 weeks represents the placebo effect, i.e., the increase in utility over the initial (double-blind) trial period, for placebo patients (Table 4).
Modeled change from baseline values for EQ-5D over the DBT and OLE phases of study BHV3000-305 are presented in Fig. 3. The curves are similar in shape to Fig. 2, in that rimegepant confers a greater increase in EQ-5D utility during the DBT phase. When patients randomized to placebo initiate rimegepant at 12 weeks, a marked improvement in HRQoL is observed. The improvement in EQ-5D is durable out to 64 weeks with continued rimegepant treatment.
Discussion
Key Findings
Compared to placebo, during the 12-week DBT phase, treatment with rimegepant 75 mg was associated with greater improvement in HRQoL when MSQv2 values were mapped to EQ-5D utilities. HRQoL improvements during the first 12 weeks were driven by the greater MMD reduction with rimegepant vs. placebo, reported previously by Croop et al. (− 4.3 days vs. − 3.5 days; least squares mean difference of − 0.8 days, 95% CI − 1.46 to − 20; p = 0.0099) [9]. This translated to greater health state utility improvement when mapped to EQ-5D.
By 24 weeks (i.e., 12-week DBT period + first 12 weeks of OLE), at which point patients who were originally randomized to the placebo arm had received rimegepant 75 mg EOD for 12 weeks, HRQoL (MSQv2 and mapped EQ-5D utilities) was similar across treatment arms, demonstrating rapid onset of treatment effect. The effect of rimegepant on improved HRQoL was durable out to 64 weeks post-randomization and was associated with an additional increase in MSQv2 and mapped health state utilities over the OLE period. The overall improvement in HRQoL at 64 weeks (as measured by all MSQv2 domains) was greater than the established minimum important differences and was therefore clinically meaningful.
Comparison to Literature
Other novel CGRP antagonists for migraine prevention have demonstrated similar long-term improvements in HRQoL in OLE studies. For example, a 9-month OLE study of galcanezumab found that MSQv2 RFR domain scores increased from baseline by a mean of 29.0–32.9 points at Month 12 (p < 0.001) [29]. A similar magnitude of increase from baseline to the end of the OLE period was observed in the current analysis of rimegepant. Clinically meaningful and durable HRQoL improvements have also been reported in OLE studies of fremanezumab and atogepant, confirming the value of this class of therapies for improving lives of patients with migraine, over longer time horizons [30, 31]. Descriptively, the magnitude of HRQoL improvements appear to be comparable across different CGRP antagonists; however, to date there have been no head-to-head trials to confirm this.
MSQv2 data from several other migraine prevention trials have been mapped to EQ-5D values using similar methods as the current post hoc analysis. For example, Di Tanna mapped MSQv2 to EQ-5D using data from three erenumab studies for migraine prevention [32]. In these studies, MMDs ranged from 8.2 ± 2.5 to 18.2 ± 4.7 at baseline, and mapped EQ-5D values from MSQv2 improved from 0.62 (0.18) at baseline to 0.74 (0.14) at week 12 with erenumab 140 mg treatment [32]. This is comparable to the improvement in mapped EQ-5D values with rimegepant in the current study. Prior studies have demonstrated a negative correlation between a number of MMDs and mapped utility values (e.g., utility values increased when the number of MMDs decreased), which is also supported by the current analysis. Lipton et al. also estimated EQ-5D-3L (UK Value set) from MSQv2 scores using the algorithm by Gillard et al. for patients in the US treated with erenumab for migraine prevention [33]. The mapped utility values were higher for migraine patients using erenumab vs. standard of care and were inversely related to baseline MMD [33].
The same MSQv2 mapping algorithm by Gillard et al. [17] has been used in health technology assessments of three migraine preventive therapies—erenumab, fremanezumab, and galcanezumab—by the National Institute for Health and Care Excellence (NICE) in the UK and was considered a valid method of estimating health state utility values for the purpose of economic modeling [34,35,36]. In one situation (erenumab technology appraisal), even though EQ-5D utilities were collected in one of the key erenumab prevention trials [37], MSQv2 mapped to EQ-5D values was the preferred model input. This was due to the longer recall period of MSQv2 (4 weeks) and that this disease-specific measure was more sensitive to change in migraine symptoms over time than the EQ-5D measure, which was limited to patient HRQoL measured on the day of the appointment [35].
EQ-5D has been directly used to measure quality of life in patients with migraine in observational research and in some trial settings. Findings vary substantially depending on underlying disease severity, whether patients are asked on a day with or without migraine, and pain severity experienced. For example, Stafford measured EQ-5D-3L in patients with migraine in the UK with a mean age of 47.5 years, 76.4% female, and a mean MMD (SD) of 5.2 (4.1) [38]. Utility scores were 0.87 in patients currently without a migraine, 0.66 in patients with mild migraine pain severity, 0.53 in patients with moderate pain severity, and -0.20 in patients with severe pain [38]. A study characterizing the burden of migraine in Sweden (n = 630) reports EQ-5D-5L index scores of 0.79 for patients on a day without migraine compared with 0.42 on a day with migraine [39]. Similarly, EQ VAS scores were significantly lower on a day with migraine (0.39) compared with a day without migraine (0.67) [39]. Migraine presence and severity at the time EQ-5D is administered therefore have a significant impact on EQ-5D values.
When measured directly, EQ-5D values are also influenced by migraine frequency as MSQv2 and EQ-5D scores improve as MMDs decrease. Patients with ≥ 4 monthly headache days (MHDs) have lower EQ-5D-5L score and poorer HRQoL than people without migraine (0.68 vs. 0.81, p < 0.001) [40]. HRQoL deteriorates with increasing migraine frequency (incremental burden), with EQ-5D scores of 0.74 for those with 4–7 MHDs, 0.7 for those with 8–14 MHDs, and 0.56 for those with chronic migraine (≥ 15 MHDs) [40]. Doane et al. compared the humanistic burden of migraine in patients with migraine in Europe according to number of monthly headache days (1–3 MHDs vs. ≥ 4 MHDs) [41]. The authors found that HRQoL was significantly lower (as measured by EQ-5D-5L and EQ-5D VAS) in patients with migraine with ≥ 4 MHDs compared to those with 1–3 MHDs [41]. EQ-5D index scores and EQ-5D VAS scores were 0.75 and 66.85, respectively, in those with 1–3 MHDs, 0.72 and 64.92, respectively, in those with 4–7 MHDs, 0.70 and 61.86, respectively, in those with 8–14 MHDs, and 0.60 and 52.66, respectively, in those with ≥ 15 MHDs [41]. The inverse is also true—i.e., as headache-free days (HFDs) increase, EQ-5D-5L scores increase as well [42]. EQ-5D index score increased by 0.01 points for every 1 HFD and by 0.04 points for 5 HFDs, while EQ VAS increased by 0.76 for every 1 HFD and by 3.79 for every 5 HFDs (p < 0.001 for both) [42].
Common trends across these studies confirm that (1) effective migraine preventive treatments improve MMDs, MSQv2 scores, and EQ-5D utilities to a greater degree than placebo and (3) MSQv2 and EQ-5D scores improve as MMDs decrease.
Implication of Findings
Very few migraine studies assess EQ-5D directly during clinical trials. The results of this study are important as, although MSQv2 is recommended as a HRQoL measurement for migraine, EQ-5D utility measurements are also relevant as they can be used for broader comparisons. These results can help guide clinical decision-making for patients as direct comparisons with other novel treatments can be made. EQ-5D is an important component of cost-effective analysis. The results from this study can therefore be used to inform economic models in the future.
Limitations
This secondary analysis included data from the 52 week OLE phase, which would not have been as rigorously controlled as the DBT phase [9]. However, it is not realistic to conduct double-blind placebo-controlled studies for periods of this length. The sample size of the current study was smaller than the calculated sample size required to provide 95% power for the primary efficacy point in the rimegepant RCT [9]. BHV3000-305 was a mixture of patients with episodic (77%) and chronic migraine (23%) [9]. This may affect the EQ-5D utilities calculated as variability may be greater than in a strictly EM population. Another limitation might be a miscalculation for chronic patients in the mapping algorithm. If patients did not report history of chronic migraine at baseline, some may have developed chronic migraines throughout the study and may have been misclassified, as only baseline characteristics were considered for this analysis. However, given the effectiveness of rimegepant to reduce MMDs, it is unlikely that many patients developed chronic migraine during the study period. Ideally, these results would be replicated in a less selected study population to increase generalizability of the results.
The mapping algorithm that was used in this study to estimate EQ-5D-3L utility values from MSQv2 may overestimate utility values for patients with migraine who have greater disease severity [17]. However, NICE considered the mapped EQ-5D-3L utility values from MSQ using the Gillard et al. algorithm to be underestimates but reasonable [35].
The algorithm used a UK valuation set [17], but this study was conducted in the US, which may affect the validity of the findings. However, the UK value set has previously been used in a US dataset [19]. Another study which calculated EQ-5D index scores using both UK and US value sets in patients in the US with HIV found that the scores generated were generally similar between these value sets [43]. However, a study that compared EQ-5D index scores using Japanese, UK, and US value sets in Thai patients with type 2 diabetes found that the US value set generated higher scores than the Japanese and UK value sets [44].
Strengths
All of the measures calculated in this study (MSQv2, EQ-5D, and mapping MSQv2 to EQ-5D) have been previously validated and published [17, 18, 21]. EQ-5D utilities are well-known HRQoL measures and represent the strength of an individual’s preference for specific health states or conditions, using a scale anchored at 1 (full health), 0 (dead), and < 0 (worse than dead). These measures have been used previously in migraine patients [25,26,27]. The methods used in the secondary analysis have also been used in a previously published article and poster which examined migraines [19, 45].
Possible Improvements
One way this study could have been approved upon is if the trial calculated EQ-5D utilities directly rather than relying on mapping. However, MSQv2 is known as a disease-specific measure of HRQoL sensitive to treatment response and this is why it was chosen in the trial. As pointed out previously, MSQv2 assesses HRQoL over a 4-week period rather than on one day like the EQ-5D, and therefore it may provide a better idea of patients’ HRQoL because EQ-5D can be greatly affected by migraine frequency, severity, and whether or not patients have a migraine or not on the day of the assessment.
Other ways in which this study can be improved include using US preference weights for the mapping as the country- or region-specific EQ-5D value set that is used can impact utility values.
Next steps involve collecting additional evidence from observational studies, such as EQ-5D measurements from those receiving rimegepant in real-world practice to provide further confirmation utilities seen in migraine patients taking this medication in a real-world setting and in a less restrictive population of patients with migraine.
Conclusions
Compared to the placebo, during the 12-week DBT phase, treatment with rimegepant 75 mg was associated with greater improvement in MSQv2 and mapped EQ-5D utilities. At 24 weeks (after 12 weeks of taking rimegepant 75 mg EOD during the OLE phase), scores of participants originally randomized to the placebo arm caught up to the rimegepant arm, demonstrating a rapid onset of treatment effect. This preventive effect was durable to 64 weeks post-randomization and was associated with an additional increase in HRQoL measures over the OLE period. Overall, the increase in MSQv2 was 3–6 times greater than the threshold for minimum important differences, depending on domain. This confirms that the magnitude of the HRQoL improvements was meaningful to patients. Results of the mapped MSQv2 to EQ-5D analysis further quantifies this improvement and can inform future economic evaluations of rimegepant and comparisons to other novel therapies.
References
Ailani J, Burch R, Robins M, on behalf of the Board of Directors of the American Headache Society. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–39.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6.
Biohaven Pharmaceutical Inc. NURTEC ODT (rimegepant) Prescribing Information. New Haven, CT: FDA; 2021.
ClinicalTrials.gov. Trial in Adult Subjects with Acute Migraine 2018 [updated March 27, 2020]. https://clinicaltrials.gov/ct2/show/NCT03461757?term=NCT03461757&draw=2&rank=1. Accessed 15 Aug 2022.
Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet (London, England). 2019;394(10200):737–45.
Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142–9.
ClinicalTrials.gov. Efficacy and Safety Trial of Rimegepant for Migraine Prevention in Adults: U.S. National Library of Medicine; 2018 [updated Aug 9, 2021. NCT03732638]. https://clinicaltrials.gov/ct2/show/NCT03732638?term=BHV3000-305&draw=2&rank=1. Accessed 15 Aug 2022.
Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2021;397(10268):51–60.
Amgen. AIMOVIGTM (erenumab-aooe) injection Prescribing Information. Thousand Oaks: FDA; 2018.
Eli Lilly and Company. EMGALITYTM (galcanezumab-gnlm) injection prescribing information. Indianapolis: FDA; 2018.
Lundbeck Seattle BioPharmaceuticals Inc. VYEPTITM (eptinezumab-jjmr) injection, for intravenous use Prescribing Information. Bothel: FDA; 2020.
Teva Pharmaceuticals USA Inc. AJOVYTM (fremanezumab-vfrm) injection prescribing information. North Wales: FDA; 2018.
Hubig L, Smith T, Powell L, Johnson K, Harris L, Litalien G, et al., editors. Patient preferences for calcitonin gene-related peptide (CGRP) inhibitors in the preventive treatment of migraine: A discrete choice experiment in the US and Germany. Poster presented at Amercian Headache Society Annual Meeting; 2022.
Diener HC, Tassorelli C, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40(10):1026–44.
McGinley JS, Houts CR, Nishida TK, Buse DC, Lipton RB, Goadsby PJ, et al. Systematic review of outcomes and endpoints in preventive migraine clinical trials. Headache. 2021;61(2):253–62.
Gillard P, Devine B, Varon S, Liu L, Sullivan S. Mapping from disease-specific measures to health-state utility values in individuals with migraine. Value Health. 2012;15(3):485–95.
Bagley C, Rendas-Baum R, Maglinte G, Yang M, Varon S, Lee J, et al. Validating Migraine-Specific Quality of Life Questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52(3):409–12.
Johnston KM, L’Italien G, Popoff E, Powell L, Croop R, Thiry A, et al. Mapping migraine-specific quality of life to health state utilities in patients receiving rimegepant. Adv Ther. 2021;38(10):5209–20.
Schulz K, Altman D, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. PLoS Med. 2010;7(3):e1000251.
Martin BC, Pathak DS, Sharfman MI, Adelman JU, Taylor F, Kwong WJ, et al. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1). Headache. 2000;40(3):204–15.
Speck RM, Yu R, Ford JH, Ayer DW, Bhandari R, Wyrwich KW. Psychometric validation and meaningful within-patient change of the Migraine-Specific Quality of Life questionnaire version 2.1 electronic patient-reported outcome in patients with episodic and chronic migraine. Headache. 2021;61(3):511–26.
Cole JC, Lin P, Rupnow MF. Minimal important differences in the Migraine-Specific Quality of Life Questionnaire (MSQ) version. Cephalalgia. 2009;29(11):1180–7.
EuroQol Research Foundation. EQ-5D-3L User Guide 2018. https://euroqol.org/publications/user-guides. Accessed 15 Aug 2022.
Xu R, Insinga R, Golden W, Hu X. EuroQol (EQ-5D) health utility scores for patients with migraine. Qual Life Res. 2011;20:601–8.
Ko Y, Coons SJ. Self-reported chronic conditions and EQ-5D index scores in the US adult population. Curr Med Res Opin. 2006;22(10):2065–71.
Luo N, Johnson JA, Shaw JW, Coons SJ. Relative efficiency of the EQ-5D, HUI2, and HUI3 index scores in measuring health burden of chronic medical conditions in a population health survey in the United States. Med Care. 2009;47(1):53–60.
Biohaven Pharmaceuticals. BHV3000–305 Clinical Study Report Addendum 01. 2021.
Detke H, Pozo-Rosich P, Reuter U, Dolezil D, Li LQ, Wang S, et al. One-year treatment with galcanezumab in patients with chronic migraine: results from the open-label phase of the REGAIN study (P2.10–010). Neurology. 2019;92(15 Supplement):P2.10–010.
Spierings ELH, Ning X, Ramirez Campos V, Cohen JM, Barash S, Buse DC. Improvements in quality of life and work productivity with up to 6 months of fremanezumab treatment in patients with episodic and chronic migraine and documented inadequate response to 2 to 4 classes of migraine-preventive medications in the phase 3b FOCUS study. (1526–4610 (Electronic)).
Ashina M, Dodick D, Goadsby PJ, Reuter U, Silberstein S, Zhang F, et al. Erenumab (AMG 334) in episodic migraine: interim analysis of an ongoing open-label study. 2017(1526–632X (Electronic)).
Di Tanna GL, Porter JK, Lipton RB, Hatswell AJ, Sapra S, Villa G. Longitudinal assessment of utilities in patients with migraine: an analysis of erenumab randomized controlled trials. Health Qual Life Outcomes. 2019;17(1):171.
Lipton RB, Brennan A, Palmer S, Hatswell AJ, Porter JK, Sapra S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–75.
NICE. Galcanezumab for preventing migraine (TA659) 2020. https://www.nice.org.uk/guidance/ta659. Accessed 15 Aug 2022.
NICE. Erenumab for preventing migraine (TA682) 2021. http://www.nice.org.uk/guidance/ta682. Accessed 15 Aug 2022.
NICE. Fremanezumab for preventing migraine (TA764) 2022. http://www.nice.org.uk/guidance/ta764. Accessed 15 Aug 2022.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7.
Stafford MR, Hareendran A, Ng-Mak DS, Insinga RP, Xu R, Stull DE. EQ-5D™-derived utility values for different levels of migraine severity from a UK sample of migraineurs. Health Qual Life Outcomes. 2012;10:65.
Hjalte F, Olofsson S, Persson U, Linde M. Burden and costs of migraine in a Swedish defined patient population—a questionnaire-based study. J Headache Pain. 2019;20(1):65.
Vo P, Fang J, Bilitou A, et al. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain. 2018;19:82.
Doane MJ, Gupta S, Fang J, Laflamme AK, Vo P. The humanistic and economic burden of migraine in Europe: a cross-sectional survey in five countries. Neurology and therapy. 2020;9(2):535–49.
Doane M, Gupta S, Vo P, et al. Associations between headache-free days and patient-reported outcomes among migraine patients: a cross-sectional analysis of survey data in Europe. Pain Ther. 2019;8(2):203–16.
Huang IC, Willke RJ, Atkinson MJ, Lenderking WR, Frangakis C, Wu AW. US and UK versions of the EQ-5D preference weights: does choice of preference weights make a difference? Qual Life Res. 2007;16(6):1065–72.
Sakthong P, Charoenvisuthiwongs R, Shabunthom R. A comparison of EQ-5D index scores using the UK, US, and Japan preference weights in a Thai sample with type 2 diabetes. Health Qual Life Outcomes. 2008;6:71.
Johnston K, L'Italien G, Popoff E, Powell L, O'Sullivan F, Harris L, et al., editors. MSQ utility mapping of rimegepant by change in monthly migraine days for preventive treatment of migraine. American Academy of Neurology 2021 Annual Meeting; 2021; Virtual Poster.
Acknowledgements
The authors acknowledge and thank the participants of study BHV3000-305 and their families.
Funding
This study was funded by Biohaven Pharmaceuticals, New Haven, CT, USA, which is the manufacturer of rimegepant. Biohaven Pharmaceuticals also funded this journal’s Rapid Service and Open Access fees.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Adinet Lock. Support for this assistance funded by Broadstreet HEOR, Vancouver, Canada.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given approval for this version to be published. Lauren Powell, Gilbert L’Italien, Evan Popoff, Karissa Johnston, Fiona O’Sullivan, Linda Harris, and Richard B. Lipton contributed significantly to the conception and design of the work, the analysis, interpretation, and drafting of the most recently submitted version of the manuscript. Robert Croop and Vladimir Coric contributed significantly to the conception and interpretation of the work. The first draft of the manuscript was written by Adinet Lock and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
This manuscript is based on work that was presented at the 64th Annual Scientific Meeting of the American Headache Society, June 9–12, 2022.
Disclosures
Karissa Johnston, Evan Popoff, Lauren Powell, and Fiona O’Sullivan are employees of Broadstreet HEOR, which received funds from Biohaven Pharmaceuticals for this work. Linda Harris, Gilbert L’Italien, Robert Croop and Vladimir Coric are employed by and own stock/stock options in Biohaven Pharmaceuticals. Richard B. Lipton receives research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He serves on the editorial board of Neurology, as a senior advisor to Headache, and as an associate editor of Cephalalgia. He has reviewed for the NIA and NINDS; holds stock options in Biohaven Holdings and Manistee; and serves as consultant, advisory board member, or has received honoraria from: American Academy of Neurology, Allergan, American Headache Society, Amgen, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Lundbeck (Alder), Merck, Pfizer, Teva, and Vedanta. He receives royalties from Oxford University Press (Wolff’s Headache and Other Head Pain, 7th Edition [2001] and 8th Edition [2007]), Wiley, and Informa.
Compliance with Ethics Guidelines
This study is a secondary analysis of a multicenter, phase 2/3, randomized, double-blind, placebo-controlled trial (BHV3000-305). Study BHV3000-305 was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulations. The protocol was approved by Advarra central Institutional Review Board (IRB) in Columbia (USA) and MedStar (a study site IRB at McLean USA). The protocol is available in the supplementary information of Croop et al. 2021 [9]. Written consent was obtained from all participants prior to screening.
Data Availability
Data utilized in this study are patient-level data collected prospectively in the context of a randomized controlled and open-label study. Under the ethics agreement and patient-level privacy considerations, data cannot be made directly available, but the authors can provide further details upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Powell, L.C., L’Italien, G., Popoff, E. et al. Health State Utility Mapping of Rimegepant for the Preventive Treatment of Migraine: Double-Blind Treatment Phase and Open Label Extension (BHV3000-305). Adv Ther 40, 585–600 (2023). https://doi.org/10.1007/s12325-022-02369-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02369-x