Abstract
Introduction
Dysmenorrhea and endometriosis are common gynecologic disorders among women of reproductive age that significantly impact health-related quality of life (HRQL) as well as productivity. Although there are treatment options listed in Japanese guidelines, a gap remains in unmet medical needs for maximizing treatment outcome. The extended regimen of ethinylestradiol and drospirenone (EE/DRSP) (taken daily for up to 120 consecutive days) has been available in Japan for treating dysmenorrhea and/or endometriosis-associated pain since 2016. Yet, the effectiveness of its usage on HRQL has not been investigated elsewhere to date. Therefore, in this study, we aim to observe changes in HRQL of Japanese women treated with an extended regimen of EE/DRSP for dysmenorrhea and/or endometriosis-associated pain.
Methods
As part of a 2-year post-marketing surveillance study, women with dysmenorrhea or endometriosis-associated pelvic pain were prescribed extended EE/DRSP during routine clinical practice. Data were collected 1 month before and 3 and 6 months after initiating treatment. Primary outcomes were the Menstrual Distress Questionnaire (MDQ) (before, during, and after menstruation) in patients with dysmenorrhea, and the Endometriosis Impact Scale (EIS) and European Quality of Life 5-dimensions 5-level instrument (EQ-5D-5L) in patients with endometriosis.
Results
The study cohort included 315 patients (mean age 28.9 years) with dysmenorrhea and 262 patients (mean age 31.3 years) with endometriosis. Mean MDQ total scores before and during menstruation decreased significantly after 6 months with extended EE/DRSP; there was no improvement in after-menstruation MDQ score. Mean EIS domain scores improved significantly by 6 months, with improvement in most EIS individual item scores. Mean EQ-5D-5L scores increased slightly during 6 months of treatment.
Conclusions
Extended EE/DRSP treatment improved HRQL outcomes in Japanese women with dysmenorrhea or endometriosis-associated pelvic pain.
Trial Registration
Registered at ClinicalTrials.gov (NCT03126747) on June 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Endometriosis and dysmenorrhea are common gynecologic disorders that affect HRQL and productivity of women of reproductive age. Therefore, generating real-world evidence of patients treated with a current treatment option, the extended regimen of EE/DRSP, is an urgent matter and would add scientific value for future treatment selection processes. |
Previous study revealed that the extended regimen of EE/DRSP improved endometriosis-related pain. However, the impact of the extended regimen on HRQL in patients with dysmenorrhea or endometriosis-associated pain in a real-world setting has not been investigated to date. |
What was learned from the study? |
The overall results demonstrated that the extended EE/DRSP regimen was associated with improvement in HRQL of women suffering from dysmenorrhea and endometriosis-associated pelvic pain. |
The extended regimen of EE/DRSP was associated with improvement in MDQ score (particularly in pain and negative affect domains) as well as the majority of EIS domains. |
Regardless of patient treatment status (either naïve or previously treated with cyclic regimen: 28-day cycles), the extended regimen of EE/DRSP presented improvement in MDQ; therefore, treatment with the extended regimen for patients with dysmenorrhea and endometriosis-associated pelvic pain may optimize treatment outcome. |
Introduction
Endometriosis is a common and chronic inflammatory disease affecting women of reproductive age. It is characterized by the presence and growth of endometrial-like tissue on organs outside of the uterus, such as the ovaries, fallopian tubes, and intestines [1,2,3], and is generally believed to be an estrogen-dependent disorder [4, 5]. The main symptoms of endometriosis are pelvic pain and/or infertility [1], with pelvic pain peaking during menstruation. Other frequently reported symptoms include lower abdominal and lower back pain, and pain outside of menstruation, during defecation/urination, and during sexual intercourse [6, 7]. As a result of these symptoms, endometriosis impairs physical activities, sexual function, and personal relationships and thus has a significant negative impact on health-related quality of life (HRQL) [1, 2].
Endometriosis has been reported to affect up to 10% of women of reproductive age [8, 9], and there is evidence of endometriosis in approximately 50–70% of women and adolescent girls who have chronic pelvic pain or dysmenorrhea [10, 11]. Up to 50% of women experiencing infertility have endometriosis [12]. In Japan, approximately 2.6 million women, 9.4% of those of reproductive age, are reported to be affected by endometriosis [13].
Dysmenorrhea is defined as lower abdominal, lower back, or pelvic pain occurring during menstruation, and can be classified as primary (without underlying pathology) or secondary (associated with pelvic pathology or a recognized medical condition) [14]. It is one of the most common gynecologic disorders [15], although the reported prevalence varies widely, from 16.8% to up to 90% [15, 16]. Dysmenorrhea can be associated with nausea, headache, dizziness, insomnia, anxiety/irritability, diarrhea, and depression [15, 17]. As with endometriosis, dysmenorrhea impacts HRQL, as it leads to school and work absenteeism, and limits social, academic, and sporting activities [18,19,20,21].
In Japan, two large surveys (n = 19,254) found that 50% of women experienced dysmenorrhea, and 17% reported associated work productivity loss (paid work, school work, and household chores) in the previous 3 months; the total annual economic burden of menstrual symptoms (including dysmenorrhea) was estimated to be Japanese yen 682.8 billion (approximately US$ 8.6 billion) [22]. A further survey (n = 3941) found that approximately one-third of Japanese women reported severe dysmenorrhea that usually required analgesic therapy, and 18% reported that their daily activities were negatively impacted by dysmenorrhea [23].
The guidelines of the Japanese Society of Obstetrics and Gynecology (JSOG) and Japanese Association of Obstetricians and Gynecologists (JAOG) recommend non-steroidal anti-inflammatory drugs (NSAIDs), low-dose estrogen/progestin (LEP), and the levonorgestrel-releasing intrauterine system (LNG-IUS) as first-line treatment options for dysmenorrhea [24]. LEP combined oral contraceptives reduces the severity of dysmenorrhea symptoms in Japanese women [25, 26]. The JSOG/JAOG guidelines also recommend NSAIDs for the treatment for pain associated with endometriosis [24]. In patients whose symptoms do not respond to analgesics, either LEP or progestin is recommended as first-line therapy and a gonadotrophin-releasing hormone (GnRH) agonist or danazol as second-line therapy; LNG-IUS can also be considered [24].
In Japan, a combined formulation of the estrogen ethinylestradiol and the progesterone drospirenone (EE/DRSP, 0.02/3 mg), taken in a cyclic 28-day regimen (24 days of active and 4 days of placebo tablets), was approved and launched for dysmenorrhea in 2010 (YAZ®). This regimen results in a monthly withdrawal bleed. Given that menstrual pain is generally more severe during menstrual withdrawal bleeding, an extended regimen of EE/DRSP (YazFlex®) was developed, which is taken for up to 120 consecutive days followed by a 4-day tablet-free interval. This latter preparation has been available in Japan for the treatment of dysmenorrhea and for endometriosis-associated pelvic pain since 2016.
Clinical studies in Japanese women have demonstrated that the cyclic regimen of EE/DRSP improved HRQL outcomes associated with dysmenorrhea [27], the cyclic and extended regimens reduced dysmenorrhea-related pain [25, 28], and the extended regimen improved endometriosis-related pain [29]. However, the impact of the extended regimen of EE/DRSP on HRQL in patients with dysmenorrhea or endometriosis-associated pain in a real-world setting has not been determined.
A 2-year post-marketing surveillance (PMS) study was conducted to collect information on the safety and effectiveness of the extended regimen of EE/DRSP for the management of dysmenorrhea or endometriosis-associated pelvic pain in Japanese women in routine clinical practice (ClinicalTrials.gov identifier NCT03126747), the results of which have been published previously [30].
The study reported here was conducted as a sub-analysis of the PMS study [30] to assess change in HRQL. The analysis includes an evaluation of HRQL improvement in patients with dysmenorrhea or endometriosis who were switched from the cyclic 28-day regimen to the extended EE/DRSP regimen, and an assessment of whether patient background characteristics affected the changes in HRQL.
Methods
Study Design and Patient Enrollment
The PMS study was a 2-year, non-interventional, multicenter, single-cohort study in Japanese women with endometriosis-associated pelvic pain or dysmenorrhea treated with EE/DRSP in the extended regimen (ClinicalTrials.gov identifier NCT03126747). The study investigated the safety and efficacy of extended-regimen EE/DRSP and covered the period from April 2017 through December 2021. The current sub-analysis presents data for the first 6 months of each participant’s treatment while in the study. Endometriosis and dysmenorrhea were diagnosed and defined as described in the previously published PMS study [30]. All treatment decisions were determined by shared decision-making between physicians and patients, and treatment was administered according to routine clinical practice. Study participants were recruited from 107 healthcare institutions.
Treatment
The study participants receiving the cyclic 28-day regimen of EE/DRSP were instructed to take one active tablet every day for 24 days, followed by a 4-day tablet-free interval, and then start another cycle of 28-day treatment. For the extended regimen, participants were instructed to take one active tablet every day until day 24; from day 25 onward, participants could continue treatment up to a maximum of 120 days, or stop treatment for 4 days if they experienced three consecutive days of bleeding or spotting and then restart the extended regimen. If participants completed 120 consecutive days of treatment with the extended regimen, they were required to take a 4-day pill break, before restarting the treatment cycle.
Hospital visits occurred 1 month prior to the start of treatment, and at 3 and 6 months after initiating treatment. These visits took place as per routine clinical practice.
Study Outcomes
Change in HRQL with the extended regimen of EE/DRSP was assessed using the Menstrual Distress Questionnaire (MDQ) [31], the Endometriosis Impact Scale (EIS) [32], and the EQ-5D-5L [33]. The primary outcome was MDQ for patients with dysmenorrhea, and EIS and EQ-5D-5L for patients with endometriosis. The secondary outcomes were MDQ in patients who switched from the cyclic 28-day regimen to the extended regimen compared to those without previous hormonal treatment, and exploratory assessment of the influence of patient background characteristics on MDQ and EIS scores.
The MDQ includes 47 items in eight health-related domains: pain related to menstruation, concentration, behavioral effect, autonomic response, water retention, negative feeling, arousal, and control, with each assessed on a 4-point Likert scale of 0 (no symptom), 1 (mild), 2 (moderate), or 3 (strong). The total score before and during menstruation, and each domain score before, during, and after menstruation, are determined [31].
The EIS is a 22-item patient-reported outcome designed to assess the impact of the primary symptoms of endometriosis on two multi-item domains: physical activities and emotional well-being. The EIS also includes an additional item that assesses the impact of endometriosis on sexual activity, and a further five items that assess its impact on concentration, sleeping, household activities, paid work and study, and social and leisure activities [32]. Each item is rated on a 5-point rating scale (“not at all”, “slightly”, “moderately”, “a lot”, and “extremely”, plus “not applicable”), with the score for each item ranging from 0 to 100; a higher value indicates a greater impact of endometriosis.
The EQ-5D-5L was developed to describe and assess health status, using questions related to mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [33].
All the aforementioned are licensed tools, and license permission to use them was obtained from the copyright holders before conducting the study.
Data Collection
The following patient background characteristics were collected at study enrollment: age, body mass index (BMI), smoking history, alcohol consumption history, pregnancy history, childbirth (delivery) history, and medical history, including gynecologic primary diseases and past drug therapy.
MDQ, EIS, and EQ-5D-5L scores were collected approximately 1 month prior to the start of treatment, and at 3 months and 6 months after the start of treatment. Data were collected as part of routine clinical practice; the physician provided each patient with a copy of the relevant questionnaires to complete.
Statistical Analysis
The target study population for the current analysis was the same as the PMS study [30]. To allow the detection of at least one adverse drug reaction with a frequency of 1% and a probability of 95%, the target number of patients to be enrolled was set at 300 for both the dysmenorrhea group and the endometriosis group in the PMS study.
Categorical variables were expressed as frequencies, while continuous variables were expressed as mean (standard deviation, SD) values. Analysis of variance (ANOVA) with repeated measures was performed within the groups for continuous variables of the MDQ and EIS. All statistical tests were two-sided, with a statistical significance level of 0.05; however, p values were adjusted using the Bonferroni correction to account for statistical multiplicity. For the EQ-5D-5L, only a descriptive analysis was carried out. Statistical tests were performed using Statistical Analysis Software (SAS) Version 9.4 (SAS Institute. Inc., Cary, NC, USA).
For subgroup analysis, ANOVA with repeated measures was performed by stratifying participants into two groups: those who had switched from the cyclic 28-day EE/DRSP regimen to the extended regimen (the “switching subgroup”), and those who were newly treated with the extended regimen (the “naïve subgroup”). As exploratory analysis, mean MDQ total score and EIS score were stratified by patient background characteristics of age, BMI, delivery history, and previous hormone treatment, to assess if patient background characteristics affected the change in HRQL.
Descriptive analysis, such as patient background characteristics, was conducted using data from all the patients enrolled in the PMS study. For the statistical tests, only those participants for whom data were collected at all data collection points (i.e., pre-treatment, and 3 and 6 months post-treatment initiation) were analyzed, and missing values were supplemented using the last observation carried forward (LOCF) method. As a result of the exploratory nature of the study, all p values were nominal.
Ethics
The PMS study was conducted in accordance with the guidelines of Japanese Good Post-marketing Study Practice. Ethics approval was obtained in accordance with the requirements of all the participating institutions. All procedures involved in this study were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and Helsinki Declaration of 1964, and its later amendments. Prior to enrollment in the study, written informed consent was obtained from each participant for documentation of their data.
Results
Patient Characteristics
This analysis included 315 patients with dysmenorrhea and 262 with endometriosis. The mean age (SD) was 28.9 (7.2) [min–max 12–49] years in patients with dysmenorrhea and 31.3 (7.6) [min–max 18–51] years in those with endometriosis (Table 1). Mean (SD) BMI was similar in each group, 20.7 (2.72) kg/m2 and 20.6 (2.87) kg/m2, respectively. More than two-thirds of the patients in each group were nulliparous; however, patients with endometriosis had higher pregnancy and delivery rates than patients with dysmenorrhea (33.6% and 30.5% vs. 27.0% and 23.2%). Most patients in each group were non-smokers (more than 88%); approximately 13% in each group reported alcohol consumption.
Of the patients with dysmenorrhea, 87.9% (n = 277) were diagnosed with primary dysmenorrhea and 10.8% (n = 34) with secondary dysmenorrhea; the latter group included 18 cases of endometriosis, 12 of uterine fibrosis, five of adenomyosis, and two of other conditions (some patients had more than one disorder).
Approximately half of the patients in each group had switched from other hormone therapies to the extended regimen of EE/DRSP; the switching rate was 49.8% in patients with dysmenorrhea and 45.8% in patients with endometriosis. The most common previous hormone therapy was cyclic EE/DRSP (used by 103/315 [32.7%] patients with dysmenorrhea and 71/262 [27.1%] patients with endometriosis).
Changes in MDQ Scores
One month prior to treatment, patients with dysmenorrhea had mean (SD) MDQ total scores before, during, and after menstruation of 33.1 (25.5), 31.8 (22.7), and 8.8 (13.3), respectively (Table 2; Fig. 1). The before- and during-menstruation MDQ scores decreased significantly at 6 months after starting treatment, to 21.6 (22.6) and 22.1 (21.3), respectively (both P < 0.0001 vs. baseline). In contrast, the mean (SD) after-menstruation MDQ score decreased only slightly, to 7.1 (12.1) at 6 months (P = 0.1667) (Table 2; Fig. 1).
The before- and during-menstruation mean scores for the pain, concentration, behavior affect, autonomic response, water retention, and negative affect domains of the MDQ decreased significantly across the study period (Table 2). There was no statistically significant change in the before- and during-menstruation scores for the arousal, control, and other domains.
Regarding the after-menstruation MDQ scores, only the arousal domain showed a statistically significant improvement with treatment (P = 0.0471) (Table 2).
Changes in EIS scores
In patients with endometriosis, most domains and items of the EIS showed substantial improvement during treatment. Mean (SD) scores for the two multi-domains (i.e., physical activities and emotional well-being) increased slightly at 3 months, but then decreased significantly at 6 months, from 10.6 (18.4) before treatment to 4.3 (11.7) at 6 months (p = 0.0015) and from 12.5 (19.4) to 6.6 (15.0) (p = 0.0025), respectively (Fig. 2). Similarly, EIS item scores for difficulty concentrating (p = 0.0001), difficulty sleeping (p = 0.0007), and impact on household activities (p = 0.0018) decreased significantly at 6 months (Fig. 2). Treatment was also associated with significantly improved scores related to impact on paid work or study and social and leisure activities at 6 months (p = 0.0127 and p = 0.0008, respectively), despite scores increasing slightly at 3 months (Fig. 2). In contrast, treatment had less impact on items related to sexual activity. At 6 months, there was no significant improvement in scores for the items that described impact on sexual activity (p = 0.3874) and guilt about avoidance of sexual intercourse (p = 0.2288); however, the limited enjoyment of sexual intercourse item score did significantly improve (p < 0.0001) (Fig. 2).
EQ-5D-5L Scores
The mean (SD) EQ-5D-5L score in patients with endometriosis at 1 month before the start of treatment was 0.914 (0.134) and increased slightly to 0.949 (0.095) after 6 months of treatment.
MDQ Subgroup Analysis
Prior treatment
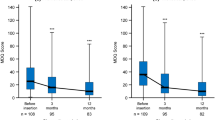
Among patients with dysmenorrhea, mean (SD) MDQ total before- and during-menstruation scores at 1 month before the start of treatment were higher in the naïve subgroup, 35.8 (26.5) and 36.4 (23.4), respectively, than in the switching subgroup, 31.6 (23.7) and 29.6 (20.4), respectively (Fig. 3a, b; Supplementary Table S1). In both subgroups, before- and during-menstruation scores decreased significantly by 6 months after the start of treatment (Fig. 3a, b). In contrast, after-menstruation scores were not significantly different at 6 months in either subgroup (Fig. 3a, b). There was no significant difference in treatment outcome (i.e., change in mean MDQ total before- and during-menstruation scores at 6 months) between the switching and naïve subgroups (Supplementary Table S1).
Change in mean Menstrual Distress Questionnaire (MDQ) total score before, during, and after menstruation in women with dysmenorrhea who a had switched from the cyclic 28-day regimen of ethinylestradiol/drospirenone (EE/DRSP) (switching subgroup), and b had not previously received hormone treatment (naïve subgroup). Error bars represent standard deviation
Patient Background Characteristics
In the exploratory analysis of MDQ total score by patient background characteristics, the before-menstruation score decreased significantly by 6 months after treatment initiation in all patient subgroups, with the exception of patients aged 40 years or older and those with a BMI of at least 21 kg/m2 (Supplementary Table S1). Similarly, during-menstruation scores decreased in all subgroups at 6 months, with the exception of patients aged 40 years or older, and those with a history of delivery (Supplementary Table S1).
However, it should be noted that the number of patients was low in the 40 years or older subgroup (n ≤ 20), and the baseline MDQ total score was lower in this subgroup than in the 29 years or younger subgroup and the 30–39 years subgroup (23.5 vs. 37.2 and 29.5, respectively). Similarly, there was a difference in baseline MDQ total score between the BMI subgroups (33.5 in those with a BMI of less than 21 kg/m2 vs. 36.6 in those with a BMI of greater than 21 kg/m2).
EIS Subgroup Analysis
The results of the subgroup analysis of EIS scores by patient baseline characteristics are shown in Supplementary Table S2.
Age, BMI, and delivery history had some impact on EIS domain scores. For example, the physical activities and emotional well-being domain scores only improved significantly in those aged 30–39 years, with a BMI less than 21 kg/m2, and with no delivery history (Supplementary Table S2). A similar pattern was seen in some of the single domains (such as those assessing an impact on concentration, sleeping [plus an effect in those aged 40 years or older], household activities, and social/leisure activities) (Supplementary Table S2).
Only patients in the switching subgroup experienced a significant improvement in the EIS scores related to physical activities, emotional well-being, and sleeping (Supplementary Table S2). In contrast, only patients in the naïve subgroup experienced a significant improvement in EIS scores for the impact on household activities, social and leisure activities, and paid work and study (Supplementary Table S2).
There was only a small number of patients in the 40 years or older subgroup (n < 40), and there was some variation in the baseline EIS scores (i.e., 1 month before the start of treatment) between the subgroups for most comparisons. For example, baseline scores for almost all domains were lower (indicating better HRQL) in the older age subgroup (40 years or older) than the younger (30–39 years) and youngest (29 years or less) age subgroups (Supplementary Table S2). Similarly, a lower (vs. higher) BMI and a positive (vs. negative) history of delivery or history of previous hormone treatment were associated with lower baseline EIS scores for all domains.
Discussion
The present PMS study was a multicenter, non-interventional, single-cohort study of patients with dysmenorrhea (n = 315) or endometriosis-associated pelvic pain (n = 262) who initiated treatment with an extended regimen of EE/DRSP. The current analysis focused on changes in HRQL for these patients, evaluated using MDQ, EIS, and EQ-5D-5L scores. The overall results demonstrated that the extended EE/DRSP regimen was associated with improved HRQL in women with dysmenorrhea and in those with endometriosis.
The mean MDQ total score improved significantly after treatment in patients with dysmenorrhea, particularly in the before- and during-menstruation periods (no clear effect was seen after menstruation). This timeline of effect is likely related to the onset of menstrual symptoms, including pain, which usually begin about 1 week before and peak 24–48 h after the onset of menstruation. Considering the concept of a minimum importance difference (MID), using the distribution-based method, a score change of 0.5 × SD is considered indicative of a clinically meaningful change [34]. The results from the current study show a decrease of 0.5 × SD in both the before- and during-menstruation MDQ scores, suggesting that these were clinically meaningful improvements. Focusing on the MDQ domains, although scores for some domains (i.e., arousal, control, and other domains) did not show improvements throughout the study period, improvements were observed in the physical domains (e.g., pain), as well as in the mental domains (e.g., negative affect). These improvements in the physical and mental domains, in turn, may have led to a significant positive change in the concentration and behavioral effect domains.
In patients with endometriosis, statistically significant differences were found for almost all EIS domains and items, with decreases of more than 50% observed for most items (including physical activity, emotional well-being, limited enjoyment of sexual intercourse, difficulty concentrating, difficulty sleeping, impact on household activity, impact on paid work or study, and impact on social and leisure activities) at 6 months. The exception was the effect on items related to sexual activity. No effect was seen on the impact on sexual activity and guilt about avoidance of sexual intercourse items, although a significant improvement was seen in the limited enjoyment of sexual intercourse item. Fewer participants responded to the questions about sexual activity than to those related to other aspects of life, possibly because not all participants had a sexual partner. Despite the small number of respondents, a large improvement (p < 0.0001) was observed in the item related to enjoyment of sexual intercourse, indicating how pain limits enjoyment of sexual activity for patients with endometriosis and how effective treatment can improve this aspect. While the domain and item scores did not change by 0.5 × SD (i.e., the MID), a change of at least 0.3 × SD was seen in most scores.
Interestingly, although a significant decrease was seen on most EIS domains and items at 6 months, the scores after 3 months’ follow-up were higher (indicating a deterioration in HRQL) than those at 1 month prior to treatment for some domains and items. The exact reason for the increase in EIS scores in the first 3 months of treatment in this study is not clear. However, some suggestions can be made; for example, the increase in EIS scores at 3 months was more marked in patients without previous hormone treatment than in those who had previously received such treatment, possibly because treatment-naïve patients are more likely to experience adverse effects such as irregular bleeding at the start of treatment, which may have had a negative impact on HRQL. Despite this temporary reduction in HRQL, the EIS domain and item scores significantly improved with ongoing treatment, emphasizing the importance of continued therapy with the extended EE/DRSP regimen.
The subgroup analysis in patients with dysmenorrhea indicated that switching from the cyclic regimen to the extended regimen results in improved mean MDQ total scores, even in patients who had previously been taking LEP in 28-day cycles. This suggests that further improvement of HRQL is possible in patients already receiving hormone treatment, as well as in those who are treatment naïve. When mean MDQ scores were stratified by age, BMI, delivery history, previous hormone treatment, and type of dysmenorrhea, patients aged 40 years or older and those with a BMI of at least 21 kg/m2 showed less improvement; however, since there were only a few patients aged 40 years or older, the small sample size may have influenced these results.
Among patients with endometriosis, the EIS physical and emotional well-being domains tended to show greater improvement at 6 months in the subgroup of patients who had previously received hormone treatment than in the naïve subgroup. In contrast, patients in the naïve subgroup had greater improvements in scores related to the impact on household activities, paid study or work, and leisure and social activities items. It is possible that the previous hormone treatment had a positive effect on these activities; thus, there may have been little potential for further improvement with the extended regimen. On the contrary, patients with no previous hormone treatment showed a greater degree of improvement in these items, indicating that pain due to endometriosis has a significant impact on housework, employment, and social activities.
When mean EIS scores were stratified by age, study participants aged 30–39 years tended to report greater improvement in HRQL than younger or older participants. While this may be explained in part by low baseline scores on most EIS domains for the older patients (and so limited potential for improvement with treatment), it suggests that pain relief for endometriosis does not necessarily improve HRQL in the youngest patients. These patients had the highest baseline scores (i.e., lowest HRQL) of the three age subgroups on most EIS domains and, while treatment resulted in numerical improvements in EIS domains, the effects were not statistically significant. This indicates that factors other than pain, such as other endometriosis-associated symptoms, may be involved in the reduction in HRQL experienced by younger individuals. As such, it may be necessary to provide a comprehensive treatment that includes mental healthcare, as well as pain relief, for younger patients with endometriosis.
In addition, patients with a BMI of at least 21 kg/m2 and those with a delivery history tended to experience smaller changes in HRQL. Given that higher BMI corresponds to a larger body volume, if all patients received the same EE/DRSP dose, those with a higher BMI may have experienced lower plasma drug concentrations and thus drug effects. Therefore, patients with a higher BMI may not experience the same improvements in HRQL as those with a lower BMI. Patients with a history of delivery might have breastfed their infants. Breastfeeding can cause hormonal changes that may relieve pain and reduce endometriosis recurrence [35]. Indeed, patients with a delivery history had lower scores at 1 month prior to the start of treatment, suggesting a better baseline HRQL, compared with those without a delivery history and, therefore, less potential for improvement in HRQL with EE/DRSP.
The results of this analysis are consistent with those of a previous real-world study of the cyclic regimen of EE/DRSP, which showed that 6–8 cycles of treatment had a positive impact on HRQL in Japanese women with dysmenorrhea (n = 186), as assessed using the 36-Item Short-Form Health Survey [27]. Similar results have also been observed in recent studies (including prospective clinical trials) with other hormonal treatments; improvements in HRQL in patients with endometriosis-associated pain or dysmenorrhea have been demonstrated with pharmacotherapies such as combined oral contraceptives, gonadotropin-releasing hormone receptor antagonists, and subdermal implant and oral formulations of progestin [36,37,38,39,40]. These studies, and the results of our sub-analysis, indicate the significant negative impact that both endometriosis-associated pain and dysmenorrhea can have on many aspects of a woman’s life (physical, social, mental, and economic), and that this can be improved with the extended EE/DRSP regimen.
The present study has some limitations. Firstly, it did not include a control group or comparison group, and thus it may be difficult to clearly establish the effectiveness of the extended regimen of EE/DRSP on the target population. Secondly, EE/DRSP, as indicated for dysmenorrhea and relief of pain associated with endometriosis, can be administered for 120 consecutive days or taken on a cyclic 28-day regimen (for 24 days, then stopped for 4 days); however, adherence to the 120-day regimen was not monitored over the course of the study period. Therefore, some data included in our analysis may be from patients who did not fully adhere to the extended regimen. In future studies, adherence to the specific regimen should be assessed to allow the impact of the type of regimen on HRQL to be fully determined. Lastly, the variation in sample size with improved response rate towards the 6-month period attributed to implementation of an additional campaign by the study team for collecting study questionnaires (MDQ and EIS) may somehow affect the statistical analysis, yet we believe that it would not impact significantly on the final study result.
Conclusion
Six months’ treatment with the extended regimen of EE/DRSP improved HRQL in Japanese patients with dysmenorrhea or endometriosis-associated pelvic pain, including those who switched from the cyclic 28-day regimen of EE/DRSP. These results suggest that an extended regimen of EE/DRSP may be recommended for these latter patients when further improvement in HRQL is required, emphasizing the importance of continuous therapy to achieve optimal outcomes.
References
Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–82.
Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7:349–57.
Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152:R63-78.
Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30:39–45.
Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79.
Sinaii N, Plumb K, Cotton L, et al. Differences in characteristics among 1000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89:538–45.
Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6:34–41.
Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin N Am. 1997;24:235–58.
Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82:453–61.
Janssen EB, Rijkers AC, Hoppenbrouwers K, Meuleman C, D’Hooghe TM. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19:570–82.
Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol. 2010;202(534):e1-6.
Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–56.
Momoeda M. Epidemiology of endometriosis. Obstet Gynecol (Tokyo). 2005;72:294–301.
American College of Obstetricians and Gynecologists (ACOG). ACOG committee opinion no. 760: dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018;132:e249–58.
Osayande AS, Mehulic S. Diagnosis and initial management of dysmenorrhea. Am Fam Physician. 2014;89:341–6.
Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87:55–8.
Zhao S, Wu W, Kang R, Wang X. Significant increase in depression in women with primary dysmenorrhea: a systematic review and cumulative analysis. Front Psychiatry. 2021;12: 686514.
Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154:1226–9.
Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115:138–45.
Pitangui AC, Gomes MR, Lima AS, Schwingel PA, Albuquerque AP, de Araujo RC. Menstruation disturbances: prevalence, characteristics, and effects on the activities of daily living among adolescent girls from Brazil. J Pediatr Adolesc Gynecol. 2013;26:148–52.
Wong CL. Health-related quality of life among Chinese adolescent girls with dysmenorrhoea. Reprod Health. 2018;15:80.
Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16:1255–66.
Osuga Y, Hayashi K, Kobayashi Y, et al. Dysmenorrhea in Japanese women. Int J Gynaecol Obstet. 2005;88:82–3.
Kawaguchi R, Matsumoto K, Akira S, et al. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2017 edition. J Obstet Gynaecol Res. 2019;45:766–86.
Momoeda M, Kondo M, Elliesen J, Yasuda M, Yamamoto S, Harada T. Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study. Int J Womens Health. 2017;9:295–305.
Harada T, Momoeda M, Terakawa N, Taketani Y, Hoshiai H. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2011;95:1928–31.
Momoeda M, Akiyama S, Tanaka K, Suzukamo Y. Quality of life in Japanese patients with dysmenorrhea treated with ethinylestradiol 20 mug/drospirenone 3 mg in a real-world setting: an observational study. Int J Womens Health. 2020;12:327–38.
Momoeda M, Akiyama S, Yamamoto S, Kondo M, Fukai T. Burden of menstrual pain measured by heatmap visualization of daily patient-reported data in Japanese patients treated with ethinylestradiol/drospirenone: a randomized controlled study. Int J Womens Health. 2020;12:175–85.
Harada T, Kosaka S, Elliesen J, Yasuda M, Ito M, Momoeda M. Ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril. 2017;108:798–805.
Yamamoto S, Ishimori S, Sunaya T, Watanabe A, Sato S. Safety and effectiveness of drospirenone/ethinyl estradiol combined tablets for endometriosis-associated pelvic pain and dysmenorrhea in real-world clinical practice: data from post-marketing surveillance in Japan. Ther Res. 2020;41:381–96.
Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30:853–67.
Gater A, Taylor F, Seitz C, Gerlinger C, Wichmann K, Haberland C. Development and content validation of two new patient-reported outcome measures for endometriosis: the Endometriosis Symptom Diary (ESD) and Endometriosis Impact Scale (EIS). J Patient Rep Outcomes. 2020;4:13.
Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92.
Prosperi Porta R, Sangiuliano C, Cavalli A, et al. Effects of breastfeeding on endometriosis-related pain: a prospective observational study. Int J Environ Res Public Health. 2021;18(20):10602.
Ambacher K, Secter M, Sanders AP. The use of progestin subdermal implants in the management of endometriosis-related pain symptoms and quality of life: a systematic review. Curr Med Res Opin. 2022;38(3):479–86.
Alcalde AM, Martinez-Zamora MA, Gracia M, et al. Assessment of quality of life, sexual quality of life, and pain symptoms in deep infiltrating endometriosis patients with or without associated adenomyosis and the influence of a flexible extended combined oral contraceptive regimen: results of a prospective, observational study. J Sex Med. 2022;19:311–8.
El Taha L, Abu Musa A, Khalifeh D, Khalil A, Abbasi S, Nassif J. Efficacy of dienogest vs combined oral contraceptive on pain associated with endometriosis: randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2021;267:205–12.
Sukhikh GT, Adamyan LV, Dubrovina SO, et al. Prolonged cyclical and continuous regimens of dydrogesterone are effective for reducing chronic pelvic pain in women with endometriosis: results of the ORCHIDEA study. Fertil Steril. 2021;116:1568–77.
Abrao MS, Surrey E, Gordon K, et al. Reductions in endometriosis-associated pain among women treated with elagolix are consistent across a range of baseline characteristics reflective of real-world patients. BMC Womens Health. 2021;21:246.
Acknowledgements
Funding
This work was supported by Bayer Yakuhin, Ltd., which actively participated in the study design and managed all operational aspects of the study, including monitoring data collection, statistical analyses, and writing of the report. Bayer Yakuhin Ltd also funded the Rapid Service Fee and Open Access Fee.
Medical Writing, Editorial, and Other Assistance
We would like to thank Kate Palmer of Springer Healthcare Communications who assisted with the development of the drafts of the manuscript in accordance with Good Publication Practice (GPP3) guideline (http://www.ismpp.org/gpp3). This medical writing assistance was funded by Bayer Yakuhin, Ltd., Tokyo, Japan.
Authors’ Contributions
Conceptualization: NT; acquisition of data: NT; analysis and interpretation of data: OY, YS, KY and NT; writing – original draft preparation: OY, YS, and NT; writing – review and editing: OY, YS, KY, and NT; statistical analysis: OY, YS, and NT; provision of study materials or patients: KY and NT; funding acquisition: NT; administrative, technical, or logistic support: KY and NT; supervision: OY and YS.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
This study was supported by Bayer Yakuhin, Ltd., Japan. The sponsor (Bayer) reviewed and provided comments on the study concept and protocol. The sponsor was also involved in the review and approval process for this manuscript. Noriko Takahashi and Keisuke Yoshihara are employees of Bayer Yakuhin, Ltd. Osamu Yoshino and Yoshimi Suzukamo declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
The study protocol was approved by the institutional review board of each participating institutions and was compliant with the Japanese Good Post-Marketing Study Practice guideline. All procedures involved in this study was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and Helsinki Declaration of 1964, and its later amendments. All participants provided written informed consent prior to study participation.
Data Availability
The dataset generated during this study is available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yoshino, O., Suzukamo, Y., Yoshihara, K. et al. Quality of Life in Japanese Patients with Dysmenorrhea or Endometriosis-Associated Pelvic Pain Treated with Extended Regimen Ethinylestradiol/Drospirenone in a Real-World Setting: A Prospective Observational Study. Adv Ther 39, 5087–5104 (2022). https://doi.org/10.1007/s12325-022-02301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02301-3