Abstract
Introduction
The present study collected 1-year follow-up patient-reported outcome data from Japanese women with dysmenorrhea and/or heavy menstrual bleeding (HMB) who underwent insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) 52 mg. We aimed to evaluate the quality of life (QOL) of Japanese women over the course of the investigational period.
Methods
This was a multicenter, non-interventional, prospective, single-cohort, post-marketing surveillance study (J-MIRAI). The primary outcome was the median change in the Menstrual Distress Questionnaire (MDQ) and Menorrhagia Multi-Attribute Scale (MMAS) scores from baseline to 3 and 12 months after LNG-IUS insertion, with decreasing and increasing scores, respectively, indicating improvement. The secondary outcomes were the statistical relationships between the MDQ and menstrual pain (measured by a visual analog scale, VAS), and between the MMAS and pictorial blood loss assessment chart (PBAC) scores by regression analysis.
Results
In total, 593 patients were evaluated; 376, 467, and 250 patients were diagnosed with dysmenorrhea, HMB, or both, respectively. The median MDQ score decreased significantly at 3 and 12 months after LNG-IUS insertion in both the premenstrual and menstrual periods (both p < 0.001 vs baseline), and the median MMAS score showed a similar improvement during the menstrual period. Changes in median MDQ and MMAS scores were observed regardless of patient background. Correlations between MDQ and menstrual pain (VAS) and between MMAS and PBAC scores were found (estimated regression coefficients 0.29 and − 0.15, respectively).
Conclusion
The LNG-IUS contributed to improvements in the QOL of patients with dysmenorrhea, HMB, and both, regardless of patient background characteristics.
Trial Registration
Registered at ClinicalTrials.gov (NCT02475356) on 18 June 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Dysmenorrhea and heavy menstrual bleeding (HMB) are common gynecological issues in women of childbearing age, and represent a substantial burden on quality of life. |
The levonorgestrel-releasing intrauterine system (LNG-IUS) has been approved in Japan for the aforementioned indications, but real-world safety and effectiveness data among Japanese women are lacking; furthermore, the association between clinical outcomes and patient-reported quality of life is not clearly understood. |
What was learned from the study? |
In this observational study (J-MIRAI), we evaluated patient-reported outcomes using the Menstrual Distress Questionnaire (MDQ) and the Menorrhagia Multi-Attribute Scale (MMAS), and we investigated the impact of changes in menstrual pain and pictorial blood loss assessment chart (PBAC) scores on patient-reported quality of life. |
The median MDQ score decreased significantly at 3 and 12 months after LNG-IUS insertion in both the premenstrual and menstrual periods, and the median MMAS score showed a similar improvement during the menstrual period; some statistical correlations were identified between the MDQ and menstrual pain (VAS) and between MMAS and PBAC scores. |
The LNG-IUS contributed to improvements in the quality of life of patients with dysmenorrhea and/or HMB, regardless of patient background characteristics. |
Introduction
Heavy menstrual bleeding (HMB) and dysmenorrhea are common gynecological symptoms that impose a substantial burden on women’s health [1, 2]. The prevalence of dysmenorrhea among women of reproductive age has been reported to range between 16% and 91% [3], and nearly a quarter of women suffer from HMB [4]. According to a previous study, nearly 20% of gynecology department visits are attributed to HMB [5]. In Japan, a large-scale study reported that nearly 75% of women experienced menstrual symptoms; these symptoms were attributed to dysmenorrhea in half of the women surveyed and to HMB in 19% of the women [5].
Primary dysmenorrhea is characterized by spasmodic, cramping menstrual pain, and discomfort in the absence of pelvic pathology [6]. In contrast, secondary dysmenorrhea is associated with a specific pelvic pathology such as endometriosis, adenomyosis, or uterine fibroids [7]. Nearly one-third of Japanese women have severe dysmenorrhea and generally require analgesic use [8]. The guideline from the Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists recommends non-steroidal anti-inflammatory drugs, low-dose estrogen–progestin, or the levonorgestrel-releasing intrauterine system (LNG-IUS) as first-line treatment for dysmenorrhea [9].
In Japan, HMB is clinically defined as a total menstrual blood loss of 140 mL or more per cycle [10], whereas overseas, a total menstrual blood loss greater than 80 mL per cycle is set as the cutoff [11]. Although these definitions requiring quantification of blood loss are useful for research, objective menstrual blood flow assessment is challenging under real-life conditions; thus, most international guidelines mention that a subjective assessment is helpful for diagnosis and treatment [12, 13]. Several guidelines include recommendations for medical treatment to reduce blood loss from HMB by addressing the underlying cause. Hormone treatments are among the most frequently recommended treatments [12].
These menstrual disorders often result in physical, behavioral, and emotional burdens in patients before and during their menstrual period [14], which can have a negative impact on a woman’s health-related quality of life (QOL), work productivity, and healthcare resource utilization [15,16,17]. Furthermore, both dysmenorrhea and HMB could impose a considerable socioeconomic burden due to direct and indirect medication costs, absenteeism from work, and reduced work performance [5]. In Japan, the annual economic burden due to menstrual symptoms was estimated at approximately 682.8 billion yen; over 70% of this burden was attributed to a loss in work productivity [5]. Therefore, efforts should be made to accurately diagnose HMB and dysmenorrhea and provide effective and prompt treatment to improve QOL and minimize the socioeconomic burden.
The LNG-IUS was originally developed as a method of contraception in the mid-1970s [18]; however, it was not available on the market until its first approval in Finland in the 1990s [19]. Mirena® (Bayer, Germany) is a LNG-IUS that delivers 20 μg/day of levonorgestrel directly into the uterine cavity [20]. Although its initial indication was contraception, further investigation into its non-contraceptive health benefits had extended its indications. A previous study showed that the use of LNG-IUS resulted in statistically and clinically significant reductions in menstrual blood loss [21]. The UK guideline for HMB recommends using LNG-IUS as a first-line treatment [13].
In Japan, LNG-IUS was approved as a contraceptive method in 2007. Later in 2014, its indication was extended to HMB and dysmenorrhea. In Japan, the approval of LNG-IUS for HMB and dysmenorrhea was fully dependent on clinical trial data obtained from studies conducted overseas. Therefore, post-launch, real-world data in Japanese patients with HMB and dysmenorrhea treated with the LNG-IUS are needed. Moreover, few studies have investigated an association between clinical outcomes and patient-reported outcomes in Japan; therefore, a study to evaluate this is also warranted.
This study primarily aimed to assess changes in the Menstrual Distress Questionnaire (MDQ) score and Menorrhagia Multi-Attribute Scale (MMAS) score throughout the investigational period. Additionally, we aimed to investigate the impact of changes in menstrual pain and pictorial blood loss assessment chart (PBAC) scores on the improvement of QOL in patients with dysmenorrhea and/or HMB.
Methods
Study Design and Patients
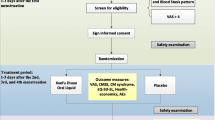
The present study was a non-interventional, prospective, single-cohort, post-marketing surveillance study conducted in 83 centers in Japan from 2015 to 2019. The study protocol was approved by the institutional review board of each participating center and was compliant with the Japanese Good Post-marketing Study Practice guidelines [22]. This study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments. All participants provided written informed consent prior to study participation. The study was registered at ClinicalTrials.gov under the identifier NCT02475356.
The patient population of the current analysis was drawn from the J-MIRAI study population. The study included women in Japan diagnosed with HMB and/or dysmenorrhea who underwent insertion of the LNG-IUS 52 mg for the treatment of HMB/dysmenorrhea. Women who had already started treatment with the LNG-IUS at the time of study initiation, as well as those using the LNG-IUS as a method of contraception, were excluded from the study. All patients who completed the MDQ and/or MMAS at each study timepoint were included in the present analysis. Each physician in charge directed the treatment decisions, and treatment was administered in the context of routine clinical practice. The observation period was 12 months from the LNG-IUS insertion.
Outcomes
The primary outcome in the current analysis was the median change in the MDQ score during the premenstrual and menstrual periods and MMAS score during the menstrual period over the course of the investigational period. The secondary outcomes were the statistical relationships between the MDQ score and menstrual pain (as measured by a visual analog scale, VAS) and between the MMAS and PBAC scores by regression analysis.
Health-related QOL for dysmenorrhea and HMB was measured using the Japanese version of the MDQ [23] and the Japanese version of the MMAS [24], respectively. The MDQ type A questionnaire includes 47 questions under the following eight health-related domains: (1) pain related to menstruation, (2) concentration, (3) behavioral effect, (4) autonomic response, (5) water retention, (6) negative affect, (7) arousal, and (8) control. The MDQ assesses the severity of symptoms on a four-point Likert scale as follows: 0, none; 1, mild; 2, moderate; and 3, severe. The total score and each domain score before and during menstruation were summarized. As the severity of symptoms improves, the MDQ score decreases. The MMAS is the QOL scale for HMB that evaluates the following six domains: (1) practical difficulties, (2) effects on social life, (3) psychological effects, (4) physical health, (5) interruption of work, and (6) family life. The total score of the MMAS is expressed on a scale from 0 (worst) to 100 (best), and the four options for each of the questions are individually weighted. The Japanese versions of the MDQ and MMAS are licensed tools and the license permission was obtained from copyright holders before conducting the study.
Menstrual pain and the amount of menstrual bleeding were measured using the VAS and PBAC. The VAS is a validated and reliable measure of chronic pain intensity [25,26,27,28], and it is usually presented as a 100-mm horizontal line on which the patient’s pain intensity is represented by a point between the extremes of “no pain at all” and “worst pain imaginable” [29]. The PBAC is a validated pictorial chart that records the number of tampons or sanitary towels (napkins) used and the degree to which they are stained with blood [30]. The score assigned is 1 for each lightly stained tampon, 5 if moderately soiled, and 10 if completely saturated with blood. The towels are given ascending scores of 1, 5, and 20. Small and large clots are scored as 1 and 5, respectively [31]. A higher PBAC score means more menstrual blood loss. The EuroQol Five Dimensions Five Levels (EQ-5D-5L) is a generic instrument used to measure health-related QOL. It is based on a descriptive system that defines health in terms of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Health status is determined using a conversion table, with 0 representing death and 1 representing perfect health. The Japanese version of the EQ-5D-5L was used [32].
Data Collection
Information such as patient demographic and clinical characteristics, including age, body mass index (BMI), pregnancy and childbirth history, treatment within 1-year of LNG-IUS insertion, and baseline EQ-5D-5L score was collected at 1 month prior to the initiation of LNG-IUS treatment to understand the general health status at baseline for each disease. MDQ and MMAS data were collected at 1 month prior to the LNG-IUS insertion and 3 and 12 months after LNG-IUS insertion. The MDQ and MMAS scores assessed at 1 month prior to the LNG-IUS insertion were considered as the baseline values. MDQ data were obtained in the premenstrual and menstrual periods, and MMAS data were obtained only during the menstrual period. The premenstrual and menstrual periods were defined as “from 7 days before menstruation to the beginning of menstruation” and “from the beginning to the end of menstruation”, respectively. VAS and PBAC scores were collected 1 month before LNG-IUS insertion and at 1, 3, 6, and 12 months following insertion. Hospital visits for data collection took place per routine clinical practice following the Japanese product label [33].
Statistical Analysis
Frequencies were calculated for categorical variables and mean (95% confidence interval, CI) or mean (standard deviation) and median (interquartile range [IQR], Q1–Q3) values were calculated for continuous variables. For continuous variables, the Wilcoxon signed-rank test was performed to test for differences between baseline and each timepoint. For the MDQ, a subgroup analysis was conducted by age, BMI, history of drug therapy, history of childbirth, history of surgery, type of dysmenorrhea, primary cause of secondary dysmenorrhea, and severity (baseline VAS score). For the MMAS, the subgroup analysis was conducted by age, BMI, history of drug therapy, history of childbirth, history of surgery, type of HMB, primary cause of secondary HMB, and severity (baseline PBAC score). In the subgroup analysis, repeated two-way analysis of variance (ANOVA) was performed to ascertain whether patient background factors could synergistically influence the effect of LNG-IUS on the time course trend of MDQ and MMAS scores. The statistical association between MDQ and menstrual pain and between MMAS and PBAC scores was investigated by log-normal regression analysis. Missing values were imputed using the last observation carried forward method. Each analysis was adjusted using Bonferroni’s correction method. A p value below 0.05 was set as the cutoff value for statistical significance. All the statistical analyses were performed using SAS version 9.4 or later (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 600 patients were enrolled in the post-marketing survey. Of these 600 patients, five patients did not undergo LNG-IUS insertion, and two patients had already used the LNG-IUS in the past. Thus, after exclusion of those patients, 593 patients were included in the final analysis. Before LNG-IUS insertion, the number of patients who completed the MDQ was 108 during the premenstrual period and 109 during the menstrual period, and that of patients who completed the MMAS was 186 during the menstrual period (Table S1 in the supplementary material).
The median age of the 593 patients was 42.0 years (IQR 38.0–45.0), and the median BMI was 21.2 kg/m2 (IQR 19.6–23.7) (Table 1). In total, 471 (79.4%) patients had a history of childbirth, and 89 (15%) patients had undergone a cesarean section. In the previous year, 263 (44.4%) patients had received pharmacotherapy for dysmenorrhea and/or HMB; the most common was an estrogen–progestin combination in 107 (18.0%) patients, followed by gonadotropin-releasing hormone agonists in 52 (8.8%) patients, and progestins alone in 51 (8.6%) patients. In addition, 186 (31.4%) patients had a history of surgery for gynecological diseases. Of these, 46 (7.8%) had surgery for endometriosis, and 37 (6.2%) for uterine fibroids, which were more common than other gynecological diseases. A total of 416 (70.2%) patients had a secondary disease. The most common secondary disease was adenomyosis (39.5%), followed by uterine fibroids (30.7%), endometriosis (13.0%), and endometrial hyperplasia (2.4%).
A total patients of 593 in the current study were classified into three groups (dysmenorrhea without or with other comorbidities, n = 376; HMB without or with other comorbidities, n = 467; and both dysmenorrhea and HMB, n = 250) to better define the patient background information for each disease. The patient background information for each group is summarized in Table 1.
The mean EQ-5D-5L score at 1 month prior to the LNG-IUS insertion for the study participants was 0.926 (95% CI 0.689, 1.000). A summary of EQ-5D-5L scores for each group is also presented in Table 1.
Changes in MDQ Scores
The changes in MDQ total scores in both the premenstrual and menstrual periods over the course of the investigational period are shown in Fig. 1 and Table S1. In the premenstrual period, the median MDQ total score was 25.5 (IQR 13.0–46.5) at 1 month prior to the LNG-IUS insertion, 16.0 (IQR 7.0–32.0) at 3 months after LNG-IUS insertion, and 10.0 (IQR 4.0–24.0) at 12 months after LNG-IUS insertion (Fig. 1a). A statistically significant improvement in MDQ scores was observed at 3 months vs baseline (p < 0.001) and at 12 months vs baseline (p < 0.001). In the menstrual period, the median total MDQ score was 36.0 (IQR 19.0–56.0) at 1 month prior to the LNG-IUS insertion, 16.0 (IQR 7.0–37.0) at 3 months after LNG-IUS insertion, and 10.0 (IQR 3.0–24.0) at 12 months after LNG-IUS insertion (Fig. 1b). Similar to the premenstrual period, a statistically significant improvement in the MDQ scores was observed during the menstrual period at 3 and 12 months after the LNG-IUS insertion vs the scores at baseline (both p < 0.001). Particularly during the menstrual period, a rapid decrease in the MDQ score was shown from baseline to 3 months after LNG-IUS insertion. This score decrease continued up to 12 months after LNG-IUS insertion. Overall, a significant improvement in the MDQ scores after LNG-IUS insertion was shown over the course of the investigational period during both the premenstrual and menstrual periods, and the improvement was more notable during the menstrual period.
All of the eight domain scores of the MDQ showed a statistically significant change at 12 months after LNG-IUS insertion in both the premenstrual and menstrual periods (Fig. 2, Table S1). In particular, improvements were observed in the domains of (1) pain and (3) behavioral effect. Among all of the domain scores of the MDQ, the median scores for (7) arousal and (8) control were the lowest at baseline, yet the results showed a statistically significant score change at the 1-year follow-up. MDQ data were analyzed comparing groups with and without missing value completion. There was no difference in the improvement trend in MDQ scores with or without missing value completion (data not shown).
Changes in MMAS Scores
The median MMAS total score during the menstrual period was 47.7 (IQR 37.4–63.0) at 1 month prior to the LNG-IUS insertion (Fig. 3, Table S1). At 3 and 12 months after LNG-IUS insertion, the total score increased significantly to 89.3 (IQR 69.3–100.0) and 93.9 (IQR 79.0–100.0), respectively (each p < 0.001 vs baseline). Of note, the MMAS score rapidly improved within 3 months of LNG-IUS insertion, followed by a gradual improvement up to 12 months post-insertion. Improvement was observed in the change over time in MMAS score with missing value completion during the menstrual period. As with the MDQ, there was no difference in the improvement trend in MMAS scores with or without missing value completion (data not shown).
All six domain scores of the MMAS showed a statistically significant improvement at 3 and 12 months after LNG-IUS insertion vs baseline (Fig. 4, Table S1). As with the MMAS total score trends, all six domain scores of the MMAS showed a rapid improvement within 3 months after the LNG-IUS insertion, and then the score gradually improved up to the 12-month follow-up.
Subgroup Analysis
The subgroup analysis of MDQ total scores by patient background during the premenstrual and menstrual periods is shown in Tables S2 and S3 in the supplementary material. Some MDQ scores at baseline differed between groups within a patient background category, such as age and history of drug therapy. For example, in the premenstrual period, the group aged less than 40 years and the group without a history of previous drug therapy had considerably higher median MDQ scores at baseline than the group aged at least 40 years and the group with a history of previous drug therapy (Table S2). Moreover, in the menstrual period, the group with BMI of at least 25 kg/m2 had a higher median MDQ score at baseline than the group with BMI of less than 25 kg/m2 (Table S3). Although there were differences in MDQ baseline scores in some patient background categories, overall, the MDQ score improved throughout the investigational period regardless of patient background characteristics, with a more notable improvement in MDQ scores within 3 months of LNG-IUS insertion.
Throughout the investigational period, an improvement in MDQ total scores was shown in both patients with primary and secondary dysmenorrhea. Patients with secondary dysmenorrhea were further categorized into three groups (uterine fibroid, adenomyosis, and endometriosis) to evaluate the effect of the type of secondary dysmenorrhea on the MDQ score trend (Tables S2 and S3). While the median MDQ score at baseline was slightly higher in the endometriosis group than the uterine fibroid and adenomyosis groups, all three groups showed a significant decrease in the MDQ score at the end of the 1-year follow-up.
Patients were categorized according to their menstrual pain at baseline into three groups (those with a VAS score of “0 to less than 30”, “30 to less than 70”, and “70 to 100”) to evaluate the effect of the baseline VAS score on the MDQ score trend (Tables S2 and S3). The MDQ total score improved significantly over the course of the investigational period in patients with a VAS score at baseline of 30 to less than 70 and 70 to 100; the MDQ total score also improved in patients with a VAS score at baseline of 0 to less than 30, but the improvement did not reach statistical significance.
A repeated two-way ANOVA was performed to investigate whether patient background characteristics and clinical categories influenced the time course trend of MDQ scores in both the premenstrual and menstrual periods. The results showed no statistically significant differences between subgroups for any patient background characteristics and clinical categories, except for the age subgroup analysis of the MDQ score during the menstrual period (interaction p = 0.0363) (Table S3).
The subgroup analysis of the MMAS total scores by patient background during the menstrual period is shown in Table S4 in the supplementary material. The MMAS score improved rapidly within the first 3 months after LNG-IUS insertion, and these score improvements continued up to 12 months after LNG-IUS insertion, regardless of patient background, except in patients with endometriosis where the MMAS score decreased from 3 to 12 months after LNG-IUS insertion. Regarding the subgroup analysis by type of HMB, in the group with primary HMB, the median MMAS score changed from 49.5 at baseline to 95.4 at 3 months following insertion; in the group with secondary HMB, the median score changed from 47.7 at baseline to 88.3 at 3 months following insertion.
Patients with secondary HMB were further categorized into three groups (uterine fibroid, adenomyosis, and endometriosis) to evaluate the effect of the type of secondary HMB on the MMAS score trend (Table S4). For all three types of secondary HMB, a rapid MMAS score improvement was shown within 3 months of LNG-IUS insertion, and then the score continued to increase up to the 12-month follow-up, except in patients with endometriosis.
The subgroup analysis of the MMAS score by the PBAC score at baseline (using a median PBAC score of at least 75 as the cutoff, which is equivalent to 60 mL of menstrual blood) during the menstrual period is shown in Table S4. The group with a baseline PBAC score below 75 was not analyzed as the sample size at each time point was too small. In the group with a baseline PBAC score of at least75, the MMAS score improved significantly at 3 months after LNG-IUS insertion, and the score continued to increase up to 12 months of follow-up.
A repeated two-way ANOVA was performed to investigate whether patient background characteristics and clinical categories influenced the time course trend of MMAS scores. The results showed no statistically significant differences between subgroups for any patient background characteristics and clinical categories (Table S4).
The Log-Normal Regression Analysis for the Relationship Between the MDQ and Menstrual Pain (VAS) and Between the MMAS and PBAC Scores
The log-normal regression analysis for the correlation between MDQ and menstrual pain and the correlation between MMAS and PBAC is shown in Fig. 5. A significant correlation between the MDQ total score and menstrual pain (VAS) was shown, with an estimated regression coefficient of 0.29 (95% CI 0.18, 0.39) (Fig. 5a). Furthermore, a significant correlation between the MMAS total score and PBAC score was also shown, with an estimated regression coefficient of − 0.15 (95% CI − 0.23, − 0.08) (Fig. 5b). The coefficients of determination for each regression analysis were 0.14 (for MDQ and menstrual pain [VAS]) and 0.20 (for MMAS and PBAC).
Discussion
The present study was a multicenter, non-interventional, prospective, single-cohort study of patients with HMB and/or dysmenorrhea who underwent LNG-IUS insertion for therapeutic (non-contraceptive) purposes. The current analysis focused on changes in the QOL of patients with dysmenorrhea and/or HMB before and after LNG-IUS insertion (1-year follow-up). To assess QOL, we investigated changes in the MDQ and MMAS scores over the course of 1 year of follow-up. Moreover, we investigated the relationship between MDQ and menstrual pain (VAS) and between MMAS and PBAC using a regression model.
The results of the present analysis showed that MDQ scores improved with statistical significance after LNG-IUS insertion compared with before LNG-IUS insertion. Of note, a substantial improvement in MDQ scores was observed, particularly within the first 3 months after LNG-IUS insertion. We further analyzed the data in groups with and without missing value completion and found that similar results were obtained in both groups. Therefore, we consider that our results showed that the LNG-IUS contributed to an improvement in the QOL of patients with dysmenorrhea.
The results of the current analysis suggest that the LNG-IUS can alleviate various symptoms of dysmenorrhea. Among the domains of the MDQ, “pain” and “behavioral effect” showed a greater degree of improvement than the other domains. This finding is important because pelvic pain is the main symptom of dysmenorrhea. In addition, the reduction of pain in patients with dysmenorrhea may have had a positive effect on patients’ behavioral aspects, thus resulting in a notable improvement in the “behavioral effect” domain.
An improvement in total MDQ scores was observed in both the premenstrual and menstrual periods, meaning that the LNG-IUS may improve various symptoms before and during menstruation. The onset of primary dysmenorrhea usually occurs on the first day of menstruation; the severity is generally higher during the initial 24–48 h [34]. This may explain why the total MDQ score at baseline in our study tended to be higher during the menstrual period than the premenstrual period. Despite this difference, the scores reached a comparable level in both the premenstrual and menstrual periods at the 3-month follow-up. This suggests that the LNG-IUS may be particularly effective in relieving pain during menstruation.
Although the total MDQ score improved considerably both during the premenstrual and menstrual periods, a slight difference in trends was identified in the domain scores. These differences in trends shown in the present study indicate that it may be difficult to capture improvement in some symptoms with short-term treatment, but improvements could be more evident with long-term treatment.
Subgroup analyses of the MDQ scores were conducted to observe changes over time according to age, BMI, history of pharmacotherapy, history of childbirth, history of surgery, type of dysmenorrhea, concomitant disease, and baseline VAS score. All subgroup analyses showed improvements in MDQ scores, regardless of patient background characteristics. Each subgroup (e.g., BMI, history of delivery, type of dysmenorrhea) showed slightly different trends in improvement. Furthermore, the analysis stratifying patients by secondary dysmenorrhea type suggested that the LNG-IUS could be useful in treating the three major causes of secondary dysmenorrhea. When we further investigated the VAS score categories at baseline, the MDQ scores showed that the LNG-IUS could be beneficial regardless of the pain level at the beginning of treatment. However, on the basis of the results of the repeated two-way ANOVA, there were no patient background characteristics that synergistically influenced the effect of the LNG-IUS, except for the age subgroup analysis of the MDQ score during the menstrual period.
The MMAS is a specific QOL measurement for HMB and consists of only six questions. Similar to our analysis of MDQ scores, we investigated the groups with and without missing value completion. The results in both groups showed a substantial improvement in MMAS scores with very similar trends over the course of the investigational period, confirming that the missing values did not affect the results of this study. As with the MDQ, a significant improvement in MMAS scores was confirmed at 3 months after LNG-IUS insertion, with a slight increase in scores between 3 and 12 months. This result is consistent with previous studies conducted in Asia and the UK. In both studies, MMAS scores were substantially improved within 3–6 months, and a slight increase in the score continued until the end of the investigational periods [35, 36]. These findings suggest that the LNG-IUS can help alleviate HMB symptoms in the early phase of treatment, which leads to improvement in the QOL of these patients.
In four of the six domains of the MMAS (practical difficulties, psychological effects, interruption of work, and family life), the IQR tended to decrease after LNG-IUS insertion. This means that there were variations in patients’ self-assessment prior to LNG-IUS insertion, though after LNG-IUS insertion, most patients’ self-assessment remained the same. Conversely, for physical health, the IQR was greater at the 3-month follow-up, but smaller at the 12-month follow-up. This means that patients’ physical health status varied at 3 months after LNG-IUS insertion; however, the score in that domain was positively improved and converged after 12 months of LNG-IUS use. However, the ceiling effect made it impossible to measure scores beyond the maximum score possible for the MMAS, resulting in many subjects choosing the same option during the follow-up assessments.
The question for “practical difficulties” asked about the impact of HMB on daily life, such as the risk of leaking blood or soiling sheets and anxiety about not having convenient access to a restroom. Our findings suggest that patients with HMB must continuously worry about leaking blood and soiling their clothes during menstruation, as well as checking if there is a toilet nearby, which may cause stress and lower their QOL. As HMB has been shown to decrease with the LNG-IUS [37], patients may have been less concerned about menstrual blood leakage after LNG-IUS insertion. In turn, this may have contributed to a greater improvement in “practical difficulties.”
Some patients with secondary HMB had concomitant adenomyosis or uterine fibroids. These patients have not only a large amount of menstrual bleeding but also considerable pain. Insertion of the LNG-IUS may have led to a decrease in menstrual pain in these patients, as well as a decrease in HMB and subsequent risk of anemia, which may explain the improvement in the “physical health” domain of the MMAS.
Subgroup analyses of the MMAS were conducted according to age, BMI, history of pharmacotherapy, history of childbirth, history of surgery, primary/secondary HMB, concomitant disease, and baseline PBAC score. The results showed a substantial improvement in MMAS score, regardless of patient background characteristics; thus, we consider that the LNG-IUS could improve the QOL of patients with HMB and different background characteristics. As with the MDQ, repeated two-way ANOVA was performed, and it showed no evidence of a time course trend of MMAS scores according to patient background. However, the LNG-IUS seemed to be beneficial in patients with a baseline PBAC score of 75 or higher.
Regarding the log-normal regression analysis to determine the statistical association between MDQ and menstrual pain, the results indicated that 10% improvement in VAS scores would be related to an approximately 3% improvement in MDQ total scores. This implies that pain improvement is related to some improvement in MDQ scores. Similarly, the log-normal regression analysis of MMAS and PBAC showed that a 10% improvement in PBAC scores would be related to an approximately 1.5% improvement in MMAS scores.
However, the coefficients of determination for both regression analyses were 0.14 (MDQ and menstrual pain) and 0.20 (MMAS and PBAC). This means that pain itself may have contributed to approximately 14% of the MDQ score, and other factors such as age and type of work contributed to 86% of the MDQ score. Similarly, the amount of menstrual bleeding may have contributed to approximately 20% of the MMAS score and other factors accounted for approximately 80%. The improvement in pain tended to correlate with the QOL of patients with dysmenorrhea, and the improvement in PBAC tended to correlate with an improvement in the MMAS score. It is noteworthy that one factor could contribute to 14–20% of the scores. However, the regression analysis results should be interpreted with caution as they suggest that other factors, besides pain and amount of blood loss, may play a role in improving the MDQ and MMAS scores.
The present analysis has some limitations. The current analysis is limited by the lack of a control group and a high risk of selection bias. The data collected at 1 month before LNG-IUS insertion could be highly variable owing to individual situations. Baseline values may require adjustment to assess MDQ and MMAS score changes more accurately; however, not enough data were collected, and thus the true baseline values remain unknown. While improvements in VAS, PBAC, MDQ, and MMAS scores were observed, whether improvements in VAS and PBAC scores directly led to improvements in MDQ and MMAS remains debatable. Furthermore, in the current study, we sought to evaluate the impact of LNG-IUS insertion on QOL among Japanese patients with dysmenorrhea or HMB.
In the current post-marketing surveillance, a combination therapy group was not considered as the current surveillance focused on evaluating the effectiveness and safety of LNG-IUS alone, without synergetic effects of other treatments. Nevertheless, we believe that evaluating the effects of combination methods in real-world settings is an important research topic to be investigated in the near future. Although previous studies investigated the impact of LNG-IUS insertion in different populations in different geographical locations [38,39,40,41,42], further evidence regarding the role of ethnicity needs to be accumulated to better understand the efficacy of LNG-IUS. Finally, whether the MDQ and MMAS scores could further improve beyond the present study’s 12-month observation period is currently unknown, and long-term 5-year follow-up studies are warranted in the future to confirm this.
Conclusions
The LNG-IUS contributed to improvements in the QOL of patients with dysmenorrhea, HMB, and both, regardless of patient background characteristics. A rapid improvement was seen within the first 3 months after LNG-IUS insertion, which persisted until the 12-month follow-up.
References
Sriprasert I, Pakrashi T, Kimble T, Archer DF. Heavy menstrual bleeding diagnosis and medical management. Contracept Reprod Med. 2017;2:20.
Armour M, Parry K, Manohar N, et al. The prevalence and academic impact of dysmenorrhea in 21,573 young women: a systematic review and meta-analysis. J Womens Health (Larchmt). 2019;28:1161–71.
Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36:104–13.
Fraser IS, Mansour D, Breymann C, Hoffman C, Mezzacasa A, Petraglia F. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynaecol Obstet. 2015;128:196–200.
Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16:1255–66.
Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21:762–78.
Nagy H, Khan MAB. Dysmenorrhea. StatPearls. 2021. https://www.ncbi.nlm.nih.gov/books/NBK560834/. Accessed 10 Mar 2021.
Osuga Y, Hayashi K, Kobayashi Y, et al. Dysmenorrhea in Japanese women. Int J Gynaecol Obstet. 2005;88:82–3.
Kawaguchi R, Matsumoto K, Ishikawa T, et al. Guideline for gynecological practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J Obstet Gynaecol Res. 2021;47:5–25.
Japan Society of Obstetrics and Gynecology. Obstetrics and gynecology glossary. 4th ed. Tokyo: Kyorinsya; 2018. (Japanese).
Davies J, Kadir RA. Heavy menstrual bleeding: an update on management. Thromb Res. 2017;151:S70–7.
Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201–25.
National Institute for Health and Care Excellence (NICE) guideline. Heavy menstrual bleeding: assessment and management. 2018. https://www.nice.org.uk/guidance/ng88/resources/heavy-menstrual-bleeding-assessment-and-management-pdf-1837701412549. Accessed 10 Mar 2021.
Laksham KB, Selvaraj R, Kar SS. Menstrual disorders and quality of life of women in an urban area of Puducherry: a community-based cross-sectional study. J Family Med Prim Care. 2019;8:137–40.
Engstrom JL, Rose R, Brill AI, Polhill KM, Lukanich CM, Fritz L. Midwifery care of the woman with menorrhagia. J Nurse Midwifery. 1999;44:89–105.
Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115:138–45.
Rodrigues AC, Gala S, Neves Â, et al. Dismenorreia em adolescentes e jovens adultas: prevalência, factores associados e limitações na vida diária [Dysmenorrhea in adolescents and young adults: prevalence, related factors and limitations in daily living]. Acta Med Port. 2011;24:383–8 (quiz 389–92. [Portuguese]).
Luukkainen T, Lähteenmäki P, Toivonen J. Levonorgestrel-releasing intrauterine device. Ann Med. 1990;22:85–90.
Costescu DJ. Levonorgestrel-releasing intrauterine systems for long-acting contraception: current perspectives, safety, and patient counseling. Int J Womens Health. 2016;8:589–98.
Bayer HealthCare Pharmaceuticals. Mirena Highlights of Prescribing Information. https://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf. Accessed 10 Mar 2021.
Endrikat J, Vilos G, Muysers C, Fortier M, Solomayer E, Lukkari-Lax E. The levonorgestrel-releasing intrauterine system provides a reliable, long-term treatment option for women with idiopathic menorrhagia. Arch Gynecol Obstet. 2012;285:117–21.
Ministry of Health, Labour and Welfare. The Japanese guideline for Good Post-marketing Study Practice. https://www.mhlw.go.jp/web/t_doc?dataId=81aa6623&dataType=0&pageNo=1. Accessed 10 Mar 2021. (Japanese).
Akiyama A, Kayasima K. The Japanese version of Menstrual Distress questionnaire. Nurs Psychol. 1979;5:272–7 (Japanese).
Momoeda M, Tanaka E, Kawaguchi M. Development of the Japanese version of the Menorrhagia Multi-Attribute Scale (MMAS): translation and linguistic validation. Sanka Fujinka [Obstetrics and Gynecology]. 2015;82:1299–305 (Japanese).
Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81.
Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis. 1979;38:560.
McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–19.
Gaston-Johansson F. Measurement of pain: the psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Symptom Manage. 1996;12:172–81.
Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001;95:1356–61.
Halimeh S, Rott H, Kappert G. PBAC score: an easy-to-use tool to predict coagulation disorders in women with idiopathic heavy menstrual bleeding. Haemophilia. 2016;22:e217–20.
Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–9.
Ikeda S, Shiroiwa T, Igarashi A, et al. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Health. 2015;64:47–55 (Japanese).
Bayer. Mirena intrauterine delivery system 52 mg, https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/630004_2529710X1027_1_07. Accessed 10 Mar 2021. (Japanese)
Momoeda M, Akiyama S, Tanaka K, Suzukamo Y. Quality of life in Japanese patients with dysmenorrhea treated with ethinylestradiol 20 μg/drospirenone 3 mg in a real-world setting: An observational study. Int J Womens Health. 2020;12:327–38.
Xu L, Lee BS, Asif S, Kraemer P, Inki P. Satisfaction and health-related quality of life in women with heavy menstrual bleeding; results from a non-interventional trial of the levonorgestrel-releasing intrauterine system or conventional medical therapy. Int J Womens Health. 2014;6:547–54.
Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368:128–37.
Lethaby A, Hussain M, Rishworth JR, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015; CD002126. Update in: Cochrane Database Syst Rev. 2020; 6:CD002126.
Wildemeersch D, Schacht E, Wildemeersch P. Treatment of primary and secondary dysmenorrhea with a novel “frameless” intrauterine levonorgestrel-releasing drug delivery system: a pilot study. Eur J Contracept Reprod Health Care. 2001;6:192–8.
Costanzi F, De Marco MP, Colombrino C, et al. The treatment with Levonorgestrel Releasing Intrauterine System (LNG-IUS) in patients affected by menometrorrhagia, dysmenorrhea and adenomimyois: clinical and ultrasonographic reports. Eur Rev Med Pharmacol Sci. 2021;25:3432–9.
Yoo HJ, Lee MA, Ko YB, Yang JB, Kang BH, Lee KH. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012;285:161–6.
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
Lu M, Yang X. Levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding in adolescents with Glanzmann’s Thrombasthenia: illustrated case series. BMC Womens Health. 2018;18:45.
Acknowledgements
Funding
This work and the journal’s Rapid Service and Open Access fees were supported by Bayer Yakuhin, Ltd., which actively participated in the study design and managed all operational aspects of the study, including monitoring data collection, statistical analyses, and writing of the report.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Michelle Belanger, MD, of Edanz Japan, for providing medical writing support, which was funded by Bayer Yakuhin, Ltd., Japan, through EMC K.K., Japan, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors would also like to thank Dr. Yoshimi Suzukamo, of Tohoku University, for providing insightful advice on analysis methods and interpretation of the results.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors participated in the study conception and design, conducted the data analysis and interpretation, and wrote and revised the manuscript. Keisuke Yoshihara and Noriko Takahashi collected and/or assembled the data. All authors made the final decision to submit the article for publication.
Disclosures
Mikio Momoeda, Shigeo Akira, Tasuku Harada, Jo Kitawaki, Nagamasa Maeda and Ikuko Ota are members of the Proper Use Advisory Committee of Mirena. Tasuku Harada has received consulting fees from Nobelpharma Co., Ltd.; honoraria from Mochida Pharmaceutical Co., Ltd., ASKA Pharmaceutical Co., Ltd., Fuji Pharma Co., Ltd., and Bayer Yakuhin, Ltd.; and has participated on a Data Safety Monitoring Board or Advisory Board for Nobelpharma Co., Ltd., ASKA Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., and Fuji Pharma Co., Ltd. Ikuko Ota has received lecture fees from Mochida Pharmaceutical Co., Ltd. Keisuke Yoshihara, and Noriko Takahashi are employees of Bayer Yakuhin, Ltd.
Compliance with Ethics Guidelines
The study protocol was approved by the institutional review board of each participating center and was compliant with the Japanese Good Post-marketing Study Practice guidelines. This study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments. All participants provided written informed consent prior to study participation.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Momoeda, M., Akira, S., Harada, T. et al. Quality of Life of Japanese Dysmenorrhea/Heavy Menstrual Bleeding Patients Treated with Levonorgestrel Intrauterine Delivery System in a Real-World Setting. Adv Ther 39, 3616–3634 (2022). https://doi.org/10.1007/s12325-022-02205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02205-2