Abstract
Introduction
Treatment continuation is essential for relapse prevention in patients with schizophrenia. The aim of this exploratory study was to compare the time to treatment discontinuation between patients with schizophrenia prescribed brexpiprazole (BRX group) and those prescribed other atypical antipsychotics (OAA group) in clinical settings in Japan using health insurance claims data.
Methods
De-identified data of working individuals with schizophrenia aged < 75 years and their dependents were assessed from April 2017 to May 2020 using a nationwide claims database. Cox proportional hazards models, adjusted for baseline patient variables, were used to compare the time to treatment discontinuation (primary outcome) for 180 days between BRX and OAA groups and to estimate the hazard ratio (HR) with 95% confidence interval (CI). The cumulative treatment continuation rates at 180 days were also estimated. Sensitivity and subgroup analyses were conducted for the primary outcome.
Results
The analysis included 978 and 4898 patients in the BRX and OAA groups, respectively. Patients in the BRX group were significantly less likely to discontinue treatment than those in the OAA group (HR 0.86, 95% CI 0.78–0.95; p = 0.0024). The cumulative treatment continuation rates were higher in the BRX group (45.9%, 95% CI 42.5–49.2]) than in the OAA group (39.5%, 95% CI 38.1–41.0; log-rank test, p < 0.0001). Based on patients matched by propensity score, the BRX group was significantly less likely to discontinue treatment than the OAA group (log-rank test, p = 0.0466). Similar results were obtained in sensitivity and subgroup analyses.
Conclusion
This real-world study showed that patients in the BRX group were less likely to discontinue treatments than those in the OAA group. These findings suggest that BRX may contribute to treatment continuation among patients with schizophrenia.
Trial Registration
University hospital Medical Information Network (UMIN) Clinical Trials Registry: UMIN000044682.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Treatment continuation is essential for relapse prevention in patients with schizophrenia. |
Brexpiprazole (BRX) has been shown to have favorable safety and tolerability profiles that may contribute to treatment continuation and further relapse prevention. |
This exploratory study compared the time to treatment discontinuation between patients with schizophrenia prescribed BRX and those prescribed other atypical antipsychotics (OAA) in real-world clinical settings in Japan. |
What was learned from the study? |
Patients prescribed BRX were less likely to discontinue treatment than those prescribed OAA. |
Our findings suggest that BRX may contribute to treatment continuation during maintenance therapy for patients with schizophrenia. |
Further prospective studies may clarify the causality between BRX and treatment continuation. |
Introduction
Schizophrenia is a chronic disorder with low rates of recovery, with only 13.5% of patients recovering in both the clinical and social domains [1]. Relapse, which hinders recovery [2], is common, with a first 5-year cumulative relapse rate of 81.9%, and a second cumulative 5-year relapse rate of 78.0% [3]. Continuous treatment is essential to prevent relapse in patients with schizophrenia [4], with one study showing that relapse risk increased by approximately fivefold when antipsychotic treatment was discontinued among patients who had achieved clinical stability [3]. As relapse leads to worse clinical outcomes and negatively affects social functioning [4, 5], relapse prevention is one of the most important goals in the maintenance treatment of patients with schizophrenia.
Along with a lack of clinical insight in this illness and negative attitudes of patients toward medication, efficacy and side effects are factors affecting medication adherence and treatment continuation [6, 7]. However, to date, few comparative studies on the efficacy and safety profiles of currently available antipsychotics are available. A network meta-analysis comparing 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia found that differences in terms of efficacy, including all-cause discontinuation, were small among second-generation antipsychotics (SGAs), while safety profiles (e.g., weight gain, QTc prolongation, sedation) differed greatly [8]. Due to the limited body of evidence to date, the treatment guidelines for schizophrenia in Japan do not recommend specific SGAs and state that medications should be chosen based on individual cases [2].
Brexpiprazole (BRX) is a serotonin-dopamine activity modulator that is a partial agonist at the serotonin 5-HT1A and dopamine D2 receptors and an antagonist at the serotonin 5-HT2A and noradrenaline alpha1B/2C receptors, all at similar potency [9]. BRX was approved and marketed for schizophrenia and the adjunctive treatment of major depressive disorder in 2015 in the USA [10], and for schizophrenia in 2018 in Japan [11]. BRX has been shown to have favorable safety and tolerability profiles [12,13,14], with a lower potential than other atypical antipsychotics (OAA) to induce D2 receptor agonist-related and antagonist-related adverse effects [15]. These profiles of BRX may contribute to treatment continuation and relapse prevention. However, to the best of our knowledge, few studies in Japan have compared BRX and other atypical antipsychotics in terms of treatment continuation, and their comparative effectiveness has not been fully clarified in clinical practice.
The objective of this exploratory study was to compare the time to treatment discontinuation between patients with schizophrenia prescribed BRX as monotherapy (BRX group) and those prescribed other atypical antipsychotics (OAA group) in clinical settings in Japan using a nationwide health insurance claims database of working individuals and their dependents. We further described patient characteristics, treatment patterns, and medication adherence assessed using the proportion of days covered (PDC) [16].
Methods
Study Design and Data Source
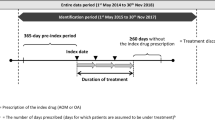
This retrospective observational cohort study used data from a health insurance claims database of working individuals aged < 75 years and their dependents (those not in full-time employment) that is managed by JMDC Inc. (Tokyo, Japan), from 18 April 2017 to 31 May 2020 (entire data period; Fig. 1). The JMDC database stores de-identified individual-level data of approximately 11 million people (as of May 2020). The database also contains patient characteristics and medical/clinical information, including date-stamped inpatient and outpatient health insurance claims (e.g., diagnosis codes in the International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10], procedures, prescriptions, medical services, costs, and medical institutions). Individual medical and treatment histories can be traced across medical institutions in the JMDC database unless an employee withdraws from the health insurance plan.
Study timeline with hypothetical patient journeys. aIndex date was defined as the date of earliest prescription of brexpiprazole, aripiprazole, olanzapine, quetiapine, risperidone, perospirone, blonanserin, paliperidone, or asenapine identified during the identification period. bThe drug prescribed at index date was defined as index drug
Ethics Statement
As this study used pre-existing, anonymized data for secondary purposes, the study does not need to apply the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, and ethics approval from the institutional review board was not required. Additionally, the claims data were classified as anonymously processed information under the Act on the Protection of Personal Information 2003 in Japan. Informed consent from individual patients was therefore not required. The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments and registered in the University Hospital Medical Information Network (UMIN Clinical Trials Registry: UMIN000044682) [17]. The data were purchased from JMDC Inc. for the purpose of this study.
Patient Selection
Patients with schizophrenia were identified from 18 April 2018 to 30 November 2019 (identification period; Fig. 1) and included in the study if they met all of the following criteria: (1) prescription records of BRX or OAA (aripiprazole [APZ], olanzapine [OLZ], quetiapine [QTP], risperidone [RIS], perospirone, blonanserin, paliperidone, and asenapine; hereafter, index drug) during the identification period (the earliest date of these prescriptions is defined as the index date), with a washout period of index drug in the last 60 days, and with patient records available for ≥ 365 days before index date; (2) diagnosis records of schizophrenia (ICD-10 code: F20) at the time of hospitalization (≥ 1 records) or outpatient visit (≥ 2 records) within 365 days before index date; and (3) ≥ 18 years of age at index date. Patients prescribed BRX before any of the other index drugs were included in the BRX group, otherwise they were included in the OAA group. Regarding criterion (1) above, QTP extended-release formulation, which does not have an indication for schizophrenia in Japan, was not examined in this study.
Patients were excluded if they met any of the following criteria: (1) diagnosis records of dementia (ICD-10 codes: F00–03, F05, G30–31), attention-deficit/hyperactivity disorder (F90), or intellectual disability (F70–73, F79) within 365 days before index date; (2) prescription records of antipsychotic drugs other than index drug during the same period as index drug for ≥ 30 days after index date; (3) prescription records of long-acting injectable drugs (APZ hydrate, paliperidone palmitate, RIS, haloperidol decanoate, and fluphenazine decanoate) within 60 days before index date; and (4) prescription records of clozapine within 365 days before index date.
Outcome Measures
The primary outcome was the time to treatment discontinuation assessed for 180 days in the BRX and OAA groups. Time to treatment discontinuation is a measure of adherence and also a measure of the efficacy and tolerability of a drug [18]. This primary outcome was selected because at the time of study inception (as of December 2020), BRX was the latest drug to become available to patients with schizophrenia in Japan, and there were no studies comparing treatment continuation among antipsychotics including BRX. Additionally, BRX was developed to overcome the side effects of APZ (e.g., akathisia, restlessness, and insomnia), which is the only other D2 partial agonist currently available and was marketed before brexpiprazole in Japan [19]. When > 1 index drugs were prescribed on the same date, they were counted as 1 day. Treatment was considered to be discontinued when the index drug was not prescribed for > 30 days. Patients were censored when treatment continued for > 180 days, or when the date of treatment discontinuation could not be assessed due to withdrawal from the JMDC database or the end of the data period.
The secondary outcome was the time to treatment discontinuation assessed for 180 days. This outcome was examined separately for patients in the BRX group and for patients in each OAA group (APZ, OLZ, QTP, or RIS). Patients prescribed perospirone, blonanserin, paliperidone, and asenapine were not examined separately because of the very small number of patients identified in each group (n < 100). In terms of discontinuation and censoring, we used the same definitions as those used for the primary outcome.
Another secondary outcome was PDC ≥ 0.8, which was defined as good medication adherence [16] to index drug. PDC ≥ 0.8 was examined separately for patients in the BRX and OAA groups, and for each APZ, OLZ, QTP, and RIS group. PDC was calculated as the total number of days’ supply of index drug divided by 180 days, and > 1 index drugs prescribed on the same date were not double-counted.
Baseline Variables
Patient characteristics assessed at baseline or at the time of index date were age, sex, benzodiazepine equivalent dose (mg/day) [20], and medications for other symptoms or disorders (antidepressants, drugs for bipolar disorder, anxiolytics, hypnotics, and anti-Parkinson’s drugs). Patient characteristics assessed included Charlson Comorbidity Index (CCI) during 365 days before the month of index date (excluding acquired immunodeficiency syndrome/human immunodeficiency virus due to the unavailability of data) [21], PDC during 365 days before index date (not including PDC = 0), chlorpromazine (CP) equivalent dose (mg/day) [20] estimated based on the last atypical antipsychotics (excluding brexpiprazole but not limited to index drugs) prescribed before index date (not including CP equivalent = 0), mean interval between outpatient visits (day), and the number and total number of days of psychiatric hospitalization during 365 days before the month of index date. The baseline PDC was calculated based on index drugs excluding BRX.
Treatment Patterns
The treatment patterns assessed during the data period included mean prescription interval between outpatient visits (days). The variables assessed during the treatment period included hospitalization in psychiatric department, BRX dose for the BRX group or CP equivalent dose of index drug for other groups (mg/day), total number of days hospitalized for psychiatric treatment at index date (including days before and after index date), and psychiatric home care.
Statistical Analysis
Baseline characteristics, treatment patterns, and PDC were descriptively summarized using the mean ± standard deviation (SD) or median and interquartile range (IQR: first quartile [Q1], third quartile [Q3]) for continuous variables, and using number and percentage for categorical variables. For the primary outcome, Cox proportional hazards models were used to estimate hazard ratio (HR) with 95% confidence interval (CI) for the BRX and OAA (reference) groups and p values. As covariates in the analysis, we used the following baseline patient characteristics: sex, age, CP equivalent doses of antipsychotic medication (mg/day), CCI, concomitant medications (yes/no; antidepressants, medications for bipolar disorder, anxiolytics, hypnotics, and anti-Parkinson's disease drugs), concomitant benzodiazepine equivalent dose (mg/day), and the number and total number of days hospitalized for psychiatric treatment. Survival curves adjusted based on the Cox proportional hazards model were generated [22]. We further used Kaplan–Meier survival analysis to estimate cumulative treatment continuation rates at 180 days, with 95% CI for the BRX and OAA (reference) groups, and to generate survival curves. The log-rank test was used to compare treatment discontinuation between the two groups. For the secondary outcome, survival curves and cumulative continuation rates with 95% CI were estimated using Kaplan–Meier survival analysis for the BRX and APZ, OLZ, QTP, and RIS groups.
We conducted sensitivity analyses to demonstrate robustness for the primary outcome, using the following statistical analysis and definitions: (1) Kaplan–Meier survival analysis based on patients matched by propensity score (BRX vs. OAA group; 1:1 caliper matching); (2) with a > 60-day gap period for assessing treatment discontinuation; and (3) with a washout period of 365 days for the inclusion criteria. For (1), the log-rank test was used to compare the treatment discontinuation between the two groups. Additionally, we further conducted the following subgroup analyses: (4) splitting data into two for patients with (18 April 2018 to 30 April 2019) and without prescription restrictions (1 May 2019 to 30 November 2019); (5) excluding patients prescribed antidepressants and CP equivalent dose < 150 mg; and (6) excluding patients diagnosed with depression at index date. For (4), we split the data because after a new drug being listed in the National Health Insurance drug price standard in Japan, each prescription is limited to a 14-day supply during the first year from the month of launch (for the case of BRX, from 18 April 2018 to 30 April 2019). This requirement ensures frequent consultation and sufficient observation by prescribing physicians. For (5), 150 mg was selected because the recommended dose of APZ, which is the only other D2 partial agonist currently available, is 6–24 mg/day [23], with the lower value equivalent to the 150 mg CP dose. We intended the included patients to have schizophrenia. All other analyses except (1) were performed using Cox proportional hazards models with the same covariates as those used for the primary outcome.
Missing data were not imputed. All statistical analyses used a significance level of 0.05, and two-sided 95% CI was estimated. All statistical analyses were performed using SAS Release 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Selection and Baseline Characteristics
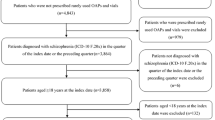
In total, 109,050 individuals diagnosed with schizophrenia were recorded in the JMDC database during the data period (Fig. 2). Of these, 1249 patients were prescribed BRX and met the other inclusion criteria, and 5885 patients were prescribed other index drugs and met the other inclusion criteria. Of the 1249 patients prescribed BRX who met all inclusion criteria, 271 were ultimately excluded because they met one or more of the exclusion criteria, leaving 978 patients to be included in the BRX group in this study. Of the 5885 patients eligible for inclusion in the OAA group (i.e., prescribed other index drugs) who met all inclusion criteria, 987 were ultimately excluded patients because they met one or more of the exclusion criteria, leaving 4898 patients to be included in the OAA group in this study. Of the 4898 patients in the OAA group, 1496, 1011, 919, and 856 patients were included in the APZ, OLZ, QTP, and RIS medication groups, respectively. A detailed patient flow chart for each group is provided in the Electronic Supplementary Material (ESM) Fig. S1.
Flow chart of patient enrollment. ADHD Attention-deficit/hyperactivity disorder, BRX brexpiprazole, OAA other atypical antipsychotics, APZ aripiprazole, OLZ olanzapine, QTP quetiapine, RIS risperidone. aIndex drug was defined as the earliest prescription of BRX, APZ, OLZ, QTP, RIS, perospirone, blonanserin, paliperidone, or asenapine identified during the identification period. Patients prescribed brexpiprazole before any of the other index drugs were included in the BRX group, otherwise they were included in the OAA group. bPatients in each of the APZ, OLZ, QTP, and RIS medication groups were included in the OAA group, and patients who were prescribed perospirone, blonanserin, paliperidone, and asenapine were not assessed separately due to the small number of patients identified in each of these medication groups
The baseline characteristics of patients are summarized in Table 1. The mean (± SD) age of patients in the BRX group was 39.8 ± 12.7 years, and 61.1% of this group were female. The mean age of patients in the OAA group was 41.9 ± 13.1 years, and 56.4% of this group were female. The other baseline characteristics are provided in ESM Table S1.
Treatment Patterns
The mean (± SD) prescription interval in the BRX group was 16.2 ± 8.7 days, and a median (Q1, Q3) BRX dose was 1.2 (1.0, 1.8) mg/day (Table 2). The majority of patients (87.3%) were not hospitalized at the time of the index date, and 5.3% received psychiatric home care during the treatment period. The mean prescription interval in the OAA group was 22.2 ± 16.2 days, and the median dose of index drug was 100.0 (56.8, 204.4) mg/day. The number of patients who were not hospitalized was slightly lower in the OAA group (77.7%) than in the BRX group, but only 2.2% received psychiatric home care during the treatment period. Other treatment patterns are reported in ESM Table S2.
Treatment Discontinuation
Among the 978 and 4898 patients in the BRX and OAA groups, respectively, 480 (49.1%) and 2705 (55.2%) patients discontinued treatments during the 180-day period. The mean (± SD) continuation period was 57.7 ± 46.6 days in the BRX group and 51.9 ± 43.3 days in the OAA group. The Cox proportional hazards model showed that patients in the BRX group were significantly less likely to discontinue treatment than those in the OAA group (HR 0.86, 95% CI 0.78–0.95; p = 0.0024; Fig. 3; ESM Table S3). The cumulative treatment continuation rates at 180 days were significantly higher in the BRX group (45.9%, 95% CI 42.5–49.2) than in the OAA group (39.5%, 95% CI 38.1–41.0; log-rank test, p < 0.0001).
A Survival curves adjusted based on a Cox proportional hazards model, B Kaplan–Meier survival curves for the time to treatment discontinuation in the BRX and OAA groups. The survival curve based on the Cox proportional hazards model was adjusted for sex and age at index date, chlorpromazine equivalent doses of antipsychotic medication (mg/day) estimated based on the last atypical antipsychotics prescribed before index date, Charlson Comorbidity Index assessed during 365 days before the month of index date, medications used at index date (yes/no: antidepressants, medications for bipolar disorder, anxiolytics, hypnotics, and anti-Parkinson’s drugs), benzodiazepine equivalent dose (mg/day) at index date, and the number and total number of days in psychiatric hospitalization during 365 days before the month of index date. HR hazard ratio, CI confidence interval
Among the 1496, 1011, 919, and 856 patients in the APZ, OLZ, QTP, and RIS medication groups, respectively, 831 (55.5%), 574 (56.8%), 497 (54.1%), and 498 (58.2%) patients, respectively, discontinued treatments during 180 days. Among the BRX group and the four medication groups of the OAA, cumulative treatment continuation rates at 180 days were higher in the BRX group (45.9%, 95% CI 42.5–49.2) than in the four groups (APZ: 40.2%, 95% CI 37.6–42.8; OLZ: 37.1%, 95% CI 33.9–40.3; QTP: 40.4%, 95% CI 37.0–43.8; RIS: 36.4%, 95% CI 32.9–39.8) (Fig. 4).
Proportion of Days Covered ≥ 0.8
The mean (± SD) PDC was 0.53 ± 0.36 in the BRX group and 0.48 ± 0.37 in the OAA group, and 35.6% and 31.9% of patients, respectively, had PDC ≥ 0.8. The mean PDC in the APZ, OLZ, QTP, and RIS medication groups was 0.51 ± 0.36, 0.46 ± 0.37, 0.48 ± 0.37, and 0.44 ± 0.37, respectively, and 33.7, 29.7, 32.2, and 28.7%, respectively, had PDC ≥ 0.8. Results for PDC < 0.5 and for 0.5 ≤ PDC < 0.8 are provided in ESM Table S4.
Sensitivity and Subgroup Analyses
Based on the sensitivity analysis with patients matched by propensity score, patients in the BRX group were significantly less likely to discontinue treatment than those in the OAA group (log-rank test, p = 0.0466; ESM Table S5; ESM Fig. S2). Other sensitivity analyses using the alternative definitions of a > 60-day gap period and a washout period of 365 days both yielded similar results; the BRX group was less likely to discontinue treatment than the OAA group (HR 0.82, 95% CI 0.74–0.92, p = 0.0005; HR 0.91, 95% CI 0.82–1.01, p = 0.0741; ESM Figs. S3, S4).
Although no significant differences were found between the BRX and OAA groups for prescription restrictions (HR 0.91, 95% CI 0.79–1.04, p = 0.1678), patients in the BRX group were significantly less likely to discontinue treatment than those in the OAA group without prescription restrictions (HR 0.85, 95% CI 0.73–0.99, p = 0.0322; ESM Fig. S5). In general, similar results favoring BRX were observed in statistical analyses excluding patients prescribed antidepressants and CP equivalents < 150 mg, and in patients diagnosed with depression (HR 0.85, 95% CI 0.76–0.95, p = 0.0041; HR 0.84, 95% CI 0.73–0.98, p = 0.0239; ESM Figs. S6, S7).
Discussion
Medication adherence is generally low in patients with schizophrenia [24], and poor adherence is associated with worse clinical outcomes including relapses [25, 26]; therefore, continuous treatment is crucial to prevent patients from relapsing and to achieve better clinical outcomes. This study is the first to exploratory compare treatment discontinuation between patients with schizophrenia prescribed BRX and those prescribed OAAs in Japanese clinical settings using a nationwide health insurance claims database. The analysis demonstrated that patients in the BRX group were significantly less likely to discontinue treatments than those in the OAA group (HR 0.86; p = 0.0024), that cumulative continuation rates at 180 days were significantly higher in the BRX group (45.9%) than in the OAA group (39.5%) and higher than in any of the four medication groups (APZ 40.2%; OLZ 37.1%; QTP 40.4%; RIS 36.4%), findings which are in line with those of a real-world study comparing continuation rates at 24 weeks (approx. 168 days) in patients prescribed BRX and asenapine [27].
The higher treatment continuation rates in the BRX group may be attributed to the efficacy and safety of BRX that have been demonstrated in clinical trials. In 6-week short-term studies in patients with acute schizophrenia, BRX showed efficacy over placebo, and favorable safety and tolerability [12, 13, 28, 29], with lower discontinuation rates due to adverse events in the BRX group than in the placebo group in global studies (8.1% vs. 12.7%) [14] and in a Japanese study (10.5–16.5% vs. 17.2%) [13]. The safety of BRX was further demonstrated in a 52-week long-term study in which the incidence of treatment-emergent adverse events, including akathisia, weight gain, and extrapyramidal disorder, was comparable to that of short-term studies [14, 30]. In addition to the efficacy and side effects, there are other factors that are known to affect medication adherence and treatment continuation, including lack of illness insight, negative attitudes toward medication, and comorbidities [6, 7].
The cumulative treatment continuation rate at 180 days (approx. 26 weeks) in the BRX group was 45.9%. In a long-term clinical trial of BRX in Japan, 59.2% and 41.8% of newly enrolled patients and patients continuing from the short-term study, respectively, completed a 52-week assessment [30]. In a long-term global study [31], 82.5% and 43.0% of the patients completed the study for 26 and 52 weeks, respectively. Our real-world study showed slightly lower continuation rates than those reported in this clinical trial, which may be due to differences between clinical trials conducted under strict inclusion/exclusion criteria and supervision, and observational studies conducted based on data obtained from daily clinical practice. A previous real-world study reported treatment continuation rates of 48.5% for BRX at 24 weeks [27]. Our results may, therefore, reflect real-world continuation rates based on big data from a nationwide health insurance claims database.
The proportion of patients with PDC ≥ 0.8 was 35.6% and 31.9% in the BRX and OAA groups, respectively; these results are generally in line with those of a previous claims database study in the USA [32]. The percentage of PDC ≥ 0.8 in the BRX group was higher than that in the OAA group and in any of the four medicine groups (APZ, 33.7%; OLZ, 29.7%; QTP, 32.2%; RIS, 28.7%). Although the severity of symptoms in our study population is unknown, the outpatient status of most patients (at the time of index date, 87.3% and 77.7% in the BRX and OAA groups, respectively, were not hospitalized) suggests that their symptoms may be managed by the current treatment. Nevertheless, further improvements in adherence may contribute to relapse prevention and improve clinical outcomes. Further studies exploring patients in need of attention are warranted.
We observed slight differences in the baseline patient characteristics between the BRX and OAA groups. As this observational study included all eligible patients prescribed index drugs in a real-world setting, selection bias may have led to such baseline differences. The sensitivity analyses based on patients matched by propensity score, however, supported the results of the main analysis, favoring BRX over OAAs. Furthermore, a subgroup analysis without prescription restrictions yielded similar favorable results for BRX. We conducted this subgroup analysis because, in the case of new drugs, prescribing physicians gradually gain an understanding of drugs’ characteristics over time. We considered that this initial stage after the drug’s launch would influence prescribing patterns and treatment discontinuation. Additionally, the mean CP equivalent dose was 186.5 and 172.2 mg/day in the two groups, respectively; these were at a lower limit of the recommended dose of APZ (150–600 mg/day CP equivalent) [23] and appeared to be lower than the previously reported figure (a median CP equivalent dose approx. slightly > 200–400 mg/day) in a real-world study using the JMDC database [33]. The lower results in our study may be because the authors of the mentioned real-world study [33] excluded patients with CP equivalent doses ≤ 75 mg/day; our results were therefore generally similar to those of the previous database study. Additionally, we found over half of the patients had comorbid depression (54.5% and 50.6% in the BRX and OAA groups, respectively; ESM Table S1), and approximately 40% of the patients in the BRX and OAA groups were prescribed antidepressants at baseline. As there is a possibility of the schizophrenia diagnosis code being entered to prescribe antipsychotics, these baseline characteristics may indicate that patients without schizophrenia were included in the analysis population. Despite these observations, all subgroup analyses showed similar results to those of the primary analysis.
The sensitivity and subgroup analyses showed similar results to those of the primary analysis. Along with the higher PDC in the BRX group, the findings of our study indicate that BRX may contribute to treatment continuation during maintenance therapy among patients with schizophrenia. Treatment continuation reflects efficacy and tolerability of a drug [18]. Combined with the aforementioned efficacy and safety profiles of BRX shown in clinical trials [12,13,14, 29, 30], these findings may suggest a potential role for BRX in relapse prevention in schizophrenia. Further studies, including prospective ones, may clarify the causality between BRX and treatment continuation and are of clinical value.
Limitations
This study has several limitations. First, it is possible that the results are not generalizable to the entire Japanese population with schizophrenia. People with severe symptoms may be less represented in the study population because we used employment-based health insurance claims data and individuals may withdraw from employment-based insurance in cases of symptom exacerbation or relapse. Although such selection bias did not apply to dependents, patients with non-severe schizophrenia symptoms may be represented in the study population. The database does not contain records of individuals aged ≥ 75 years. Furthermore, the proportion of patients aged ≥ 65 years in the data source (3.0%) [34] is considerably lower than the proportion of persons aged ≥ 65 years in the Japanese general population (approximately 29%) [35]. Therefore, the findings of this study may not be consistent with the general situation in Japan, and the potential confounding effect of the differences in the proportion of older people should be noted. Additionally, as discussed above, we observed slight between-group differences in patient characteristics, but such differences may reflect real-world situations. Although polypharmacy is more common in Japan than in other countries among patients with schizophrenia, we compared the time to treatment discontinuation between patients prescribed monotherapy with BRX and OAAs. Due to the nature of the health insurance claims database, diagnosis records of schizophrenia entered for billing purposes may not reflect actual diagnosis. However, the sensitivity and subgroup analyses showed similar results to those of the primary analysis. The treatment discontinuation and PDC we examined using prescription records do not reflect actual intake of prescribed medication. Additionally, severity of schizophrenia is not recorded in the database. Treatment discontinuation and the level of adherence may differ with severity and reasons unknown to us. Since the patient background and baseline are biased and there are unmeasured confounding factors, we conducted analyses to remove confounding as much as possible, but it is unclear whether we were able to remove all confounding factors. However, we consider that confounding effects of polypharmacy were limited to patients who were prescribed antipsychotics other than index drugs for < 30 days after index date. We have further taken into account the confounding effect of polypharmacy in all statistical analyses by adjusting for baseline variables, including the total CP equivalent dose of antipsychotic medications.
Conclusion
In this real-world study using nationwide claims data of working individuals and their dependents, we found that patients in the BRX group were significantly less likely to discontinue treatments than those in the OAA group, and that the treatment continuation rate at 180 days was higher in the BRX group. Although we noted slight between-group differences in baseline patient characteristics, sensitivity and subgroup analyses showed similar results to those of the primary analysis. These findings suggest that BRX may contribute to treatment continuation during maintenance therapy for patients with schizophrenia. Further studies including prospective ones may clarify the causality between BRX and treatment continuation.
References
Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–306.
Japanese Society of Neuropsychopharmacology. Japanese Society of Neuropsychopharmacology: guideline for pharmacological therapy of schizophrenia. Neuropsychopharmacol Rep. 2021;41(3):266–324.
Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–7.
Kane JM. Treatment strategies to prevent relapse and encourage remission. J Clin Psychiatry. 2007;68(Suppl 14):27–30.
Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–51.
Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46; quiz 47–8.
Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–68.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51.
Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604.
US Food and Drug Administration. REXULTI® (brexpiprazole) tablets, for oral use. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/205422s007lbl.pdf. Accessed 26 July 2022.
Otsuka Pharmaceutical Co., Ltd. Rexulti tablet 1mg/ rexulti tablet 2mg. (Brexpiprazole) Prescribing information. 2018. https://www.info.pmda.go.jp/go/pack/1179058F1020_1_04/ (in Japanese). Accessed 26 July 2022.
Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-Week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–80.
Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2018;72(9):692–700.
Kane JM, Skuban A, Hobart M, et al. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016;174(1–3):93–8.
Maeda K, Lerdrup L, Sugino H, et al. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605–14.
Pharmacy Quality Alliance. PQA adherence measures [updated 2018/8/28]. https://www.pqaalliance.org/adherence-measures. Accessed 10 Feb 2022.
University Hospital Medical Information Network Center (UMIN). Clinical Trials Registry (CTR) ID: UMIN000044682. Treatment persistence and medication adherence in patients with schizophrenia treated with brexpiprazole versus other oral atypical antipsychotic therapy—cohort study using claim database. 2021. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000051007&type=summary&language=J (in Japanese). Accessed 10 Feb 2022.
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23.
Watanabe Y, Yamada S, Otsubo T, Kikuchi T. Brexpiprazole for the treatment of schizophrenia in adults: An overview of its clinical efficacy and safety and a psychiatrist’s perspective. Drug Des Devel Ther. 2020;14:5559–74.
Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–7.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Methodology). 1972;34(2):187–220.
Otsuka Pharmaceutical Co., Ltd. Abilify. (Aripiprazole) Prescribing information 2022. https://www.info.pmda.go.jp/go/pack/1179045B1021_1_35/ (in Japanese). Accessed 17 Feb 2022.
García S, Martínez-Cengotitabengoa M, López-Zurbano S, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36(4):355–71.
Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2.
Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32.
Inoue Y, Suzuki H, Hibino H, et al. Continuation rate for asenapine and brexpiprazole treatment in patients with schizophrenia. Brain Behav. 2021;11(5):e02109.
Correll CU, Skuban A, Hobart M, et al. Efficacy of brexpiprazole in patients with acute schizophrenia: Review of three randomized, double-blind, placebo-controlled studies. Schizophr Res. 2016;174(1–3):82–92.
Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1–3):127–35.
Ishigooka J, Iwashita S, Tadori Y. Long-term safety and effectiveness of brexpiprazole in Japanese patients with schizophrenia: a 52-week, open-label study. Psychiatry Clin Neurosci. 2018;72(6):445–53.
Forbes A, Hobart M, Ouyang J, Shi L, Pfister S, Hakala M. A long-term, open-label study to evaluate the safety and tolerability of brexpiprazole as maintenance treatment in adults with schizophrenia. Int J Neuropsychopharmacol. 2018;21(5):433–41.
Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–34.
Hata T, Kanazawa T, Hamada T, et al. The 12-year trend report of antipsychotic usage in a nationwide claims database derived from four million people in Japan. J Psychiatr Res. 2020;127:28–34.
Japanese Society for Pharmacoepidemiology. Database available for pharmacoepidemiology researches in Japan: information obtained from survey answers as of August 2019. 2019. https://drive.google.com/file/d/1wlX96kYd-rPT_OgBRjjt-Yq1XHeL0qLN/view. Accessed 7 Feb 2022.
Statistics of Japan. Population estimates: population by age (5-year age group) and sex, monthly estimates—Total population, Japanese population, the first day, each month 2020. https://www.e-stat.go.jp/ (in Japanese). Accessed 8 Mar 2022.
Acknowledgements
Funding
This work was supported by Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan. The Rapid Service and Open Access fees were also provided by Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan.
Medical Writing and Other Assistance
Assistance in the development of the statistical analysis plan and in medical writing was provided by Tatsuo Sakashita and Rie Hagihara, respectively, at Clinical Study Support, Inc., and were funded by Otsuka Pharmaceutical Co., Ltd. We thank Mr. Noriyuki Mamiya for his support in study planning and statistical analysis planning. Mr. Noriyuki Mamiya is a former employee of Otsuka Pharmaceutical Co., Ltd. from which he has received advisory fee.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship; made substantial contributions as detailed in the Author Contributions; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work.
Author Contributions
All authors contributed to the conceptualization, methodology, and validation of the study. Akitoyo Hishimoto, Norio Yasui-Furukori, and Sakiko Yamada were involved in supervision. Daisuke Sekine was involved in writing the original draft and project administration. Miyuki Matsukawa was involved in software and data analysis. All authors critically revised the manuscript, commented on drafts of the manuscript, and approved the final version of the manuscript to be published.
Disclosures
Akitoyo Hishimoto has received speaker's honoraria from Eisai, Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Mochida Pharmaceutical, Janssen Pharmaceutical K.K., Takeda Pharmaceutical, Meiji Seika Pharma, and Lundbeck Japan K.K. Norio Yasui-Furukori has received speaker's honoraria from Mochida Pharmaceutical, Takeda Pharmaceutical, and Otsuka Pharmaceutical. Daisuke Sekine, Miyuki Matsukawa, and Sakiko Yamada are employees of Otsuka Pharmaceutical Co., Ltd., Japan.
Compliance with Ethics Guidelines
As this study used pre-existing, anonymized data for secondary purposes, the study does not need to apply the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, and ethics approval from the institutional review board was not required. Additionally, the claims data were classified as anonymously processed information under the Act on the Protection of Personal Information 2003 in Japan. Informed consent from individual patients was therefore not required. The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments and registered in the University Hospital Medical Information Network (UMIN Clinical Trials Registry: UMIN000044682, 2021). The data were purchased from JMDC Inc. for the purpose of this study.
Data Availability
The data that support the findings of this study are available for purchase from the JMDC Inc., and restrictions apply to the availability of the data due to contractual agreements between JMDC and health insurance associations. For inquiries about access to the dataset used in this study, please contact JMDC (website: https://www.jmdc.co.jp/en/index; e-mail: mdbhelp@jmdc.co.jp).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hishimoto, A., Yasui-Furukori, N., Sekine, D. et al. Treatment Discontinuation Among Patients with Schizophrenia Treated with Brexpiprazole and Other Oral Atypical Antipsychotics in Japan: A Retrospective Observational Study. Adv Ther 39, 4299–4314 (2022). https://doi.org/10.1007/s12325-022-02252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02252-9