Abstract
Introduction
Beta-blockers are recommended by the European Society of Cardiology as first-line antianginal therapy for reducing heart rate (HR) and symptoms in patients with chronic coronary syndrome, despite a lack of data showing superiority to other antianginal agents. Most patients with angina pectoris require combination therapy to manage symptoms, with a second-line agent chosen to manage the predominant cardiovascular problem. Ivabradine, a selective sinus node If channel inhibitor shown to reduce HR and protect against anginal symptoms, has previously demonstrated noninferior anti-ischaemic and antianginal efficacy to beta-blockers.
Methods
This systematic review and meta-analysis assessed the efficacy and safety of ivabradine in patients with stable angina pectoris who remained symptomatic despite receiving beta-blockers. Keyword searches of PubMed, The Cochrane Central Library Register, ClinicalTrials.gov, The World Health Organization International Clinical Trials Registry Platform (ICTRP) and Google Scholar identified studies comparing ivabradine plus beta-blockers with placebo or other first- or second-line antianginal agents in patients with stable angina pectoris. No date limits or language restrictions were applied. Outcomes were evaluated after 1 and 4 months of treatment, including changes in HR, angina attacks, use of short-acting nitrates, quality of life and safety. Risk of bias was evaluated on the basis of recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.
Results
Seven relevant studies were identified (N = 6821). Ivabradine plus a beta-blocker consistently reduced HR, anginal symptoms and short-acting nitrate consumption within 1 month of initiating therapy, with continued reductions for up to 4 months. Furthermore, ivabradine plus beta-blocker therapy was well tolerated, with bradycardia rarely reported (0.1% of patients overall). This study is limited by the inclusion of only two randomised studies, which may lead to result interpretation bias.
Conclusions
Ivabradine may be valuable for tailoring early antianginal treatment when used in combination with beta-blockers for chronic stable angina inadequately controlled by beta-blockers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Controlling the symptoms of angina in patients with chronic stable angina remains challenging, with quality of life and economic consequences for patients. |
This systematic review and meta-analysis evaluated the efficacy and safety of ivabradine in patients with stable angina pectoris who remain symptomatic while receiving beta-blockers. |
What was learned from the study? |
The combination of ivabradine plus beta-blocker therapy effectively reduced heart rate, angina symptoms and the use of short-acting nitrates from the first month of treatment and was well tolerated in real-life clinical practice. |
Ivabradine plus beta-blockers may be a useful antianginal treatment option in patients with chronic stable angina that is inadequately controlled with beta-blocker-based therapy. |
Introduction
Ischaemic heart disease (IHD) is a leading cause of mortality worldwide [1]. Estimated to affect almost 71 million people globally in 2017, angina pectoris (AP) is a common symptom of IHD [2]. Indeed, angina causes greater disability than other cardiovascular diseases associated with IHD, such as heart failure (HF) or myocardial infarction (MI) [2]. Prognosis in patients with chronic coronary syndrome is worsened by the presence of anginal symptoms in daily life (with or without ischaemia), with an increased risk of cardiovascular death or MI compared with ischaemia alone [3].
Heart rate (HR) is an important regulator of oxygen consumption in myocytes. An increase in HR increases the number of cardiac cycles per given time frame and, therefore, cardiac performance and myocardial metabolism [4]. In contrast, a reduction in HR decreases oxygen demand and maintains the viability of myocytes, and prolongs diastolic perfusion time and improves coronary flow velocity reserve, thus increasing the ischaemic threshold [5]. Ambulatory ischaemic episodes in patients with chronic coronary syndrome are preceded by elevated HR (i.e. greater than 70 bpm), with the risk of developing ischaemia related to baseline HR, as well as the magnitude and duration of the HR increase [6, 7]. Also, the frequency of ambulatory ischaemic episodes in patients with chronic coronary syndrome is related to mean HR, with an almost twofold increase in the risk of ischaemia in patients with an HR of 80 bpm versus 70 bpm [8]. Consequently, a reduction in HR is recognised as an important target for managing angina [9] and has been demonstrated to reduce angina symptoms [5].
In the latest European Society of Cardiology (ESC) guidelines, a reduction in resting HR to a target of at most 60 bpm remains an essential goal in order to reduce ischaemia and angina when treating patients with chronic coronary syndrome [10]. Despite a lack of data showing superiority to other antianginal agents, beta-adrenergic blocking agents (beta-blockers) are currently recommended as first-line antianginal therapy for reducing HR and symptoms in patients with chronic coronary syndrome [10]. However, many patients with angina pectoris require combination therapy to manage symptoms, and the ESC guidelines recommend choosing second- and third-line agents on the basis of the patient’s predominant cardiovascular problem (e.g. high or low HR, low blood pressure, or ventricular dysfunction) [10].
Ivabradine is a hyperpolarisation-activated cyclic nucleotide-gated (HCN) channel blocker that selectively inhibits the ‘funny’ current (If) in the sinoatrial node [9, 11, 12]; ivabradine is recommended as an add-on treatment in patients with angina and elevated HR or left ventricular (LV) dysfunction [10]. It reduces HR and myocardial oxygen demand while increasing both coronary filling time and blood flow, thus protecting against ischaemia and angina symptoms [12]. Ivabradine has been shown to be noninferior to beta-blockers in terms of anti-ischaemic and antianginal efficacy [13]. Combining ivabradine with a beta-blocker further reduces HR, myocardial ischaemia and the symptoms of angina in patients with stable angina [14, 15]. A previous pooled analysis of real-world data indicated that ivabradine was effective and well tolerated in a range of patients with angina during routine clinical practice [16], but this analysis included patients receiving ivabradine with and without concomitant beta-blockers.
The current systematic review and meta-analysis assessed the efficacy and safety of ivabradine in patients with stable angina pectoris who remained symptomatic despite receiving beta-blocker-based therapy.
Methods
Study Design
This systematic review and meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17], in conjunction with Sciencefiles LLC (Ekaterinburg, Russia). A systematic search of the published literature was used to identify studies that compared ivabradine plus beta-blocker therapy with placebo or other first-line (i.e. adrenergic beta antagonists, calcium channel blockers) or second-line (i.e. long-acting nitrates, ivabradine monotherapy, nicorandil, trimetazidine, ranolazine) antianginal agents. Use of other antianginal drugs (i.e. statins, antiplatelet agents, antihypertensive agents and surgical interventions for coronary artery disease [CAD]) were permitted as concomitant therapy.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Criteria and Study Selection
Keyword searches were conducted in June 2021 using several databases: PubMed (https://www.nlm.nih.gov); The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (https://www.cochranelibrary.com/central); The National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov); The World Health Organization International Clinical Trials Registry Platform (ICTRP) (https://www.who.int/ictrp/en/); and Google Scholar (https://scholar.google.ru/). The search criteria used are shown in Supplementary Table S1 in the electronic supplementary material.
Studies of patients with stable angina pectoris with HR greater than 70 bpm were included. Excluded were studies of patients with unstable angina, any serious decompensated concomitant diseases requiring regular medical therapy or not in sinus cardiac rhythm. Selection of studies was completed independently in duplicate by E. Lantsova and I. Nikitina of Sciencefiles, with any disagreements resolved through discussion and consensus or, if required, arbitrated by a third researcher. No date limits were applied and publications in any language were included. Titles and abstracts of all search hits were reviewed to identify potentially eligible trials for retrieval. Full-text copies of all retrieved trial reports/publications were then reviewed to identify final studies for inclusion. Reasons for exclusion of ineligible studies were recorded. Duplicate publications were identified and excluded, and multiple reports of the same trial were collated into a single report.
Risk of bias was evaluated by grading each potential source of bias as high, moderate or low on the basis of recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [18] and the Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) tool [19], and summarised by domains. An additional hand search was performed to identify published study protocols to check for selective reporting bias.
Data Extraction
Data from each relevant study were extracted manually into an Excel spreadsheet (Windows MSO 365; Microsoft Corporation, Redmond, WA, USA). Data were extracted separately by two individuals, E. Lantsova and I. Nikitina, with differences resolved by discussion and consensus, or arbitrated by a third researcher. Extracted data included study methods (e.g. duration of trial, details of any run-in period, date of publication); participants (e.g. number analysed, subgroups, demographics [age, sex], clinical characteristics [previous cardiovascular events, comorbidities, congestive HF class]); interventions and concomitant medications; outcomes at 1 and 4 months (i.e. HR, decrease in HR of greater than 10 bpm or to 70 bpm or less, improvement in angina functional class, number of angina attacks per week, units of short-acting nitrates per week, quality of life [QoL], adverse events [AEs], bradycardia); and notes (e.g. trial funding, notable conflicts of interest).
Outcomes Evaluated
Primary efficacy outcomes of the study were to determine differences from baseline in HR, weekly angina attacks and weekly use of short-acting nitrates after 1 month of ivabradine plus beta-blocker therapy. Secondary efficacy outcomes included the aforementioned outcomes after 4 months of ivabradine plus beta-blocker therapy, as well as the change from baseline in functional class of angina (according to Canadian Cardiovascular Society [CCS] Angina Grading Scale) after 4 months of treatment. QoL was also assessed using the EuroQol-5D (EQ-5D) questionnaire and/or a visual analogue scale (VAS) index. Safety outcomes included the evaluation of AEs, including the incidence of bradycardia.
Statistical Analyses
Data from the spreadsheets were transformed into STATA data sets and analysed using STATA version 14 (StataCorp LLC; College Station, TX, USA). Continuous data were presented as weighted average differences between groups with 95% confidence intervals (CIs). Categorical data (frequency of events) were described using a risk ratio with 95% CI for randomised studies, or mean ± standard deviation (SD) for observational studies. Qualitative indicators were assessed using frequencies and percentage values, with a P value of less than 0.05 considered to be statistically significant. Cohen’s standardized mean differences were used to calculate treatment effect sizes. The characteristics and risk of bias for each contributing study (results not shown) were taken into account when considering treatment effects for individual outcomes. Forest plots were used to visually assess for signs of heterogeneity, and statistical heterogeneity was assessed using chi-squared test (threshold P < 0.10) and I2 statistics, to quantify inconsistency across studies.
Results
Identified Studies
Of 810 reports identified in the searches, seven studies including a total of 6821 patients fulfilled all criteria and were included in the analysis. The reasons for study exclusion are given in Fig. 1, and the details of the included studies are shown in Supplementary Table S2. One of the included studies was a randomised controlled trial [20], while the remaining six were prospective, non-interventional studies (Supplementary Table S2). All seven studies were included in the analysis of HR [15, 20,21,22,23,24,25], six studies were evaluable for angina symptoms [15, 20,21,22,23, 25], five studies were evaluable for weekly use of short-acting nitrates [15, 20, 22, 23, 25], three studies were evaluable for QoL [15, 20, 25], six studies were evaluable for change in CCS classification [15, 20, 22,23,24,25] and five studies were evaluable for safety [15, 20, 22, 23, 25].
The baseline characteristics of patients enrolled in the included studies were generally similar between studies, except in one study which only enrolled men [24], while weekly angina attacks and concomitant medications varied across studies (Table 1). Overall, patients had a mean HR of 83 bpm, a mean of two angina attacks per week and used short-acting nitrates a mean of 1.8 times per week. The majority of patients (60–88%) had hypertension and approximately one-third of patients in most studies had diabetes mellitus.
The majority of studies were rated as “low risk” (N = 1) or “some concerns” (N = 4) for bias risk (Fig. 2; Supplementary Fig. S1).
Primary Efficacy Outcomes
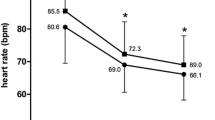
Among the seven evaluable studies, five demonstrated reduced HR after 1 month of ivabradine plus beta-blocker therapy [15, 20, 21, 23, 25]. Statistical analysis of HR effect size among these five studies revealed a decrease in HR in all studies (Fig. 3a), with an overall decrease in HR of 12.6 bpm in patients receiving ivabradine plus beta-blocker therapy (P < 0.0001).
Among five evaluable studies [15, 20, 21, 23, 25], treatment with ivabradine plus a beta-blocker was associated with a progressive reduction in the mean number of angina attacks per week at 1 month (Fig. 4a).
Similarly, among three evaluable studies, consumption of short-acting nitrates during the first month of treatment was significantly reduced from baseline (Fig. 5a) [15, 20, 25]. Evaluation of the effect size showed an overall decrease in the use of short-acting nitrates of 1.25 times per week (P < 0.0001).
Secondary Efficacy Outcomes
Among six evaluable studies, all showed a reduction in HR from baseline to 4 months (Fig. 3b), with four studies showing a decrease in HR after 1 month of treatment with continued HR reduction after 4 months [15, 20, 23, 25]. The overall mean reduction in HR from baseline to month 4 was 18 bpm (P < 0.0001).
The frequency of weekly angina attacks was significantly reduced from baseline to 4 months in all five evaluable studies [15, 20, 22, 23, 25]. Four studies showed reductions in weekly angina attacks after 1 month that continued for up to 4 months [15, 20, 23, 25]. Statistical analysis of the effect size revealed the reduction ranged from 1.1 to 2.3 angina attacks per week among the individual studies, with an overall decrease of 1.56 angina attacks per week after 4 months of treatment (P < 0.0001) (Fig. 4b).
Among five evaluable studies, weekly use of short-acting nitrates was reduced from baseline to month 4 in all studies [15, 20, 22, 23, 25], with three studies showing continued reductions in weekly short-acting nitrates from 1 month through to 4 months [15, 20, 25]. Statistical evaluation of the treatment effect size showed an overall decrease in short-acting nitrate use of 1.58 times per week (P < 0.0001) (Fig. 5b).
The combination of ivabradine plus a beta-blocker was associated with clinical improvement after 4 months of treatment, as evidenced by a change in the distribution of CCS classification among patients. An increase in the proportion of patients with CCS class I angina was reported in the six evaluable studies [15, 20, 22,23,24,25], with an overall proportional increase in patients with CCS class I angina of 42% (Fig. 6).
Improvement in QoL was observed with ivabradine plus beta-blocker therapy in three evaluable studies, but formal statistical analysis of the data was not possible [15, 20, 25]. In the study by Zarifis et al. [25], the mean ± SD EQ-5D index score improved at study completion by 0.3 points compared with baseline (from 0.6 ± 0.3 to 0.9 ± 0.2), while mean ± SD VAS score improved by approximately 16 points (from 62.8 ± 16.3 to 78.9 ± 17.0). Similarly, in the study by Werdan et al. [15] the mean ± SD EQ-5D index score improved from 0.661 ± 0.267 at baseline to 0.749 ± 0.222 at 1 month and 0.827 ± 0.196 at 4 months. The mean ± SD VAS score improved from 57.4 ± 18.3 points at baseline to 65.6 ± 16.0 points at 1 month and 72.7 ± 15.4 points at 4 months. Finally, in the study by Glezer et al. [20], median (interquartile range) VAS patient health status scores improved from 65 (48–78) points at baseline to 32 (18–47) points at the end of treatment.
Safety Outcomes
Among five evaluable studies, the overall frequency of AEs ranged from 0.6% to 9.4% across studies (Table 2). The most common AE was bradycardia, which was reported in 0.04–1.3% of patients across studies and in 0.1% of patients overall. Other AEs were rare, being reported in no more than 1.8% of patients across studies.
Discussion
This systematic review evaluated the efficacy and safety of adjunctive ivabradine in patients with angina inadequately controlled with beta-blocker-based therapy. This review was based largely on data from several prospective, observational studies, including a variety of settings in real-life clinical practice. Addition of ivabradine to beta-blocker therapy consistently reduced HR, anginal symptoms and short-acting nitrate consumption within 1 month of initiating treatment, with continued improvements for up to 4 months, resulting in improved CCS class after 4 months of therapy. Furthermore, ivabradine plus beta-blocker therapy was well tolerated. These data suggest that ivabradine may play a valuable role in tailoring early antianginal treatment, particularly when used in combination with beta-blockers in patients with chronic stable angina. Optimisation of early antianginal treatment will ensure more rapid and effective HR and symptomatic control with improved tolerability.
The results of this systematic review are consistent with those of two previously conducted pooled analyses [16, 26]. A pooled analysis based on five randomised controlled trials in approximately 2400 patients with symptomatic stable angina [26] and a pooled analysis of data from three observational clinical studies in approximately 8500 patients with symptomatic angina who received ivabradine for 3–4 months [16] both demonstrated significant reductions in the frequency of angina attacks and the consumption of short-acting nitrates with ivabradine across a variety of subpopulations of patients with angina seen in clinical practice, independent of patient age, comorbidities and use of beta-blockers.
Despite the advancement and development of medical therapy and revascularisation techniques during the past decades, controlling the symptoms of angina is still challenging. Angina remains prevalent in ambulatory patients with non-negligible QoL and economic consequences for these patients. For instance, in the BRIDGE registry, more than 50% of patients reported monthly angina, with a high prevalence of angina equivalents during exercise [27]. Interestingly, the presence of revascularisation in the patient history was not linked to a better symptomatic status, 40% of patients reported deterioration of QoL (which was significantly higher in recently diagnosed patients) and 78% reported a negative impact on their employment. Patients in this registry were treated with a mean of fewer than two antianginal drugs [27], which may partially explain the insufficient control of angina symptoms in these patients.
In the CLARIFY registry, most patients with symptomatic angina at baseline were treated with a mean of two antianginal drugs, predominantly first-line antianginal therapy (i.e. beta-blockers and calcium channel blockers) and long-acting nitrates [28]. However, 50% remained symptomatic within 1 year of follow-up, regardless of treatment and the application of revascularisation procedures (when considered appropriate by the treating physician) [28]. Moreover, in the placebo-controlled ORBITA study, more than 50% of patients remained symptomatic despite more than 90% receiving triple-combination therapy with a beta-blocker, calcium channel blocker and a long-acting nitrate, a 6-week antianginal therapy optimisation period and undergoing revascularisation [29,30,31].
The 2020 ESC chronic coronary syndrome guidelines confirmed the lack of superiority evidence among different antianginal classes, as well as the limited evidence of the benefits of combining first-line antianginals, with no data on their use in combination with long-acting nitrates [10]. The ESC guidelines endorse the concept of antianginal therapy that is tailored to the individual patient, their haemodynamic status and the presence of comorbidities, recommending the early combination of first- and second-line antianginal drugs, like ivabradine, in order to achieve fast alleviation of angina symptoms within 2–4 weeks, if possible [10]. Optimal medical therapy in angina is defined by the ESC as one that can provide efficacy with an excellent tolerability profile to ensure long-term adherence to therapy [10], which in this case is for life.
First-line haemodynamic antianginal drugs are associated with many limitations in terms of reaching the necessary dose in a real-life setting. In the CLARIFY registry of more than 33,000 patients with coronary artery disease, 44% of symptomatic patients maintained resting HR of 70 bpm or higher, despite the majority (75%) receiving beta-blocker therapy [32, 33]. Of note, mean beta-blocker dosages in CLARIFY were 35–76% of the maximum recommended levels [32]. Reaching the beta-blocker target dose is challenging, even in a clinical study setting like ORBITA, in which 46% of patients did not reach the beta-blocker target dose within 6 weeks of optimising antianginal therapy [31]. The limitations of beta-blocker up-titration as an approach to reach target HR were further highlighted in the CONTROL-2 study [20]. While such up-titration substantially reduced HR (from 83.2 ± 10.9 bpm to 63 ± 8 bpm), and target HR (55–60 bpm) was achieved in approximately 50% of patients, adverse events were frequent, being significantly more common than in patients in whom the treatment approach was ivabradine plus beta-blockers (18.4% vs. 9.4%, P < 0.001). Rates of asthma, dyspnoea, hypotension and fatigue were significantly higher in the beta-blocker up-titration group.
A strategy of early treatment with a combination of first- and second-line antianginal agents (like ivabradine) in order to achieve more rapid control of HR and angina symptoms seems a more practical approach than first-line therapy alone, especially in real-life clinical practice. In this setting, patients represent varied clinical situations and comorbidities, which may limit the application and effect of beta-blockers or their combination with calcium channel blockers or long-acting nitrates. Moreover, while ivabradine has a different primary mechanism of action to beta-blockers, beta-blockers may also affect If amplitude and action potential duration of the nodal cells (via indirect inhibition of cyclic adenosine monophosphate generation) [34], possibly providing a complementary therapeutic effect when the drugs are used in combination. Ivabradine also has a good safety profile, does not affect blood pressure and, unlike beta-blockers, has been shown to preserve coronary vasodilatation during effort [35]. In addition, ivabradine provides better myocardial perfusion for each HR bpm reduction than beta-blockers [36], with no risk of bradycardia, as HR is reduced according to the patient’s pretreatment HR [37].
There are some limitations of this systematic review and meta-analysis. Firstly, only two randomised studies were included in the analysis. Additionally, the open and observational design of the included studies may represent a bias for result interpretation. Risk of this bias may be partly reduced by the fact that the intervention’s large clinical effect was observed within relatively large sample sizes. Our review focused mainly on real-life, open-label studies that lacked a placebo-control group, which may also be perceived as a limitation; however, real-life studies have been shown to have external validity, and to provide information that can complement data from randomised controlled trials that enrol very selective patient populations [38]. Real-life data are now considered by some regulatory agencies as robust enough to support the regulatory decision-making and approval process [39]. The average HR of patients in the studies in this analysis was between 80.6 and 86.9 bpm, which is the population in whom beta-blocker plus ivabradine therapy is recommended in the European guidelines [10]. Therefore, our data should not be generalised to a patient population with well-controlled HR during beta-blocker therapy.
Conclusions
This systematic review and meta-analysis indicated that combination therapy with ivabradine plus a beta-blocker was effective from the first month of treatment and was well tolerated in real-life clinical practice in patients with angina that was inadequately controlled with beta-blocker-based therapy, including those with multiple comorbidities. In addition to guideline-recommended treatments, ivabradine plus beta-blocker therapy may be a useful antianginal treatment to optimise symptomatic control in patients with chronic stable angina.
References
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Steg PG, Greenlaw N, Tendera M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the international observational CLARIFY registry. JAMA Intern Med. 2014;174:1651–9.
Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153:1589–601.
Ferrari R, Fox K. Heart rate reduction in coronary artery disease and heart failure. Nat Rev Cardiol. 2016;13:493–501.
Andrews TC, Fenton T, Toyosaki N, et al. Subsets of ambulatory myocardial ischemia based on heart rate activity. Circadian distribution and response to anti-ischemic medication. The Angina and Silent Ischemia Study Group (ASIS). Circulation. 1993;88:92–100.
Kop WJ, Verdino RJ, Gottdiener JS, O’Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events(1). J Am Coll Cardiol. 2001;38:742–9.
Pratt CM, McMahon RP, Goldstein S, et al. Comparison of subgroups assigned to medical regimens used to suppress cardiac ischemia (the Asymptomatic Cardiac Ischemia Pilot [ACIP] study). Am J Cardiol. 1996;77:1302–9.
Bertero E, Heusch G, Munzel T, Maack C. A pathophysiological compass to personalize antianginal drug treatment. Nat Rev Cardiol. 2021;18:838–52.
Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
DiFrancesco D. Cardiac pacemaker I(f) current and its inhibition by heart rate-reducing agents. Curr Med Res Opin. 2005;21:1115–22.
DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–65.
Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, INITIATIVE Investigators. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–36.
Tardif JC, Ponikowski P, Kahan T, ASSOCIATE Study Investigators. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–8.
Werdan K, Ebelt H, Nuding S, Hopfner F, Hack G, Muller-Werdan U. Ivabradine in combination with beta-blocker improves symptoms and quality of life in patients with stable angina pectoris: results from the ADDITIONS study. Clin Res Cardiol. 2012;101:365–73.
Werdan K, Perings S, Koster R, et al. Effectiveness of ivabradine treatment in different subpopulations with stable angina in clinical practice: a pooled analysis of observational studies. Cardiology. 2016;135:141–50.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Version 6.2. 2021. https://doi.org/10.1002/14651858.ED000142. Accessed 13 Apr 2022.
Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Glezer M, Vasyuk Y, Karpov Y. Efficacy of ivabradine in combination with beta-blockers versus uptitration of beta-blockers in patients with stable angina (CONTROL-2 study). Adv Ther. 2018;35:341–52.
Kashtalap VV, Barbarash OL, Sedykh DY, Krivoshapova KE, Tsygankova DP, Tsygankova OV. Possibilities of combination of beta-blockers and ivabradine in patients with stable angina pectoris. Rational Pharmacother Cardiol. 2019;15:663–9.
Koester R, Kaehler J, Ebelt H, Soeffker G, Werdan K, Meinertz T. Ivabradine in combination with beta-blocker therapy for the treatment of stable angina pectoris in every day clinical practice. Clin Res Cardiol. 2010;99:665–72.
Perings S, Stockl G, Kelm M, RESPONSIfVE study investigators. Effectiveness and tolerability of ivabradine with or without concomitant beta-blocker therapy in patients with chronic stable angina in routine clinical practice. Adv Ther. 2016;33:1550–64.
Zaky H, Elzein H, Alsheikh-Ali AA, Al-Mulla A. Short-term effects of ivabradine in patients with chronic stable ischemic heart disease. Heart Views. 2013;14:53–5.
Zarifis J, Grammatikou V, Kallistratos M, Katsivas A, on behalf of the Investigators of the Prospective, Noninterventional Observational Study of the Antianginal Efficacy of Ivabradine During a Month Treatment of a Greek Population With Coronary Artery Disease. Treatment of stable angina pectoris with ivabradine in everyday practice: a pan-hellenic, prospective, noninterventional study. Clin Cardiol. 2015;38:725–32.
Tendera M, Borer JS, Tardif JC. Efficacy of I(f) inhibition with ivabradine in different subpopulations with stable angina pectoris. Cardiology. 2009;114:116–25.
Manolis AJ, Ambrosio G, Collins P, et al. Impact of stable angina on health status and quality of life perception of currently treated patients. The BRIDGE 2 survey. Eur J Intern Med. 2019;70:60–7.
Mesnier J, Ducrocq G, Danchin N, et al. International observational analysis of evolution and outcomes of chronic stable angina: the multinational CLARIFY study. Circulation. 2021;144:512–23.
Al-Lamee R, Howard JP, Shun-Shin MJ, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation. 2018;138:1780–92.
Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40.
Foley M, Rajkumar CA, Shun-Shin M, et al. Achieving optimal medical therapy: insights from the ORBITA trial. J Am Heart Assoc. 2021;10: e017381.
Tendera M, Fox K, Ferrari R, et al. Inadequate heart rate control despite widespread use of beta-blockers in outpatients with stable CAD: findings from the international prospective CLARIFY registry. Int J Cardiol. 2014;176:119–24.
Steg PG, Ferrari R, Ford I, et al. Heart rate and use of beta-blockers in stable outpatients with coronary artery disease. PLoS ONE. 2012;7: e36284.
DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. 2007;67(Suppl 2):15–24.
Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–66.
Tagliamonte E, Cirillo T, Rigo F, et al. Ivabradine and bisoprolol on doppler-derived coronary flow velocity reserve in patients with stable coronary artery disease: beyond the heart rate. Adv Ther. 2015;32:757–67.
Borer JS, Le Heuzey JY. Characterization of the heart rate-lowering action of ivabradine, a selective I(f) current inhibitor. Am J Ther. 2008;15:461–73.
Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495.
Purpura CA, Garry EM, Honig N, Case A, Rassen JA. The role of real-world evidence in FDA-approved new drug and biologics license applications. Clin Pharmacol Ther. 2022;111:135–44.
Acknowledgements
Funding
Servier, France, sponsored this review and paid the journal’s Rapid Service and Open Access fees.
Medical Writing, Editorial, and Other Assistance
Data extraction and statistical analysis were performed by Elena Lantsova, Irina Nikitina and Alexander Akimov of ScienceFiles, assisted by Yuriy Burtsev of Servier, Russia. Editorial assistance in the preparation and post-submission revision of this article was provided by Andrea Bothwell and Kate Palmer, respectively, on behalf of Springer Healthcare Communications. This assistance was funded by Servier, France.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the design of the analysis and evaluation of the results. The outline of the manuscript was prepared by Parvoleta Petrova. All authors commented on previous versions of the manuscript, and read and approved all drafts.
Disclosures
Alexander Nedoshivin is a committee member of the Euroobservational Research Programme: Oversight Committee (EORP), the general secretary of the NS Board- Russian Society of Cardiology, and has received speaker fees from Berlin Chemie AG, Menarini and Servier. Parvoleta Petrova is an employee of Servier. Yuri Karpov has served as an advisory board expert, national study coordinator and speaker for Servier, and has served as a speaker for AstraZeneca, Novartis, Stada, Zentiva, Novo Nordisk, Bayer, Berlin Chemie, Sanofi and Teva.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nedoshivin, A., Petrova, P.T.S. & Karpov, Y. Efficacy and Safety of Ivabradine in Combination with Beta-Blockers in Patients with Stable Angina Pectoris: A Systematic Review and Meta-analysis. Adv Ther 39, 4189–4204 (2022). https://doi.org/10.1007/s12325-022-02222-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02222-1