Abstract
Introduction
Hyperkalemia is associated with increased morbidity and mortality in patients with chronic kidney disease (CKD). Patiromer (Veltassa®) is an oral potassium binder indicated for the treatment of hyperkalemia in adults. We evaluated the impact of patiromer on the Swiss healthcare resources when used in patients with CKD and hyperkalemia who were on renin–angiotensin–aldosterone system inhibitor (RAASi) treatment.
Methods
We built a decision tree and calculated the number needed to treat (NNT) to prevent hyperkalemia, hospitalization, and death based on published aggregated data. The decision tree was populated with available data from relevant patiromer clinical trials and data were applied to create a simple model showing the expected effectiveness of adding patiromer to the treatment of patients with medium-to-severe stage CKD on RAASi compared to RAASi only. Adapting the model to the Swiss healthcare system allowed us to estimate the impact of the new treatment on healthcare expenditures from a payer as well as a Swiss public healthcare perspective.
Results
Patiromer reduced the absolute risk for recurrent hyperkalemia by 48% within 8 weeks, resulting in an NNT of 2.1 [95% CI 1.4, 3.7]. If one assumes that 90%, 50%, or 10% of all moderate-to-severe hyperkalemic events lead to hospitalization, the NNT to prevent one hospitalization would be 2.5, 4.4, and 22.2, respectively. On the basis of the death rate of patients with mild or moderate-to-severe hyperkalemia, and the prevalence of mild or moderate-to-severe hyperkalemia in the treatment and control groups, the NNT was 78.7 [95% CI 64.0, 99.3] to prevent one death. Patiromer resulted in expected cost offsets of CHF 303 (1 CHF = 0.95 EUR as of 2022) per patient over 8 weeks in Switzerland.
Conclusion
Patiromer used for the treatment of CKD reduces hyperkalemia recurrence leading to improved patient care. This results in substantial offset costs for the Swiss healthcare system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hyperkalemia is a common electrolyte disorder and particularly affects patients with chronic kidney disease (CKD). Patiromer, an oral potassium binder, is a new treatment option to prevent hyperkalemic events. |
Patiromer opens the possibility to provide savings in healthcare expenditures for not having to treat prevented hyperkalemias. We investigate how cost-effective the use of patiromer is in patients with CKD treated with RAASi in the Swiss healthcare setting. |
Clinical trials showed that patiromer can reduce the recurrence of hyperkalemia. This leads to lower outpatient and inpatient cost and therefore to expected cost offsets of CHF 303 per patient over 8 weeks. |
A preventive treatment with patiromer not only improves patients’ health but it also seems to contribute to lower healthcare cost in Switzerland. However, the lower limits of the confidence intervals for risk reduction and treatment costs are used, healthcare costs may also slightly increase. Treatment with patiromer might, however, still be worthwhile when taking into account the value of saved lives. |
Introduction
Hyperkalemia is one of the most common electrolyte disorders and particularly affects patients with chronic kidney disease (CKD). Risk factors for hyperkalemia can be life-threatening and cause serious heart problems and sudden death [1]. Potassium regulates the electrochemical balance of cells as well as the stability of the resting membrane potential. The majority of total body potassium is located in the intracellular compartment, and serum potassium concentrations are closely correlated to kidney function.
Serum potassium changes are usually determined by potassium shifts between intra- and extracellular space. Even small changes in serum potassium concentration can lead to substantial changes in the resting membrane potential. In line with the pro-arrhythmic effects of hypo- and hyperkalemia, observational studies have reported a U-shaped association between serum potassium and mortality in patients with CKD [2,3,4]. Furthermore, the analysis by Goyal et al. showed for patients with acute myocardial infarction a significant increase in mortality for serum potassium levels below 3.0 mmol/l or above 5.0 mmol/l, whereas death rates were lowest for serum potassium levels ranging between 4.0 and 4.5 mmol/l [5]. A study of patients in intensive care units also showed a strong correlation between elevated serum potassium levels above 4.5 mmol/l and increased mortality, whereas serum potassium levels lowered by at least 1 mmol/l within 48 h were associated with reduced mortality [6].
Common causes of hyperkalemia include kidney failure, hypoaldosteronism, and rhabdomyolysis. A number of medications can increase serum potassium, including spironolactone, NSAIDs, and angiotensin-converting enzyme inhibitors, such as renin–angiotensin–aldosterone system inhibitors (RAASi) [1].
Various therapies are available for the treatment of hyperkalemia. Acute, severe, and life-threatening hyperkalemia is treated with calcium gluconate, insulin and glucose, beta2-adrenoceptor agonists, and bicarbonate. If kidney function is severely impaired, potassium can be eliminated by dialysis. Treatment options for patients with recurrent hyperkalemia who do not require intensive medical care include the administration of loop diuretics and enteral potassium exchangers. Enteral potassium exchangers currently available in Switzerland consist of sodium- or calcium-containing polystyrene sulfonate. Despite limited data on their effectiveness and potentially life-threatening side effects (e.g., intestinal necrosis), they are commonly used, sometimes over long periods of time. A study performed in 2014 showed that in patients with hyperkalemia, the predominant treatment was sodium polystyrene sulfonate as monotherapy [7].
Patiromer (Veltassa®) is an oral potassium binder that received approval in December 2017 in Switzerland for the treatment of hyperkalemia in adults, and was accepted for reimbursement on 1 August 2020. Patiromer administrated to patients with CKD for up to 52 weeks has proven effective in preventing hyperkalemia by effectively reducing serum potassium levels [8]. Furthermore, patiromer enabled continuation of RAASi in patients with CKD stages 3–4 with or without heart insufficiency [8,9,10,11].
As substantial healthcare resources are needed to fight occurring hyperkalemias, the use of patiromer opens the possibility to not only improve patient health but also provide additional savings in healthcare expenditure.
The objective of this analysis therefore was to create a simple model to evaluate the cost-effectiveness of treating patients with CKD stages 3–4 with patiromer added to RAASi compared to RAASi only. We use this focus because in Switzerland reimbursement is limited to these patient groups. Adapting the model to the Swiss healthcare system allowed us not only to document benefits of either treatment regimen with regard to patient outcomes but also to evaluate potential financial savings for the Swiss healthcare system.
Our research questions were the following:
-
1.
In patients with CKD treated with RAASi, how effective is patiromer in preventing the recurrence of a hyperkalemic event?
-
2.
In patients with CKD treated with RAASi, how effective is patiromer in preventing premature death?
-
3.
In patients with CKD treated with RAASi, how cost-effective is the addition of patiromer in the Swiss healthcare setting?
The comparator for our analysis is no treatment. We chose this because the use of other treatments of hyperkalemia in an outpatient setting is limited either because of lack of efficacy or as a result of serious adverse events as pointed out earlier. The same reasoning is used in the patiromer pivotal trial that uses a placebo as a comparator [9].
Methods
To answer our research questions, we designed and populated a one-period decision tree, focussing on the short-term effects of treatment which best fitted the data analysis in this setting.
Until now, only short-term results have been published from placebo-controlled clinical trials on the use of patiromer (4 weeks in PEARL-HF [12], 8 weeks in OPAL-HK [9], and 12 weeks in AMBER [13]). In the absence of long-term placebo-controlled study data, building a Markov model showing the long-term progression of CKD with recurring hyperkalemic events was unfeasible. The use of a one-period decision tree focuses on the short-term effects of a treatment and was therefore considered to best fit the available data for this study.
We built our decision tree with the goal to compare three endpoints for patients with CKD stage 3 or 4 who had recurrent hyperkalemia and were treated with RAASi only or RAASi and patiromer. The three endpoints were:
-
1.
Recurrence of hyperkalemia
-
2.
Hospitalization
-
3.
Death
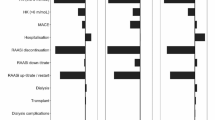
Figure 1 shows the model in the form of a decision tree. The decision node (blue square) is the path separator for the two treatment groups (RAASi only vs. RAASi with patiromer). The green circles show the chance nodes with the events’ rate probabilities during the observation period. There could be a mild or severe hyperkalemia or no hyperkalemia at all. Conditional on the occurrence of a hyperkalemic event, there is the option of an outpatient treatment, and in the case of a severe hyperkalemia, there is an additional option of an inpatient treatment (hospitalization). The red triangles show the end nodes of the model, which are no hyperkalemia, recovered, and death.
As conditional probabilities for the chance nodes, our model used results from recent studies to assess the effectiveness of patiromer added to RAASi in preventing recurrent hyperkalemia. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The main data source was the OPAL-HK clinical trial, a randomized, placebo-controlled, single-blind two-phase trial. The study includes patients with CKD stages 3–4 with a history of mild, moderate, or severe hyperkalemic event who were receiving RAAS inhibitors. In phase 1 (initial treatment phase), patients received patiromer for 4 weeks. In phase 2 (randomized withdrawal phase), patients were randomly assigned to 8 weeks of patiromer dosing added to RAASi (treatment group) or RAASi only (control group) [9]. Our model refers to the 8-week withdrawal phase. In accordance with the OPAL-HK study, we defined hyperkalemia as a potassium level of at least 5.1 mmol/l. Furthermore, we differentiated between mild hyperkalemia (potassium levels of at least 5.1 mmol/l and less than 5.5 mmol/l) and moderate-to-severe hyperkalemia (potassium levels of at least 5.5 mmol/l).
In addition to the endpoint of recurrent hyperkalemia, we compared the number of hospitalizations and deaths resulting from hyperkalemia. Again, the conditional probabilities result from recent studies which are described in the following sections. Where necessary, we supplemented the values from the literature with results from expert discussions with specialists with own practice. Table 1 shows an overview of all the probabilities used in the model with their sources.
Number needed to treat (NNT) was used to assess the effectiveness of the treatment with patiromer. The NNT describes the number of patients that have to be treated with patiromer to prevent one event (hyperkalemia, hospitalization, or death). This statistical measure is calculated from the inverse of the absolute risk reduction (ARR), which is the difference between the treatment event rate and the control event rate:
In addition to treatment effectiveness, we analyzed treatment outcomes in the form of cost effects. Here, we only looked at the first endpoint (recurrent hyperkalemia) and calculated the changes in treatment costs of one prevented hyperkalemic event. The costs of hospitalizations were included in the computation of cost effects for a prevented hyperkalemic event. We did not include in our analysis the cost derived from death.
Prevention of a Recurring Hyperkalemic Event
The probabilities of recurrence of hyperkalemia were based on the withdrawal phase of the OPAL-HK study. The probability of a recurring hyperkalemic event in the study’s treatment group was significantly lower than in the placebo group (PHK = 0.43 vs. 0.91 PHKsev = 0.15 vs. 0.60). Since only the probabilities for potassium levels of at least 5.1 mmol/l (PHK) and at least 5.5 mmol/l (PHKsev) were shown in the study, we calculated the missing probabilities for potassium levels between 5.1 and 5.5 mmol/l by subtracting the corresponding values (PHKmild = 0.28 vs. 0.31). For the sensitivity analysis, we calculated the maximal (minimal) risk reduction using the lower (upper) limit of the confidence interval of the treatment group and the upper (lower) limit of the confidence interval of the control group.
The NNT for preventing the recurrence of hyperkalemia was then calculated according to the formula:
In the case of mild or moderate-to-severe hyperkalemia, only the corresponding probabilities for these events remained in the formula.
Prevention of a Hospitalization
Successful management of hyperkalemia requires monitoring of serum potassium and treatment may include non-pharmacological and pharmacological interventions. Thus, we assumed that every hyperkalemic event was managed at least on an outpatient basis (\({P}_{\mathrm{OutpMild}}=1\), \({P}_{\mathrm{OutpSev}}=1\). Thus, the NNT to prevent an outpatient treatment corresponds to the NNT to prevent a hyperkalemic event:
However, hyperkalemia can also lead to a hospitalization. Usually, a mild hyperkalemic event can be treated entirely on an outpatient basis, meaning that no hospital stay is necessary in this case. We therefore assumed that
in the case of mild hyperkalemia. However, a positive probability of a hospitalization was assumed for moderate-to-severe hyperkalemic events:
The NNT to prevent a hospitalization due to hyperkalemia is hence
Einhorn et al. showed in a retrospective analysis of a national cohort of 2,103,422 records from 245,808 veterans in the USA that 52.7% of all hyperkalemic events (at least 5.5 mmol/l) occurred during hospitalization [1]. If only patients with CKD stages 3–5 were considered, the share of inpatients events was slightly lower (50.6%). However, the study only included patients with at least one hospitalization and therefore the share may have been overestimated. In Smith et al.’s study [14] the share of inpatient treatments among all treatments was 67% for patients with CKD, given a hyperkalemic or acute renal failure event. However, as acute renal failure was also included, this might also have been an overestimation. As a result of the rather limited data available, we used the hyperkalemic event rate of 50.6% of Einhorn et al. [1] as a starting point, and showed the sensitivity of this assumption by using the lower and upper values of 10% and 90%, respectively. We chose this range because the true values were highly likely to lie within it.
Prevention of a Death
In the worst case, hyperkalemia can lead to the death of a patient, with the probability of death depending on the severity of the hyperkalemic event. In a retrospective observational study with 55,266 patients with CKD stage 3–5, Luo et al. found a death rate of 3.4% for patients with mild hyperkalemia and 5.7% for patients with moderate-to-severe hyperkalemia (5.0–5.4 mmol/l and at least 5.5 mmol/l, respectively). Patients with normal potassium levels of 4–4.9 mmol/l had a death rate of 2.9% [4]. For our model we considered the difference between the two values (0.5% = 3.4% − 2.8% and 2.8% = 5.7% − 2.8%). In accordance with the study, we calculated the 95% confidence interval with a Poisson distribution.
The NNT for preventing one death was calculated in the same way as for preventing a hospitalization, with the probability of a hospitalization being replaced by the probability of death:
Costs
Besides the efficacy of the treatment (measured as NNT), we analyzed costs, i.e., healthcare expenditure resulting from outpatient and inpatient treatments of a hyperkalemic event. We conducted this analysis from a healthcare payer perspective, in this case the health insurance. Costs were based on the relevant insurance claims made to the Swiss mandatory health insurance (MHI). We considered outpatient and inpatient costs. Since we were interested in the difference in treatment costs between the two patient groups, we did not need treatment costs for managing CKD outside a hyperkalemic event, as we assumed that there was no difference for these costs between the two groups.Footnote 1
There is no diagnosis information available for outpatient treatments in Switzerland. We therefore calculated outpatient costs based on the tariff system for outpatient medical services (TARMED, version 1.09) by using model cases depending on the severity of the event. The model cases are based on a 15-min standard examination by a specialist, and resulted in outpatient costs of \({C}_{\mathrm{OutpMild}}\) = CHF 73 and \({C}_{\mathrm{OutpSev}}=\) CHF 139 (1 CHF = 0.95 EUR as of 2022), respectively. The tariff positions for such an outpatient standard diagnosis and treatment of a mild or moderate-to-severe hyperkalemia are listed in Tables S1 and S2 in the electronic supplementary material. The cost estimate of the outpatient treatment for moderate-to-severe hyperkalemia is rather conservative, as we only considered one consultation, whereas in actual fact 2–3 consultations are often necessary.
For inpatient treatments, we used the cost weights of the SwissDRG, the tariff system for inpatient treatments. The insurance has to pay the same amount for each case in a specific diagnosis-related group (DRG) according to its cost weights. Cases with main diagnosis of hyperkalemia (ICD-10 E87.5) are generally grouped into a DRG in the Major Diagnostic Category (MDC) 10Footnote 2 [15]. To calculate inpatient costs we used the average of the cost weights from these DRGs [16], weighted with the respective number of cases with main diagnosis hyperkalemia and multiplied by a base rate of CHF 9840, which is the weighted average for Switzerland ([17], own calculations). Since inpatient costs are jointly financed by health insurers and the cantons, we only considered the part financed by the mandatory health insurance (45%), which resulted in inpatient treatment costs for a moderate-to-severe hyperkalemia of CHF 3139 (see Table S3 in the electronic supplementary material for details).
Based on these outpatient and inpatient treatment costs, the expected treatment costs for a mild and moderate-to-severe hyperkalemic event could be calculated as follows:
This led to the expected treatment costs for the two patient groups treated with and without patiromer:
Finally, the expected cost offsets (ECO) due to a prevented hyperkalemic event were
Inclusion of the costs of the patiromer treatment (\({C}_{\mathrm{pat}}\)) led to cost differences calculated as
Results
Main Results
Treatment with patiromer resulted in an absolute risk reduction of 48 percentage points for recurrent hyperkalemia (43% of the patients in the treatment group compared to 91% in the control group had at least one potassium value of 5.1 mmol/l or higher). This led to an NNT as low as 2.1, i.e., 2.1 [1.4–3.7] patients would need to be treated with patiromer for 8 weeks to prevent recurrence of hyperkalemia.
In moderate-to-severe hyperkalemia, the absolute risk reduction was 45 percentage points (15% of patients in the treatment group with at least one potassium value of 5.5 mmol/l or higher, compared to 60% in the control group), leading to an NNT of 2.2 [1.5–4.3]. Weir et al. only report the values for all hyperkalemia and for moderate-to-severe hyperkalemia [9]. Therefore, we assume that at least 28% of the patients in the treatment group have a mild hyperkalemia (= 43% − 15%) and at least 31% of the patients in the control group (= 91% − 60%). This in turn led to an absolute risk reduction of 3 percentage points and an NNT of 33.3. As the calculated 95% confidence interval of the treatment and control groups overlapped, the differences between the groups were not statistically significant. The NNT ranged from 2.9 to − 3.5, where the negative value indicates that fewer hyperkalemic events occurred in the control group at the upper level of the confidence interval.
If one assumes that 50.6% of all moderate-to-severe hyperkalemic events lead to a hospitalization, the NNT to prevent one hospitalization is 4.4. A 90% hospitalization rate would lead to an NNT of 2.5, whereas a 10% hospitalization rate would result in an NNT of 22.2.
The death rates for patients with mild or moderate-to-severe hyperkalemia were 0.5% and 2.8%, respectively. Given the prevalence of a mild and moderate-to-severe hyperkalemia in the treatment and control group, this resulted in an NNT of 78.7 to prevent one death (Table 2).
Cost Difference
Expected cost difference was calculated on the basis of prevented outpatient and inpatient treatment costs of a hyperkalemic event minus the cost of patiromer medication. We did not take into account the costs of RAASi, as we assumed that these were the same for both treatment groups. The reimbursement price for patiromer in the indication CKD was set at CHF 255 for 30 days in Switzerland.Footnote 3 This resulted in a cost of CHF 476 per 8-week period (56 days).Footnote 4 Applying these amounts to our model resulted in an expected cost saving with patiromer of CHF 303. Using the lower and upper values in risk reduction and treatment cost resulted in expected cost offsets ranging between CHF − 137 and 843 (see Table S4 in the electronic supplementary material for details). The negative value indicates that treatment with patiromer led to additional costs. We did not include the cost of a death or any other intangible costs, such as reduced quality of life, or indirect costs due to reduced ability to work. Rates of hospitalization estimated at 10% and 90% resulted in expected cost offsets of CHF − 270 and 860, respectively (at the main values for risk reduction and treatment cost). These values only take into consideration the costs financed by the mandatory health insurance (MHI). Furthermore, when the costs financed by the cantons and thus by taxpayers were also taken into account, the expected cost offsets in the main scenario increased from CHF 303 to 1176 (Fig. 2).
Expected offset costs of a treatment with RAASi and patiromer compared to a treatment with only RAASi over 8 weeks in CHF. MHI mandatory health insurance. Positive values indicate expected offset costs, negative values indicate expected cost increase for a treatment of one patient with patiromer over 8 weeks. 1 CHF = 0.95 EUR as of 2022
Discussion
Clinical trials were able to demonstrate that in patients with CKD stage 3–4 on RAASi with a past prior event of hyperkalemia, patiromer was effective in reducing hyperkalemic events. The resulting NNT of 2.1 implies that the recurrence of hyperkalemia could be prevented in every second patient treated with patiromer for 8 weeks. The effectiveness of patiromer stems mainly from its efficacy in treating moderate-to-severe hyperkalemia (potassium levels of at least 5.5 mmol/l), with absolute risk reduction values of 45 percentage points. In contrast the risk of mild hyperkalemia was only reduced by 3 percentage points.
Since the treatment of moderate-to-severe hyperkalemia can require inpatient care, there is the potential for cost offsets. In our model we calculated that patiromer prevented one hospitalization for just over four patients within 8 weeks of treatment (NNT = 4.4). In addition, considering the offsets coming from the prevented outpatient treatments for all hyperkalemic events, this resulted in cost reductions for the MHI of CHF 303 per patient for an 8-week treatment period in our baseline scenario. Projected at 1 year, this would result in a cost-savings of approximately CHF 1970 per patient.
If the price for patiromer is lower than the reduction of inpatient and outpatient costs due to prevented hyperkalemia, the cost–benefit ratio will always be favorable because in addition to the health benefits there is a net saving of healthcare costs resulting from patiromer treatment. When the lower limits of our sensitivity analysis are used, there are some scenarios in which the price for patiromer was higher than the expected cost offsets, leading to increased healthcare costs. In this case, treatment with patiromer may still be worthwhile depending on expected effects beyond healthcare costs, such as the number of avoided deaths. Our model shows that an 8-week treatment with patiromer prevents one death per about 80 patients (NNT = 78.7). When the lower limits of risk reduction and treatment cost are used, healthcare costs for treating a patient for an 8-week period would rise by CHF 137 (scenario 4 in Table S4 in the electronic supplementary material).Footnote 5 In this example, the prevention of one death would cost CHF 10,960 (80 patients treated at an additional cost of CHF 137 per patient). A recent study showed that the willingness of the Swiss population to pay for prolonging the life of terminally ill patients is roughly CHF 10,000 per gained month [18]. So, even if treatment with patiromer were to extend life only by 1 month, the cost–benefit ratio would again look favorable on the basis of this example.
Our results have to be interpreted in the context of available clinical trial data. First, the randomized withdrawal phase in the OPAL-HK study lasted only 8 weeks. This is a major limitation of the study, as it is uncertain whether the effect can be extrapolated. However, retrospective cohort studies have shown that continuous treatment with patiromer can be effective well beyond 8 weeks. Patiromer over a 6-month period enabled RAASi therapy to be continued more often than in patients not receiving patiromer [19]. In addition, the hospitalization rate after a 6-month treatment period with patiromer has also shown to decrease compared to the 6 months before patiromer administration [20]. The treatment phase of patiromer in the DIAMOND study, expected to last more than 2 years, is expected to bring answers with regard to the benefit of long-term treatment with patiromer. Second, only about half of the patients in the OPAL-HK study were included in the second, randomized section of the study. In the first 4 weeks of the study, all patients received patiromer. Briefly, a total of 109 patients (of the 219 patients who completed the initial treatment phase) were not eligible to enter the randomized withdrawal phase, mainly because of a baseline potassium level below 5.5 mmol/l. A further 22 patients were excluded because of an out-of-range serum potassium level (cf. [9], Fig. S1). If one assumes that these patients had the same outcome as the control group, the prevalence for a hyperkalemic event would increase from 0.43 to 0.51 = 0.43 × [107/(107 + 22)] + 0.91 × [22/(107 + 22)] in the treatment group. This would reduce the absolute risk reduction from 48 to 40 percentage points and increase the NNT for a recurring hyperkalemic event from 2.1 to 2.5. Third, the generalizability of the OPAL-HK trial results may also be limited because patients with CKD were only selected if their estimated glomerular filtration rate (eGFR) was between 15 and less than 60 ml/min/1.73 m2. However, hyperkalemia is uncommon when eGFR is greater than 60 ml/min/1.73 m2 and increases in prevalence with lower eGFR [21]. When eGFR is less than 15 ml/min/1.73 m2, small incremental losses in kidney function can cause a steep rise in serum potassium concentration, leading to potentially fatal outcomes, and thus the RAASi prescription rate at this stage of CKD is low even when treatment was guided by nephrologists [22]. Fourth, patients included in the OPAL-HK study had initial potassium levels comprised between 5.1 and less than 6.5 mmol/l. However, the definition of hyperkalemia can vary; in the recent KDIGO (Kidney Disease: Improving Global Outcomes) conference reporting on dyskalemia in kidney disease, potassium levels ranging from 5.0 to 6.0 mmol/l were defined as mild-to-moderate, and potassium levels ranging from 6.0 to 6.4 mmol/l as moderate-to-severe hyperkalemia [23]. Fifth, the underlying risk assumptions for hospitalizations or death are subject to uncertainty. Patiromer is approved for adult patients with hyperkalemia in Switzerland. Long-term outpatient treatment of hyperkalemia is mostly needed for elderly patients suffering from CKD stage 3 to 5. Although the populations used for risk assumptions mirror these facts, representativeness of the population may be limited. Available data on the probability of hospitalization in case of hyperkalemia is especially sparse, as hyperkalemia itself is often not recorded as a reason for hospitalization. But even if the true hospitalization rate for hyperkalemia is not known, it is known that hyperkalemia increases the risk for future hospitalizations of any kind. Thomsen et al. [24] showed that patients with CKD and potassium levels greater than 5.5 mmol/l had a 75% higher risk for hospitalizations 6 months after a hyperkalemic event compared to 6 months before (73% vs. 42%, respectively). As a result of the uncertainty of this input parameter, we applied a wide range of 10–90% in the sensitivity analysis, while being aware that the lower and upper bounds were clearly under- and overestimations, respectively. As with hospitalizations, there is limited data on deaths. Thomsen et al. [24] showed that for patients with CKD and hyperkalemia (greater than 5.0 mmol/l), the 6-month risk of death was 20 percentage points higher than for matched patients without hyperkalemia (26% vs. 6%). Compared to these numbers, our input data for the model (death rate of 2.8% and 0.5% for moderate-severe and mild hyperkalemia, respectively) seemed to be an appropriate estimate. Furthermore, there may be additional benefits of a treatment with patiromer that were not included in our model. Most promising in this context is the fact that RAASi treatment could be maintained in the patiromer group. Therapy with RAASi prevents progression of CKD and reduces all-cause mortality in patients with CKD or chronic heart failure [25]. However, there is a gap between RAASi treatment recommendations and prescribing patterns for RAASi because patients who would benefit most from RAASi treatment are also those at risk of developing hyperkalemia [26, 27]. Indeed, among adult patients with CKD who were prescribed a RAASi, approximately 50% had to have their RAASi treatment regimen modified following the first hyperkalemic event, including treatment discontinuation in over one-third of cases [23]. This is of key importance as the risk of death among patients with CKD appears to double within a period of 13 months if RAASi are used at suboptimal doses or discontinued [27]. This in turn strongly supports continued use of RAASi in this vulnerable population. The addition of patiromer to RAASi in this setting was effective in preventing discontinuation of RAASi treatment (6% in the patiromer vs. 56% in the placebo group discontinued RAASi treatment in the OPAL-HK trial) [9].
Conclusion
Patiromer was effective in preventing hyperkalemia in patients with CKD. Our cost–benefit calculation also demonstrated that by reducing the risk of hyperkalemia, patiromer prescribed at the current reimbursement price resulted in significant cost offsets for the Swiss healthcare system.
Notes
Patiromer can, however, enable RAASi treatment to be maintained. This would lead to more use of RAASi and consequently higher drug costs in the patiromer group. On the other hand, discontinuation or suboptimal use of RAASi can lead to poorer health which again leads to increased healthcare expenditures. In order to simplify the analysis, both the potential higher cost of medication in the treatment group and the poorer health status and the resulting increase in the healthcare expenditures in the control group were not taken into account.
Endocrine, nutritional, and metabolic diseases.
As of 01.08.2020 the pharmacy retail price of a packet of 30 tablets 8.4 g or 16.8 g is CHF 255. A maximum of one package per month is reimbursed.
If the treatment lasted only 8 weeks it would be more appropriate for the calculation to use the price of two packets (60 days). This would increase the cost of patiromer medication by CHF 34. However, since we assume that patiromer is used for a longer period, we use the drug price per day for the calculation.
The cost reduction in our baseline scenario was calculated on the basis of healthcare expenditures covered by the mandatory health insurance (MHI share), which would be 45% of the costs for inpatient treatments. If total healthcare expenditures were to be taken into account, cost reductions would be higher.
References
Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–62.
Kovesdy CP, Matsushita K, Sang Y, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J. 2018;39:1535–42.
Gasparini A, Evans M, Barany P, et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34:1534–41.
Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100.
Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–64.
McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–42.
Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347:93–100.
Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-dn randomized clinical trial. JAMA. 2015;314:151–61.
Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–21.
Leoncini G, Viazzi F, Pontremoli R. RAAS inhibition and renal protection. Curr Pharm Des. 2012;18:971–80.
Hoogwerf BJ. Renin–angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 2010;105:30A-35A.
Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–8.
Agarwal R, Rossignol P, Romero A, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540–50.
Smith DH, Raebel MA, Chan KA, et al. An economic evaluation of a laboratory monitoring program for renin-angiotensin system agents. Med Decis Mak. 2011;31:315–24.
SwissDRG AG. SwissDRG 11.0 Katalogversion (2019/2022)—Swiss Diagnosis Related Groups Version 11.0—Definitionshandbuch. Bern; 2021. https://www.swissdrg.org/de/akutsomatik/swissdrg-system-1102022/definitionshandbuch. Accessed 2 Mar 2022.
SwissDRG AG. Datenspiegel SwissDRG 11.0. 2021. https://datenspiegel110.swissdrg.org/?locale=de. Accessed 2 Mar 2022.
Cosandey J, Roten N, Rutz S. Gesunde Spitalpolitik—Mehr Transparenz, mehr Patientensouveränität, weniger “Kantönligeist”. Zürich: Avenir Suisse; 2018. www.avenir-suisse.ch/publication/gesunde-spitalpolitik/. Accessed 1 July 2022.
Fischer B, Telser H, Zweifel P. End-of-life healthcare expenditure: testing economic explanations using a discrete choice experiment. J Health Econ. 2018;60:30–8.
Desai NR, Rowan CG, Alvarez PJ, Fogli J, Toto RD. Hyperkalemia treatment modalities: a descriptive observational study focused on medication and healthcare resource utilization. PLoS ONE. 2020;15:e0226844.
Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States Veterans. Postgrad Med. 2020;132:176–83.
Foley RN, Wang C, Ishani A, Ibrahim HN, Collins AJ. Creatinine-based glomerular filtration rates and microalbuminuria for detecting metabolic abnormalities in US adults: the National Health and Nutrition Examination Survey 2003–2004. AJN. 2008;28:431–7.
Pecoits-Filho R, Fliser D, et al. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens (Greenwich). 2019;21:991–1001.
Jun M, Jardine MJ, Perkovic V, et al. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS ONE. 2019;14:e0213192.
Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrol Dial Transplant. 2018;33:1610–20.
Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10(1):e003529.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147-239.
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin–angiotensin–aldosterone system inhibitors. Am J Manag Care. 2015;21:S212–20.
Acknowledgements
Funding
Sponsorship for this study and the journal’s Rapid Service and Open Access Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Medical background was provided by Andreas Serra. Data analysis was performed by Barbara Fischer and Harry Telser. The manuscript was co-authored by all authors. All authors read and approved the final manuscript.
Disclosures
This study was financially supported by Vifor Pharma Switzerland. The funders of the study had no role in the study design; the collection, analysis, and interpretation of data; and the writing of this article. The authors declare that they have no competing interests. Barbara Fischer, Andreas Serra, and Harry Telser have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fischer, B., Serra, A. & Telser, H. Cost-Effectiveness of Treating Patients with Chronic Kidney Disease and Prior Hyperkalemia with Renin–Angiotensin–Aldosterone System Inhibitor and Patiromer: A Swiss Public Healthcare Perspective. Adv Ther 39, 2717–2730 (2022). https://doi.org/10.1007/s12325-022-02123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02123-3