Abstract
Introduction
Levodopa/carbidopa intestinal gel (LCIG; carbidopa/levodopa enteral suspension) has been widely used and studied for the treatment of motor fluctuations in levodopa-responsive patients with advanced Parkinson’s disease (PD) when other treatments have not given satisfactory results. Reduction in ‘off’-time is a common primary endpoint in studies of LCIG, and it is important to assess the durability of this response. This systematic literature review was conducted to qualitatively summarise the data on the long-term effects of LCIG therapy on ‘off’-time.
Methods
Studies were identified by searching PubMed, EMBASE and Ovid on 30 September 2019. Studies were included if they reported on patients with PD, had a sample size of ≥ 10, LCIG was an active intervention and ‘off’-time was reported for ≥ 12 months after initiation of LCIG treatment. Randomised clinical trials, retrospective and prospective observational studies, and other interventional studies were included for selection. Data were collected on: ‘off’-time (at pre-specified time periods and the end of follow-up), study characteristics, Unified Parkinson’s Disease Rating Scale (UPDRS) II, III and IV total scores, dyskinesia duration, quality of life scores, non-motor symptoms and safety outcomes.
Results
Twenty-seven studies were included in this review. The improvement in ‘off’-time observed shortly after initiating LCIG was maintained and was statistically significant at the end of follow-up in 24 of 27 studies. ‘Off’-time was reduced from baseline to end of follow-up by 38–84% and was accompanied by a clinically meaningful improvement in quality of life. Stratified analysis of ‘off’-time demonstrated mean relative reductions of 47–82% at 3–6 months and up to 83% reduction at 3–5 years of follow-up. Most studies reported significant improvements in activities of daily living and motor complications. Most frequent adverse events were related to the procedure or the device.
Conclusion
In one of the largest qualitative syntheses of published LCIG studies, LCIG treatment was observed to provide a durable effect in reducing ‘off’-time.
Infographic

Video Abstract
Plain Language Summary
By synthesising publications from scientific journals, this article shows that levodopa/carbidopa intestinal gel (LCIG; also known as carbidopa/levodopa enteral suspension or the tradenames Duodopa® and Duopa®) may have benefits for patients with advanced Parkinson’s disease that last for 12 months or more. Pills taken by mouth for Parkinson’s disease often do not work as well after a few years. This means the symptoms of Parkinson’s disease, such as shaking or slow movements, etc., re-emerge despite medication (known as ‘off’-time). To reduce the amount of ‘off’-time, people with advancing Parkinson’s disease may switch from pills to other types of treatments, for example, those that use devices to deliver the drug into the body, such as LCIG. LCIG has been available for many years and is known to help patients by reducing ‘off’-time. Despite this, less is known about how long the benefits of LCIG last. By summarising all information available on the long-term use of LCIG, this report shows that when patients have been taking LCIG for at least 12 months, they have 2–4 h less ‘off’-time each day than they did before starting the LCIG treatment. This effect is maintained for 3–5 years after starting LCIG treatment. There were no unexpected side effects with long-term use of LCIG. The time not spent in ‘off’ may allow people with advanced Parkinson’s to increase their independence in daily activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic review of the literature, which includes 27 studies, is the most comprehensive qualitative synthesis of data on the long-term (≥ 12 months follow-up from treatment initiation) impact of levodopa/carbidopa intestinal gel on ‘off’-time in patients with advanced Parkinson’s disease |
Of the 27 studies, 14 (52%) were multicentre studies and 10 (37%) had a sample size of ≥ 50 patients. Study follow-ups ranged from 12–120 months with 15 (56%) studies having follow-ups ≥ 24 months |
Treatment of advanced Parkinson’s disease with levodopa/carbidopa intestinal gel was observed to be consistently effective in significantly reducing ‘off’-time within 3 months, and this improvement is maintained in the long-term, even after 24 months |
The improvement in ‘off’-time may be associated with clinically meaningful improvement in health-related quality of life in the long term |
Safety issues with levodopa/carbidopa intestinal gel are most frequently related to the procedure or the device, and the emergence of unexpected adverse events in the long-term is not frequent |
Dose optimisation of levodopa/carbidopa intestinal gel allows personalisation of treatment that should further enhance the maintenance of long-term efficacy |
Digital Features
This article is published with digital features, including a summary slide, plain language summary, infographic and video abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13056008.
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder that in the long-term presents with motor and non-motor fluctuations in many patients [1, 2]. As symptoms worsen with disease progression, daily activities and quality of life (QoL) are negatively affected [3]. Most patients progress to a disease state often referred to as ‘advanced PD’. While there is no consensus for the definition of advanced PD, it is generally characterised by motor (and non-motor) symptoms that respond poorly to optimised oral medication, longer ‘off’-time per day, shorter ‘on’-time per day and dyskinesia [4,5,6,7,8,9,10,11], which in turn may result in limited mobility and risk of falls. In addition, many people with advanced PD have cognitive and psychotic problems [9].
Levodopa combined with peripheral decarboxylase inhibitor remains the most effective symptomatic therapy for PD [12], but one defining aspect of advanced PD is the inability to provide sustained benefit with oral levodopa. As PD advances, presynaptic storage of levodopa/dopamine in striatal dopaminergic neurons, which buffers synaptic transmission against the fluctuations in plasma levodopa levels, is lost and response to levodopa more closely follows plasma concentrations [13]. Due to the short half-life of levodopa and erratic absorption caused by unpredictable gastric emptying, fractionated and intermittent oral dosing of levodopa results in fluctuating plasma levels as well as motor fluctuations and complications, limiting its benefit for patients with advanced PD [9, 14,15,16].
Levodopa/carbidopa intestinal gel (LCIG; Duodopa®, carbidopa/levodopa enteral suspension; Duopa®, AbbVie Inc., North Chicago, IL, USA) is a stable gel suspension suitable for continuous delivery to the proximal jejunum through percutaneous endoscopic gastrostomy and a jejunal extension tube (PEG-J) via a portable pump [17]. Continuous infusion of LCIG bypasses the stomach and hence removes the influence of gastric emptying on plasma levels of levodopa [18], stabilises plasma levodopa concentrations and avoids the peaks and troughs that lead to motor fluctuations and dyskinesia [16]. Many studies have demonstrated that LCIG can significantly reduce ‘off’-time, increase ‘on’-time (without troublesome dyskinesia) and improve activities of daily living (ADL) and QoL in patients with advanced PD [19,20,21,22]. The length of follow-up in published studies varies from 3 months to > 5 years. The flexible and personalised dosing that LCIG offers, including adjustable flow rate, ability to administer bolus doses and benefits of using as monotherapy or with other anti-PD medications [17, 23], means that good long-term efficacy is achievable.
LCIG was first approved in 2004 (in the EU) and there is, therefore, long-term experience with this treatment. As PD is a progressive disease, it is important to ascertain how long the benefits of LCIG are sustained. Furthermore, a systematic review of LCIG was published in 2016 to assess outcomes compared with conventional therapy, apomorphine infusion and deep-brain stimulation [24], but this did not provide detailed information on the long-term outcomes with LCIG therapy. The results of several studies and registries of LCIG therapy have been reported since 2016; therefore, a review of the data on LICG therapy is overdue. This systematic literature review summarises data on the long-term (≥ 12 months) efficacy of LCIG in PD, with a focus on ‘off’-time.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [25]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Sources
Searches were conducted on PubMed, EMBASE and Ovid on 30 September 2019.
Search Strategy
Search strategy was limited to studies involving humans and published in English language. An example of the full search strategy is given here: (("Parkinson Disease"[Majr]) OR (parkinson’s disease[tiab] OR parkinsons disease[tiab] OR parkinson disease[tiab] OR parkinsons[tiab] OR parkinson’s disease[ot] OR parkinsons disease[ot] OR parkinson disease[ot] OR parkinsons[ot])) AND (duopa[tiab] OR carbidopa and levodopa enteral suspension[tiab] OR CLES[tiab] OR duodopa[tiab] OR levodopa/carbidopa intestinal gel[tiab] OR levodopa-carbidopa intestinal gel[tiab] OR LCIG[tiab] OR L-dopa-infusion[tiab] OR levodopa infusion[tiab] OR duodenal levodopa infusion[tiab] OR duodenal l-dopa infusion[tiab] OR duopa[ot] OR carbidopa and levodopa enteral suspension[ot] OR CLES[ot] OR duodopa[ot] OR levodopa/carbidopa intestinal gel[ot] OR levodopa-carbidopa intestinal gel[ot] OR LCIG[ot] OR L-dopa-infusion[ot] OR levodopa infusion[ot] OR duodenal levodopa infusion[ot] OR duodenal l-dopa infusion[ot]). Searches were made for major article topic terms (‘Majr’), free text terms in title or abstract (‘tiab’) and other terms (‘ot’). Any important papers known by the authors that were not identified with this search strategy were included in the search results (‘hand search’).
Eligibility Criteria
The following criteria (PICOS) were used for inclusion of studies: patients with PD, sample size of ≥ 10 and LCIG as an active intervention irrespective of the inclusion of a comparator arm. The main outcome measure assessed was ‘off’-time if reported for at least 12 months after initiation of LCIG treatment. Randomised clinical trials (RCTs), retrospective and prospective observational studies and other interventional studies were included for selection, if published between 1 January 2000 and 30 September 2019.
Screening, Selection and Data Extraction
Identified publications were initially screened by title to remove duplicates and papers of a type not meeting with the PICOS eligibility criteria. Screening and data extraction were conducted independently by two reviewers (A. Alobaidi and S. Inguva). Results were matched between reviewers and discordance was resolved by consensus through a third reviewer (referring to the original publication if necessary).
Data on ‘off’-time at all reported timepoints were collected from each selected publication. Other available information that was extracted from the selected publications, where reported and at all reported timepoints, was: study characteristics (study design and setting, treatment regimen, length of follow-up, sample size, the number of patients receiving each regimen and key inclusion/exclusion criteria), change from baseline in motor symptoms (Unified Parkinson’s Disease Rating Scale [UPDRS] III total score), change from baseline in motor complications (UPDRS IV total score), change from baseline in dyskinesia duration, change from baseline in motor experiences of daily living (UPDRS II total score), change from baseline in QoL scores, change from baseline in non-motor symptoms (NMS) and safety outcomes.
These data were extracted independently by both reviewers from selected publications using a standardised Microsoft Excel-based form. Extracted data were verified in the drafting of this manuscript by a third reviewer. As identified studies were not RCTs, the domains to address in a risk of bias assessment, according to the Cochrane Collaboration [26], were absent in most studies. Therefore, we did not draw a funnel plot or conduct a formal assessment of the risk of bias of included publications.
Results
Search Results and Study Selection
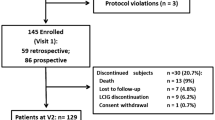
The literature search identified 344 records, and 27 studies fulfilled the eligibility criteria and are included in this review (Fig. 1) [19, 20, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The characteristics of the 27 studies are summarised in the Supplementary Table 1. Fourteen of the 27 studies (52%) were multicentre studies and ten of 27 (37%) had a sample size of ≥ 50 patients. Data were not extracted on co-medication/LCIG monotherapy use, LCIG dose, or previous therapies. None of the identified studies was a RCT (one study was an open-label extension of a pivotal RCT [41] and one was an open-label study that included patients from the aforementioned open-label extension study and a separate open-label study [48]); therefore, no assessment of risk bias was made.
Long-Term ‘Off’-time
Attrition rate in the studies varied by the length of follow-up, with follow-ups ranging from 12–120 months (Table 1).
The effect of LCIG therapy on ‘off’-time was evaluated using UPDRS IV item 39 (n = 15, but with 1 study using Movement Disorder Society (MDS)-UPDRS item 4.3 to also assess h/waking day), PD patient diary (n = 9), healthcare professional assessment (n = 3) and MDS-UPDRS item 4.3 (n = 2; used in addition to UPDRS IV item 39) (Table 1). Mean baseline ‘off’-time ranged from 1.1 to 7.6 h/waking day when assessed by the healthcare provider and 4.7–8.0 h/waking day when assessed by patient diary, and mean baseline UPDRS IV item 39 scores ranged from 1.6 to 2.9 (Table 1).
Mean ‘off’-time was significantly reduced by the end of follow-up (i.e., at least 12 months after starting LCIG therapy) in 24 of the 27 studies, with reductions from baseline in ‘off’-time of 38–84% (weighted average 61.0%; Table 1; Fig. 2). Reductions in ‘off’-time at the end of follow-up were consistent across studies irrespective of the method used for measuring ‘off’-time. When UPDRS IV item 39 was used, the percentage of the waking day spent in ‘off’-time was reduced from baseline by 36–68% (Table 1; Fig. 2). In studies using patient diaries or healthcare provider assessment to determine the hours of the waking day spent in ‘off’-time, the reduction from baseline was 43–84 and 56–71%, respectively (Table 1; Fig. 2).
Percentage reduction in ‘off’-time from baseline to last follow-up in the studies included in this review. *Denotes non-significant change from baseline, all other changes are statistically significant. ‡‘Off’-time improvement at end of follow-up of each individual study (minimum 12 months; range 12–120 months). Horizontal dotted line represents the weighted average reduction in ‘off’-time across all studies. HCP healthcare professional. UPDRS Unified Parkinson’s Disease Rating Scale
All 16 studies with a mean follow-up of at least 24 months had statistically significant ‘off’-time reductions at the end of this longer follow-up, ranging from 38–83% reduction from baseline (Table 1). In nine studies reporting change in ‘off’-time 3–6 months after initiating LCIG therapy, reductions from baseline ranged from 47 to 82%; in two studies reporting change in ‘off’-time at 3–5 years of follow-up, reductions from baseline were 68 and 83% (Table 1).
Other Motor Symptoms
Motor symptoms assessed by UPDRS III total score (includes ratings for tremor, bradykinesia, rigidity and balance) were measured inconsistently in the ‘on’ or ‘off’ state. In the 20 studies reporting UPDRS III total score changes, statistically significant improvements were observed in seven studies at follow-ups ranging from 3 to 32 months (three of these seven studies showed improvement in UPDRS III scores at follow-ups of ≥ 24 months; Table 2).
ADL assessed by UPDRS II total score were reported in 18 studies (Table 2). These scores were statistically significantly improved with LCIG therapy in nine studies (follow-up: 12–36 months), significantly worsened in three studies, and there was no statistically significant change in six studies (Table 2).
Motor complications assessed by UPDRS IV total score were reported in 14 studies and significantly improved in 13 studies (follow-up: 12–52 months; Table 3). One of the 14 studies did not report on the significance of change from baseline in UPDRS IV total score as it was a comparator trial [28]. Change in dyskinesia duration was reported in 26 studies, but the method of measuring dyskinesia varied considerably between studies (Table 3). In the 12 studies reporting UPDRS IV item 32 score (dyskinesia duration—in some studies this was modified to h/day), nine studies reported statistically significant improvements at follow-ups ranging from 6 to 36 months, and no study showed an increase in dyskinesia duration (Table 3).
Non-Motor Symptoms and Quality of Life
NMS endpoints were reported in 14 of the selected studies (Table 4). A wide variety of assessment tools were used, making general conclusions difficult. The most frequently used scales were the non-motor symptom scale (NMSS), the mini-mental state examination (MMSE) and part I of the UPDRS (mentation, behaviour and mood).
Of six studies reporting NMSS total score, four showed statistically significant improvements at follow-up with LCIG (Table 4). Improvements in the NMSS sub-scale scores were also observed but with no clear trend across studies. The MMSE scores significantly worsened in four studies (at follow-ups of 24–52 months), and three studies reported no statistically significant change (Table 4). UPDRS I total score improved in four studies with follow-ups of 3 weeks to 36 months and there was no statistically significant change in two studies (Table 4).
Health-related QoL (HRQoL) outcomes were reported in 17 studies (Table 5). Most studies reporting HRQoL used the Parkinson’s Disease Questionnaire (PDQ)-39 and 8 of 11 studies reported statistically significant improvements in PDQ-39 scores from baseline 12–36 months after starting LCIG therapy (Table 5). Of the studies reporting PDQ-39 or PDQ-8, 12 studies reported the change from baseline at end of follow-up, and while there may be too few data points to conclude on correlations, there appears to be a trend for a greater improvement in HRQoL in studies reporting a greater reduction in long-term ‘off’-time (Fig. 3).
Percentage reduction in ‘off’-time from baseline plotted against the improvement in health-related quality of life (HRQoL according to PDQ-39 or PDQ-8) in the studies reporting both endpoints at end of follow-up. a Change in HRQoL as percentage change from baseline; b change in HRQoL as actual change in PDQ score from baseline. *Denotes statistically significant change from baseline in HRQoL (p < 0.05). ‡Horvath et al. [66]. HRQoL health-related quality of life. 1. Standaert et al. 2017 [50]. 2. Antonini et al. 2017 [19]. 3. De Fabregues et al. 2017 [38]. 4. Juhasz et al. 2017 [39]. 5. Chang et al. 2016 [40]. 6. Caceres-Redondo et al. 2014 [30]. 7. Sensi et al. 2014 [32]. 8. Antonini et al. 2013 [33]. 9. Zibetti et al. 2013 [34]. 10. Foltynie et al. 2013 [42]. 11. Fasano et al. 2012 [47]. 12. Antonini et al. 2008 [37]
Safety and Tolerability
The frequency of LCIG-related adverse events (AEs) varied widely in the selected studies because of the way in which data were collected or reported (Table 6). In many studies, the most frequent AEs were related to the PEG procedure or the device, such as wound/stoma infection, abdominal/procedural pain or problems with the tubing such as dislocation (Table 6). AEs that were considered levodopa-related included weight loss, hallucinations and neuropathy (Table 6). However, discontinuation rates due to AEs were lower than the rates of AE occurrence.
Discussion
To our knowledge, this is the largest qualitative synthesis of published studies evaluating the long-term efficacy of LCIG on ‘off’-time in advanced PD. Most studies showed significant reduction in ‘off’-time by end of follow-up (38–84%), with the longest follow-up being 120 months. As PD progresses, people with PD spend a greater proportion of the waking day in the ‘off’ state, which limits mobility and impacts on QoL. The reduction in ‘off’-time is, therefore, a key aim of PD management. Key clinical trials of LCIG have demonstrated significant reductions of ‘off’-time for 12 weeks after treatment initiation [21]. However, since PD is a progressive disease, it is important to assess the long-term impact of LCIG on ‘off’-time, and often such evidence stems from studies that are not RCTs.
The results of this literature review suggest that LCIG extends the benefit of levodopa (in terms of reduced ‘off’-time) for at least 2–5 years. This supports the suggestion that the long-term efficacy of LCIG is similar to efficacy at 3 months that was demonstrated in a pivotal RCT [21]. Treatment patterns were not reported consistently in the selected studies; therefore, it is not possible to determine the impact of treatment changes on the sustained effects of LCIG on ‘off’-time. The flexibility of dosing provided by LCIG (the ability to adjust the flow rate of the pump and give one-off bolus doses, as well as the possibility to use it as monotherapy or in combination with other anti-PD medications) aids long-term optimisation of outcomes. Data from phase 3 trials suggest that dose optimisation is achieved within 7 days of initiating LCIG and doses remain relatively stable for > 12 months [52]. However, treatment patterns in routine practice are likely to vary between countries and centres. Several publications have provided detailed guidance on patient selection, dose conversion factors, and dose titration and adjustment [53,54,55], and with this guidance and experience, longer-term LCIG dose adjustment is relatively straightforward [56, 57]. The individualisation of LCIG treatment regimens over time may be an important factor for maintaining the long-term reductions in ‘off’-time presented in this review.
The studies reviewed here show that after 1 year or more, the reductions of ‘off’-time that occur after starting LCIG therapy remain, i.e., the proportion of the waking day spent in ‘off’ is reduced compared with baseline by > 2 h, and in many cases > 4 h. These improvements were accompanied by more time spent in the ‘on’ state, and in most studies there was also a reduction in the duration of dyskinesia. Such changes in ‘on/off’-time are likely to have an impact on the patients’ ADL and QoL (and that of the care partner). In some of the studies, UPDRS II total scores (ADL) and HRQoL scales did show improvement (that potentially correlates with reduction in ‘off’-time), but assessing these endpoints was not the primary goal of this review.
The most frequently used measure of ‘off’-time in these studies was UPDRS IV item 39. While capturing ‘off’-time at hospital visits with UPDRS items is convenient, especially in the clinical trial setting, this method suffers from recall bias. Most of the remaining studies in this review assessed ‘off’-time using patient diaries (n = 9), which requires good education of patients and care partners, and compliance with frequent diary entries may be a limitation to their accuracy. Thus, the methodologies used in different studies may have influenced the extent of ‘off’-time reduction reported. However, several factors may have influenced the magnitude of ‘off’-time reduction across studies, such as the baseline ‘off’-time.
Since long-term ‘off’-time was the primary outcome measure used to select studies for this review, information on other motor symptoms, NMS and HRQoL was reported less consistently. Motor complications (dyskinesia) and ADL were mostly unchanged or improved in these studies, while some aspect of NMS and HRQoL improved in many studies. It should be stressed that since these endpoints were not part of the PICOS selection criteria, the findings presented here do not represent a comprehensive picture of the effects of LCIG treatment on these endpoints. Dedicated systematic reviews or meta-analyses would be needed to draw firm conclusions on the long-term effect of LCIG on other motor symptoms, dyskinesia, NMS and HRQoL.
The AE profiles reported in these studies were consistent with the known safety profile of LCIG. The methodological variability in AE reporting makes it difficult to provide a quantitative overview of AE frequency. The most frequent AEs were generally device- and PEG procedure-related including wound/stoma infection, abdominal/procedural pain, erythema and tube dislocation. Generally, discontinuations due to device-related issues or infection occurred in < 5% of patients, and while serious AEs (SAEs) relating to the procedure are known to occur [58], these were of a low frequency in most studies included here. The reason for a higher incidence of device-related SAEs in some studies [29, 38] is not known, but in general an experienced multidisciplinary team is needed to reduce the risk of PEG-related complications. In the pivotal RCT of LCIG, common AEs occurred most frequently in the first 1–2 weeks after initiating LCIG therapy and subsequently declined to considerably lower frequencies [21]. While most studies included in this review reported overall AE frequency and did not specify the timing of AEs, it is likely that the most frequent AEs related to the procedure occurred mostly in the first week [59]. However, other AEs including device-related AEs may have occurred consistently throughout the follow-ups of these studies. Peripheral neuropathy has become recognised as an AE of levodopa-based therapies, and group B vitamin deficiency is thought to play a role [60,61,62]. In recent years, therefore, vitamin B supplementation has been used to manage and/or prevent this complication [63, 64], and it would be useful analyse the effect of increased awareness and improved management of neuropathy on its incidence in patients receiving LCIG. In this analysis, neuropathy was reported in studies from 2008 [37] to 2019 [51] (Table 6), but because AEs were not the primary outcome used for selection of articles in this review, we cannot draw firm conclusions. A recent review of levodopa-induced neuropathy did not assess the impact of the introduction of vitamin B supplementation on the incidence of neuropathy [65].
Studies were mostly retrospective and observational in nature. While this limits the strength of the evidence, long-term RCTs may be impractical with device-aided therapies for advanced PD. Future evaluations of the heterogeneity between studies are warranted to conduct a pooled analysis of long-term ‘off’-time reduction with LCIG treatment in advanced PD patients. However, this qualitative review provides valuable confirmation that long-term ‘off’-time reduction with LCIG treatment is relatively consistent in studies using different measures of ‘off’-time, in different geographical locations, in the controlled trial setting versus routine practice settings and over the last 12 years.
In conclusion, this large qualitative synthesis of 27 published studies shows that continuous dopaminergic stimulation provided by LCIG reduces ‘off’-time and improves other motor complications that were not well controlled on oral levodopa, and these improvements are sustained for > 12 months. People with advanced PD that is not well controlled by oral treatment may gain long-lasting improvements in many aspects of their lives with LCIG therapy via reduced ‘off’-time.
References
Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74.
Fasano A, Fung VSC, Lopiano L, et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19:50.
Brooks DJ. Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat. 2008;4:39–47.
Giugni JC, Okun MS. Treatment of advanced Parkinson’s disease. Curr Opin Neurol. 2014;27:450–60.
Kulisevsky J, Luquin MR, Arbelo JM, et al. Advanced Parkinson’s disease: clinical characteristics and treatment. Part II. Neurologia. 2013;28:558–83.
Lokk J. Lack of information and access to advanced treatment for Parkinson’s disease patients. J Multidiscip Healthc. 2011;4:433–9.
Olanow CW, Obeso JA, Stocchi F. Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat Clin Pract Neurol. 2006;2:382–92.
Varanese S, Birnbaum Z, Rossi R, Di Rocco A. Treatment of advanced Parkinson’s disease. Parkinson’s Disease. 2011;2010:480260.
Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34:2063–73.
Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson’s disease. Mov Disord. 2018;33:900–8.
Agid Y, Ahlskog E, Albanese A, et al. Levodopa in the treatment of Parkinson’s disease: a consensus meeting. Mov Disord. 1999;14:911–3.
Mouradian MM. The continuous dopaminergic stimulation concept and evidence to date. In: Aquilonius SM, Mouradian MM, editors. Parkinson’s disease. Role of continuous dopaminergic stimulation. ESP Bioscience Ltd; 2012. p. 66–82.
Nyholm D, Lennernas H, Gomes-Trolin C, Aquilonius SM. Levodopa pharmacokinetics and motor performance during activities of daily living in patients with Parkinson’s disease on individual drug combinations. Clin Neuropharmacol. 2002;25:89–96.
Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72:S1-136.
Nyholm D. The rationale for continuous dopaminergic stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl):S13–7.
Duodopa summary of product characteristics. November 2017. https://www.medicines.org.uk/emc/product/6231/smpc. Accessed 1 Aug 2020.
Nyholm D, Lennernas H. Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2008;4:193–203.
Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20.
Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30:500–9.
Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141–9.
Kruger R, Lingor P, Doskas T, et al. An observational study of the effect of levodopa-carbidopa intestinal gel on activities of daily living and quality of life in advanced Parkinson’s disease patients. Adv Ther. 2017;34:1741–52.
Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A. Levodopa-carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy, and safety. J Parkinsons Dis. 2019;9:531–41.
Wirdefeldt K, Odin P, Nyholm D. Levodopa-carbidopa intestinal gel in patients with parkinson’s disease: a systematic review. CNS Drugs. 2016;30:381–404.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011.
Zibetti M, Angrisano S, Dematteis F, et al. Effects of intestinal Levodopa infusion on freezing of gait in Parkinson disease. J Neurol Sci. 2018;385:105–8.
Merola A, Espay AJ, Romagnolo A, et al. Advanced therapies in Parkinson’s disease: long-term retrospective study. Parkinsonism Relat Disord. 2016;29:104–8.
Calandrella D, Romito LM, Elia AE, et al. Causes of withdrawal of duodenal levodopa infusion in advanced Parkinson disease. Neurology. 2015;84:1669–72.
Cáceres-Redondo MT, Carrillo F, Lama MJ, et al. Long-term levodopa/carbidopa intestinal gel in advanced Parkinson’s disease. J Neurol. 2014;261:561–9.
Zibetti M, Merola A, Artusi CA, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. Eur J Neurol. 2014;21:312–8.
Sensi M, Preda F, Trevisani L, et al. Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. J Neural Transm. 2014;121:633–42.
Antonini A, Odin P, Lopiano L, et al. Effect and safety of duodenal levodopa infusion in advanced Parkinson’s disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm (Vienna). 2013;120:1553–8.
Zibetti M, Merola A, Ricchi V, et al. Long-term duodenal Levodopa infusion in Parkinson’s disease: a 3-year motor and cognitive follow-up study. J Neurol. 2013;260:105–14.
Merola A, Zibetti M, Angrisano S, Rizzi L, Lanotte M, Lopiano L. Comparison of subthalamic nucleus deep brain stimulation and Duodopa in the treatment of advanced Parkinson’s disease. Mov Disord. 2011;26:664–70.
Antonini A, Bondiolotti G, Natuzzi F, Bareggi SR. Levodopa and 3-OMD levels in Parkinson patients treated with Duodopa. Eur Neuropsychopharmacol. 2010;20:683–7.
Antonini A, Mancini F, Canesi M, et al. Duodenal Levodopa infusion improves quality of life in advanced Parkinson’s disease. Neurodegener Dis. 2008;5:244–6.
De Fabregues O, Dot J, Abu-Suboh M, et al. Long-term safety and effectiveness of Levodopa-carbidopa intestinal gel infusion. Brain Behav. 2017;7:e00758.
Juhasz A, Aschermann Z, Acs P, et al. Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: an open-label study. Parkinsonism Relat Disord. 2017;37:79–86.
Chang FC, Kwan V, van der Poorten D, et al. Intraduodenal levodopa-carbidopa intestinal gel infusion improves both motor performance and quality of life in advanced Parkinson’s disease. J Clin Neurosci. 2016;25:41–5.
Slevin JT, Fernandez HH, Zadikoff C, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis. 2015;5:165–74.
Foltynie T, Magee C, James C, Webster GJ, Lees AJ, Limousin P. Impact of Duodopa on quality of life in advanced Parkinson’s disease: a UK case series. Parkinsons Dis. 2013;2013:362908.
Eggert K, Schrader C, Hahn M, et al. Continuous jejunal levodopa infusion in patients with advanced Parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol. 2008;31:151–66.
Valldeoriola F, Grandas F, Santos-Garcia D, et al. Long-term effectiveness of levodopa-carbidopa intestinal gel in 177 Spanish patients with advanced Parkinson’s disease. Neurodegener Dis Manag. 2016;6:289–98.
Buongiorno M, Antonelli F, Camara A, et al. Long-term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord. 2015;21:871–6.
Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol. 2014;261:2438–45.
Fasano A, Ricciardi L, Lena F, Bentivoglio AR, Modugno N. Intrajejunal levodopa infusion in advanced Parkinson’s disease: long-term effects on motor and non-motor symptoms and impact on patient’s and caregiver’s quality of life. Eur Rev Med Pharmacol Sci. 2012;16:79–89.
Fernandez HH, Boyd JT, Fung VSC, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. Mov Disord. 2018;33:928–36.
Fabbri M, Zibetti M, Beccaria L, et al. Levodopa/carbidopa intestinal gel infusion and weight loss in Parkinson’s disease. Eur J Neurol. 2019;26:490–6.
Standaert DG, Rodriguez RL, Slevin JT, et al. Effect of levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advanced parkinson’s disease. Mov Disord Clin Pract. 2017;4:829–37.
Lopiano L, Modugno N, Marano P, et al. Motor and non-motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol. 2019;266:2164–76.
Lew MF, Slevin JT, Kruger R, et al. Initiation and dose optimization for levodopa-carbidopa intestinal gel: insights from phase 3 clinical trials. Parkinsonism Relat Disord. 2015;21:742–8.
Pahwa R, Lyons KE. Outpatient titration of carbidopa/levodopa enteral suspension (Duopa). Int J Neurosci. 2017;127:459–65.
Pedersen SW, Clausen J, Gregerslund MM. Practical guidance on how to handle levodopa/carbidopa intestinal gel therapy of advanced PD in a movement disorder clinic. Open Neurol J. 2012;6:37–50.
Burack M, Aldred J, Zadikoff C, et al. Implementing levodopa-carbidopa intestinal gel for parkinson disease: insights from US practitioners. Mov Disord Clin Pract. 2018;5:383–93.
Amjad F, Bhatti D, Davis TL, et al. Current practices for outpatient initiation of levodopa-carbidopa intestinal gel for management of advanced Parkinson’s disease in the United States. Adv Ther. 2019;36:2233–46.
Santos Garcia D, Martinez Castrillo JC, Puente Periz V, et al. Clinical management of patients with advanced Parkinson’s disease treated with continuous intestinal infusion of levodopa/carbidopa. Neurodegener Dis Manag. 2016;6:187–202.
Klostermann F, Jugel C, Bomelburg M, Marzinzik F, Ebersbach G, Muller T. Severe gastrointestinal complications in patients with levodopa/carbidopa intestinal gel infusion. Mov Disord. 2012;27:1704–5.
Lang AE, Rodriguez RL, Boyd JT, et al. Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Mov Disord. 2016;31:538–46.
Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson’s disease. Mov Disord. 2008;23:1850–9.
Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav. 2017;7:e00698.
Muller T, van Laar T, Cornblath DR, et al. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19:501–17 (discussion).
Santos-Garcia D, de la Fuente-Fernandez R, Valldeoriola F, et al. Polyneuropathy while on duodenal levodopa infusion in Parkinson’s disease patients: we must be alert. J Neurol. 2012;259:1668–72.
Rispoli V, Simioni V, Capone JG, et al. Peripheral neuropathy in 30 duodopa patients with vitamins B supplementation. Acta Neurol Scand. 2017;136:660–7.
Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L. Levodopa-induced neuropathy: a systematic review. Mov Disord Clin Pract. 2019;6:96–103.
Horvath K, Aschermann Z, Kovacs M, et al. Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology. 2017;48:1–8.
Acknowledgements
Funding
Financial support for the study and the journal’s Rapid Service and Open Access Fees were provided by AbbVie. AbbVie participated in study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication.
Medical Writing and Other Editorial Assistance
The authors would like to thank Niodita Gupta, who is an employee of AbbVie, for her support with data synthesis. We acknowledge editorial support in the preparation of this manuscript from Martin Gilmour, PhD, CMPP of ESP Bioscience, funded by AbbVie.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Angelo Antonini has received compensation for consultancy and speaker related activities from UCB, Bial, GE, Boehringer Ingelheim, Biogen, Theravance Biopharma, AbbVie, Zambon, Neuroderm. He also received research support from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020—PD_Pal Grant 825785, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. Per Odin has received compensation for consultancy and speaker related activities from AbbVie, Bial, Britannia, Ever Pharma, Lobsor, Nordic Infucare, Stada, and Zambon. He has received royalties from Uni-Med Verlag. Rajesh Pahwa has received consulting fees from AbbVie, ACADIA, Acorda, Adamas, Cynapses, Global Kinetics, Lundbeck, Neurocrine, Pfizer, Sage, Sunovion, Teva Neuroscience and US World Meds. He has received research grants from AbbVie, Adamas, Avid, Biotie, Boston Scientific, Civitas, Cynapses, Kyowa, National Parkinson Foundation, NIH/NINDS, Parkinson Study Group. Jason Aldred has been a consultant and received honorarium from AbbVie, Acorda, Adamas, Allergan, Boston Scientific, Teva, Medtronic, and US World Meds. He has also received research support from NINDS, Abbott, AbbVie, Acadia, Biogen, Boston Scientific, Denali, Impax/Amneal, Sunovion, Neuroderm, Novartis, and Theravance. K Ray Chaudhuri has received educational funding from UCB, and honoraria for sponsored symposia from UCB, AbbVie, Britannia, US Worldmeds, Otsuka, Medtronic, Zambon and acted as a consultant for AbbVie, UCB, Britannia, Medtronic and Mundipharma.
Sushmita Inguva was employed with AbbVie at the time of the study. Ali Alobaidi, Yash J Jalundhwala, Pavnit Kukreja, Yanjun Bao, Lars Bergmann are employees of AbbVie and may own stock/shares in the company.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this manuscript as no datasets were generated or analysed during the reported study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Antonini, A., Odin, P., Pahwa, R. et al. The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on ‘Off’-time in Patients with Advanced Parkinson’s Disease: A Systematic Review. Adv Ther 38, 2854–2890 (2021). https://doi.org/10.1007/s12325-021-01747-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01747-1