Abstract
The introduction of immunotherapy has fundamentally transformed the treatment landscape in cancer, providing long-term survival benefit for patients with advanced disease across multiple tumor types, including non-small cell lung cancer (NSCLC). In the placebo-controlled phase 3 PACIFIC trial, the PD-L1 inhibitor durvalumab demonstrated significant improvements in progression-free survival and overall survival in patients with unresectable, stage III NSCLC who had not progressed after platinum-based chemoradiotherapy (CRT). These findings have led to the widespread acceptance of the ‘PACIFIC regimen’ (durvalumab after CRT) as the standard of care in this setting. Moreover, the PACIFIC trial is the first study to demonstrate a proven survival advantage with an immunotherapy in a curative-intent setting, thereby providing a strong rationale for further investigation of durvalumab in early-stage cancers. Herein, we describe the extensive clinical development program for durvalumab across multiple tumor types in curative-intent settings, outlining the scientific rationale(s) for its use and highlighting the innovative research (e.g., personalized cancer monitoring) advanced by these trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immunotherapy has fundamentally transformed the treatment landscape in cancer. |
In the placebo-controlled phase 3 PACIFIC trial, the programmed cell death-ligand 1 inhibitor durvalumab demonstrated significant improvements in survival in patients with unresectable, stage III non-small lung cancer. |
This has led to the widespread acceptance of the ‘PACIFIC regimen’ (durvalumab after chemoradiotherapy) as the standard of care in this setting. |
As durvalumab is the first immunotherapy with a proven survival advantage in a curative-intent setting, there is a strong rationale for its further investigation in early-stage cancers. |
An extensive clinical development program, as described herein, is investigating durvalumab across multiple tumor types in curative-intent settings. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.14035628.
Introduction
The introduction of immunotherapy has fundamentally transformed the treatment landscape in cancer, providing long-term survival benefit for patients with metastatic disease across a range of tumor types. In particular, immune checkpoint blockade (ICB) of the programmed cell death-ligand 1 (PD-L1) and PD-1 pathway, and of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), has shown survival benefit in many types of advanced cancer, including non-small cell lung cancer (NSCLC) [1, 2]. The success of these agents has led to several regulatory approvals and, in advanced NSCLC, has recently led to a shift in the use of ICB therapy from the second- to first-line setting as the preferred treatment option (either alone or in combination with chemotherapy [CT]) for patients without genomic-driven tumors [3].
Durvalumab is a selective, high-affinity, human IgG1 monoclonal antibody that targets PD-L1 and occludes its binding to PD-1 and CD80, allowing T cells to recognize and kill tumor cells [4,5,6]. In an early phase 1/2 study of patients with a range of advanced solid tumors, including NSCLC [7, 8], durvalumab demonstrated encouraging antitumor activity and, based on this study, was granted accelerated approval in the US for patients with locally advanced or metastatic urothelial carcinoma after failure of platinum-based CT [9, 10]. In the subsequent phase 3 PACIFIC trial, durvalumab versus placebo demonstrated significant improvements in the primary end points of progression-free survival (PFS) and overall survival (OS) in patients with unresectable, stage III NSCLC who had not progressed after platinum-based chemoradiotherapy (CRT) [11,12,13,14].
These findings have led to global regulatory approvals and the widespread acceptance of the ‘PACIFIC regimen’ (durvalumab after CRT) as the standard of care in this setting. Moreover, the PACIFIC trial is the first study to demonstrate a proven survival advantage with an immunotherapy in a curative-intent setting, thereby providing a strong rationale for further investigation of durvalumab in early-stage cancers. Two other checkpoint inhibitors have been approved by global regulatory authorities for use in the curative-intent setting: nivolumab and pembrolizumab as adjuvant therapy in patients with resected melanoma [15, 16] and pembrolizumab as treatment of patients with bacillus Calmette-Guerin (BCG)-unresponsive, high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS), who are ineligible for or elect not to undergo cystectomy [16]. However, the focus of this review is further evaluation of durvalumab in early-stage cancers.
Herein, we describe the extensive clinical development program for durvalumab across multiple tumor types in curative-intent settings, including several trials in early-stage lung cancer, as well as in other cancers (e.g., bladder cancer, hepatocellular carcinoma [HCC], and gastric and esophageal cancer). In addition, we will outline the scientific rationale(s) for use of durvalumab in these settings and highlight the innovative research in personalized cancer monitoring that will be advanced by these studies. This program will not only shed light on the potential use of durvalumab to reduce recurrence rates and improve survival outcomes, but also increase our understanding of patient selection and optimal use of immunotherapy in this setting where we have the best chance to cure patients.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Early-Stage Cancer: Diagnosis, Prognosis and Rationale for Curative-Intent Immunotherapy
A patient’s treatment goals and prognosis can vary significantly, depending on his/her disease stage at diagnosis. Localized, early-stage disease can be treated with curative intent, whereas the goal for metastatic disease is to extend survival and, in some cases, is palliative only [17,18,19]. For patients diagnosed with early-stage cancer, surgery remains the mainstay of curative treatment for most common tumor types. However, recurrence after curative surgery remains a major concern, with or without the use of neoadjuvant or adjuvant treatment [20,21,22,23,24,25,26]. It is hypothesized that, based on the more amenable biology of early-stage cancer, there may be an increased likelihood of cure with immunotherapy.
Diagnosis and Treatment Outcomes in Early-Stage Cancer
Across all tumor types, a common goal is screening and early detection in order to cure patients by revealing the malignancy, or precursor lesion, at an early prior stage, when treatment is most effective. This is reflected in survival rates, which can vary significantly depending on the stage at diagnosis. For example, among patients with bladder cancer, the 5-year survival rates range from as high as 96% for patients with in situ disease to 36% for patients with regional disease and as low as 5% for patients with metastatic disease [27]. Similarly, among NSCLC patients, 5-year survival rates can reach up to 92% for patients with stage IA1 diagnosis to as low as 10% for patients with stage IV diagnosis (i.e., metastatic disease), with significant variation by, and within, the stages in between [28].
However, the proportion of patients diagnosed with early-stage cancer varies by tumor type and depends on several factors, including ease of detection, the stage at which patients become symptomatic (e.g., early-stage disease may remain asymptomatic and undetected) and whether reliable screening programs are in place [20, 27, 29, 30]. For example, only 30% of patients with NSCLC present with resectable disease at diagnosis [20, 29, 31, 32]; most patients are diagnosed at a later stage, associated with an unfavorable prognosis, as reflected in the overall 5-year survival rate of 18% [33]. In contrast, for example, the overall 5-year survival rate for patients with bladder cancer is 77%, since > 90% of patients are diagnosed with resectable disease [19, 27].
Screening has been shown to reduce mortality for several types of cancer. For example, the National Lung Screening Trial in the US and the Dutch-Belgian NELSON lung cancer screening trial have shown a reduction in lung cancer-related deaths of 20–25% using low-dose computed tomography compared with control groups [30]. Further efforts to increase lung screening and earlier diagnosis are being championed by the Lung Ambition Alliance [34], of which AstraZeneca is a founding partner.
Finally, a major area of research in improving earlier diagnosis (i.e., ‘stage shifting’) is utilization of blood-based circulating tumor cell-free DNA (cfDNA) to simultaneously detect and localize multiple cancer types. In a recently published analysis from the Circulating Cell-free Genome Atlas study, based on 6689 participants (2482 with previously untreated cancer and 4207 without cancer), target methylation analysis of plasma cfDNA was used to simultaneously detect > 50 cancer types, across all stages, and localize the tissue of origin with > 90% accuracy [35].
Rationale for Curative-Intent Immunotherapy
First, there are several theoretical advantages for use of immunotherapy, early in the course of tumor development, including (1) lower tumor volume, (2) reduced tumor heterogeneity [36,37,38,39,40] and (3) largely intact host fitness, not yet impaired by multiple rounds of CT.
There is also a strong scientific rationale for the use of immunotherapy in the curative-intent setting, based on potential synergistic effects when used as part of a multi-model treatment approach. For example, it has been suspected for some time that tumor resection may somewhat paradoxically promote tumor growth. Recent findings suggest that surgery may suppress antitumor immunity and induce the development of new postoperative metastases as well as promote the growth of micrometastases and residual disease [41,42,43]. However, in a preclinical model for surgical stress, treatment with a PD-1 inhibitor boosted CD8+ T cell numbers and function [44]. In addition, preclinical evidence suggests that CRT causes upregulation of PD-L1 expression and induction of immunogenic cell death as well as activation of a post-CRT inflammatory response [45,46,47,48,49,50].
Finally, based on several studies of postoperative adjuvant CT in NSCLC [51,52,53], which have demonstrated absolute risk reductions in 5-year survival rates of 5–15%, we can extrapolate that immunotherapy in the early-stage setting may confer similar benefit, given the improvement already demonstrated by immunotherapy in the treatment of some metastatic cancers. However, because such benefit is reliant upon immune cell infiltration of the tumor, it cannot be guaranteed that this will be replicated in all early-stage cancers (e.g., if infiltration is deficient in so-called ‘cold tumors’) [54].
That said, all of these factors could serve to improve the likelihood of a cure, using a treatment capable of harnessing the body’s immune mechanisms.
The Pacific Study: Evidence of Durvalumab’s Potential as Curative-Intent Treatment
Historically, among stage III NSCLC patients who are considered unresectable, platinum-based doublet CT, administered with definitive-dose RT (concurrent CRT), was the standard of care. The treatment goal was with curative intent; however, outcomes were poor because most patients had disease progression after CRT, with approximately 15–32% of patients alive at 5 years [55, 56]. Prior to the PACIFIC trial, several studies had investigated systemic therapies with curative intent after patients had disease control with CRT. However, none had proven effective [57,58,59,60,61].
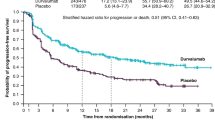
The phase 3 PACIFIC trial was designed to exploit the potential synergy between immunotherapy and CRT (Fig. 1). In PACIFIC, patients with unresectable, stage III NSCLC (and any tumor PD-L1 status) who had not progressed after platinum-based CRT were randomized in a 2:1 ratio to receive durvalumab 10 mg/kg or matching placebo given intravenously every 2 weeks for up to 12 months [11,12,13,14]. Durvalumab versus placebo significantly improved the primary end points of PFS (stratified HR 0.52, 95% CI 0.42–0.65; P < 0.001; median 16.8 vs. 5.6 months; data cutoff [DCO], 13 February 2017) and OS (stratified HR 0.68, 95% CI 0.53–0.87; P = 0.0025; median not reached vs. 28.7 months; DCO, 22 March 2018) [11, 12] and was observed across most patient subgroups. In addition, durvalumab exhibited a manageable safety profile and had no detrimental effect on patient-reported outcomes (PROs) [63], which was notable since the previous standard of care in this setting was observation alone with no presumed detriment to PROs.
In addition, updated survival analyses at approximately 4 years after the last patient was randomized (median follow-up, 34.2 months; DCO, 20 March 2020) demonstrated consistent and durable benefit with durvalumab [14]. Updated OS (stratified HR, 0.71; 95% CI 0.57–0.88) and PFS (stratified HR, 0.55; 95% CI 0.44–0.67) remained consistent with the primary analyses, and median OS for durvalumab was reached, 47.5 months (versus 29.1 months for placebo). The estimated 4-year OS rates were 49.6% for durvalumab versus 36.3% for placebo, and the 4-year PFS rates were 35.3% versus 19.5%, respectively. With approximately 50% of patients alive at 4 years and approximately 35% progression-free, despite completing their last treatment, 3+ years earlier, these findings have further established the PACIFIC regimen as the standard of care in this setting.
The PACIFIC trial, and subsequent worldwide approvals of the PACIFIC regimen for patients in this setting, has resulted in a paradigm shift in the treatment landscape, setting the stage for the investigation and potential use of durvalumab as curative-intent treatment in other early-stage cancer contexts.
Durvalumab Clinical Development Program in Early-Stage Lung Cancer
The role of durvalumab treatment in early-stage lung cancer is undergoing extensive investigation within several ongoing and planned phase 2 and 3 trials as (1) neoadjuvant or adjuvant treatment of resectable NSCLC, (2) concurrent treatment with or following CRT in unresectable NSCLC and (3) treatment after CRT in limited stage SCLC (Table 1).
Resectable Stage I–III NSCLC
The ongoing phase 3 BR.31 study [68] (Table 1), led by the Canadian Cancer Trials Group, will be a key trial in the adjuvant setting, enrolling approximately 1360 patients with NSCLC, classified as stage IB (≥ 4 cm in the longest diameter), II or IIIA, to be randomized to either durvalumab or placebo; prior, postoperative platinum-based doublet CT, per standard of care, is allowed but not mandatory. The BR.31 study will investigate the importance of PD-L1 testing at time of diagnosis, assessing disease-free survival (DFS) in all patients and in patients with either PD-L1 tumor cell (TC) expression ≥ 25% or ≥ 1%.
In addition to the effects of surgery, recent evidence indicates that, like advanced tumors, early-stage tumors may have an immunosuppressive microenvironment [84]. Emerging clinical data show that PD-1/PD-L1 inhibition (alone or combined with CT) in the neoadjuvant setting in patients with resectable NSCLC is associated with expansion of CD8+ T cell clones in both tumor and peripheral blood expansion and with encouraging rates of major pathologic response (mPR) [85,86,87].
The phase 3 AEGEAN trial [66, 67, Data on file] (Table 1) will assess the clinical benefit of combining durvalumab with CT in the neoadjuvant setting in patients with resectable stage IIA to select IIIB NSCLC; patients will continue their randomized treatment (durvalumab or placebo) after surgery. The primary end points are pathologic complete response (pCR) and event-free survival (EFS).
Minimal residual disease (MRD), as indicated by the presence of circulating tumor DNA (ctDNA), has shown promise as a prognostic marker for disease recurrence [88]. Its detection, in the absence of radiologic evidence of disease, may improve clinical outcomes via earlier detection and selective intervention with adjuvant therapy, minimizing overtreatment of MRD-negative (MRD–) patients, most of whom are expected to have been cured by their surgery. In addition, MRD detection may help identify patients at high risk of clinical relapse who may benefit from more intense or earlier therapeutic intervention. Such a novel proposed management strategy would replace the current paradigms in which treatment is often reactive, unable to catch-up with the progressing disease, and allow identification of patients at risk for relapse who may need additional therapies (e.g., to maximize curative intent) in a setting where cure is still potentially achievable (Fig. 2).
The phase 3 MERMAID-1 and -2 studies [69, 70] (Table 1) will aim to build a better understanding of the value of MRD in identifying the risk of relapse and the need for therapeutic intervention in patients with completely resected stage II–III NSCLC. In both studies, each patient’s post-surgery MRD status will be assessed using an innovative, highly sensitive, personalized assay developed by ArcherDX that uses whole exome sequencing (WES) of the patient’s resected tumor tissue to detect ctDNA in collected plasma samples. In MERMAID-1, a ‘landmark’ approach with respect to MRD detection immediately after surgery will be utilized, whereas, in MERMAID-2, longitudinal monitoring for ‘emergent’ relapse will be used.
In MERMAID-1 [69], patients will be randomized following successful surgery to standard-of-care adjuvant CT plus either durvalumab or placebo. The primary end point is DFS in patients who have detectable MRD (MRD+) following surgery, who are at high risk of relapse. In MERMAID-2 [70], patients who have completed curative-intent therapy (complete resection + optional neoadjuvant and/or adjuvant therapy) will be enrolled in a 96-week surveillance period during which they will be monitored for the emergence of MRD; patients who subsequently become MRD+ will then be rescreened and, if eligible, randomized to treatment. The primary end point in MERMAID-2 is DFS in patients with PD-L1 TC ≥ 1%.
Although combinations of neoadjuvant PD-1/PD-L1 inhibitors with CT seem to improve efficacy (as measured by mPR rate) compared with immunotherapy alone [89], improved efficacy may come at risk of combined and increased toxicity; as such, novel combinations of immunotherapy may reduce the level of preoperative toxicity.
In this context, the phase 2 multidrug platform NeoCOAST study [64, 65] (Table 1) will explore the efficacy of durvalumab alone or combined with novel agents (known to be additive to or synergistic with durvalumab) in the neoadjuvant setting. These agents include oleclumab, a monoclonal antibody that binds to CD73, inhibiting production of immunosuppressive adenosine; monalizumab, an immune checkpoint inhibitor targeting NKG2A receptors; or danvatirsen, an antisense oligonucleotide that inhibits the STAT3 transcription factor.
Inoperable Stage I/II NSCLC
Most patients with unresectable NSCLC have stage III disease, although some patients with localized, stage I or II NSCLC will be medically inoperable because of comorbidities such as heart disease or emphysema. Stereotactic body RT (SBRT) is a recommended standard-of-care treatment for these patients [20, 31], with local tumor control of > 90% at 5 years, and a 5-year OS rate of 40% in stage I medically inoperable patients [90]. The efficacy of durvalumab, compared with placebo, given concurrently with definitive SBRT to patients who have refused surgery or with medically inoperable stage I/II, lymph node-negative NSCLC, will be assessed in the phase 3 PACIFIC-4 trial [71, 72] (Table 1).
Unresectable Stage III NSCLC
Stage III NSCLC is heterogeneous in presentation, and the majority of cases are unsuitable for curative surgery as the primary treatment modality [91]. Historically, definitive treatment for unresectable stage III NSCLC had been concurrent CRT; however, based on the PACIFIC trial, durvalumab after CRT is now the standard of care for eligible patients who have not progressed following platinum-based concurrent CRT.
Several hypothesized mechanisms may have contributed to the unprecedented efficacy observed with durvalumab in the PACIFIC trial. These include upregulation of tumor PD-L1 expression by CT and/or RT [45,46,47,48] and/or increased availability of tumor neo-antigens (as a result of DNA damage and cell death from RT), promoting both the priming and effector phases of the antitumor immune response mediated by T cells [49, 50]. Moreover, in vivo experiments have demonstrated the synergistic effects of RT and, specifically, PD-1/PD-L1 blockade [46, 47]. However, the optimum sequencing of durvalumab and CRT was not explored in PACIFIC and has not yet been fully investigated in a clinical setting; in addition, preclinical studies suggest that efficacy may be increased when immune checkpoint inhibitors are administered concurrently with RT [47, 50, 92].
In this context, the next key study anticipated to read out in this setting is the PACIFIC-2 trial [79, 80] (Table 1), which will assess whether durvalumab given concurrently with CRT (followed by durvalumab consolidation treatment) provides additional benefit versus concurrent CRT alone.
Although concurrent CRT is superior to sequential CRT for treatment of unresectable stage III NSCLC with respect to survival, it is associated with greater acute esophageal toxicity [93]; sequential CRT may therefore be preferable for some older patients, those with poorer performance status or those with specific comorbidities unable to tolerate the increased toxicity [31, 94, 95]. The ongoing phase 3 PACIFIC-5 trial [73, 74] (Table 1) was therefore designed to assess the efficacy and safety of durvalumab in patients with no disease progression following either sequential or concurrent CRT. However, unlike the PACIFIC trial, the window for randomization in PACIFIC-5 is up to 28 days post-CRT, rather than up to 42 days, and consolidation treatment with durvalumab or placebo is continued until disease progression (because of the poorer 5-year OS and DFS rates seen for this broader population, which includes patients who progress during the induction CRT therapy [11,12,13,14, 57, 93, 96, 97]), unacceptable toxicity or other discontinuation criteria.
Finally, additional studies of durvalumab in patients with unresectable, stage III NSCLC include the three phase 2 trials, PACIFIC-6 [75, 76], COAST [77, 78] and DUART [81] (Table 1).
Limited-Stage SCLC
Approximately one-third of patients diagnosed with SCLC present with limited-stage disease, for which the curative-intent standard of care is generally platinum-based CT with concurrent thoracic RT, followed by prophylactic cranial irradiation (PCI) if indicated [98, 99]. However, recurrence of disease is common with a 5-year survival rate of 31–34% [100].
The phase 3 CASPIAN study demonstrated a significant improvement in OS for patients with extensive-stage SCLC who received first-line durvalumab, in combination with a choice of platinum-etoposide (EP) chemotherapy (followed by durvalumab maintenance) versus EP alone (HR, 0.73, 95% CI 0.59–0.91; P = 0.0047) [101], leading to worldwide regulatory approvals for durvalumab as a new standard of care in this setting.
The positive OS outcomes demonstrated in both the CASPIAN and PACIFIC studies suggest the potential for durvalumab to provide benefit for the treatment of limited-stage SCLC. The ADRIATIC study [82, 83] (Table 1), therefore, builds on these studies and will enroll patients with limited-stage SCLC (stages I–III) and no disease progression after concurrent CRT; patients will be randomized to receive durvalumab, durvalumab plus tremelimumab or placebo up to 24 months or until disease progression or intolerable toxicity.
Durvalumab Clinical Development Program in other Early-Stage Cancers
Bladder Cancer
As noted above, there is significant variation in survival rates for patients with bladder cancer, depending on the stage at diagnosis. Management of low-risk disease focuses on preventing recurrence or progression to HR-NMIBC or muscle-invasive bladder cancer (MIBC) for which the 5-year mortality rate is 50–70% (even after radical cystectomy) [102, 103]. The most common treatment for early-stage or superficial (non-muscle invasive) bladder cancers is transurethral resection of the bladder tumor (TURBT), which is commonly followed by intravesical therapy with CT or immunotherapy (as induction and/or maintenance therapy) [19].
It has long been known that bladder cancer is immuno-responsive, when it was demonstrated that intravesical instillation of BCG, a live-attenuated tuberculosis-related bacteria, could be used to stimulate an immunologic reaction in NMIBC, thereby, inducing a pro-inflammatory cytokine and direct cell-to-cell cytotoxicity [104]. BCG is the most common intravesical immunotherapy for treating early-stage bladder cancer. In addition, among all cancer types, bladder has one of the highest mutational burdens and, as such, is likely to elicit a relatively large T-cell mediated antitumor immune response and benefit from checkpoint inhibition [105]. Higher mutation rates have been shown to be associated with higher responses to ICB therapy in several cancer types, including melanoma, lung cancer and urothelial carcinoma [106,107,108]. Notably, recent analyses of patients with urothelial carcinoma from a phase 1 trial of durvalumab plus tremelimumab and a phase 1/2 trial of durvalumab monotherapy demonstrated that the tumor mutation burden (TMB) was associated with survival benefit [109]. Finally, upregulation of the PD-1 pathway has also been observed in BCG-resistant NMIBC [110].
Based on the preceding data, combined with the incentive to treat patients with bladder cancer early, there is a strong rationale to study durvalumab in this setting. As such, durvalumab is being assessed in two early-stage bladder cancer trials (Table 2). The ongoing phase 3 NIAGARA trial [111, 112] in patients with resectable MIBC is investigating the effect of adding durvalumab to standard-of-care cisplatin-based neoadjuvant treatment [113, 114] followed by radical cystectomy with extended lymphadenopathy and then adjuvant durvalumab monotherapy. The phase 3 POTOMAC trial [115, 116] will assess whether the addition of durvalumab to standard-of-care BCG, after complete resection of papillary tumors, improves DFS compared with BCG alone.
Liver Cancer
The development of HCC is facilitated by the intrinsic intra-hepatic immunosuppressive microenvironment and chronic inflammation, making HCC an appropriate candidate for immune checkpoint inhibitors [117]. A phase 2 study of patients with advanced HCC (Study 22) recently demonstrated promising clinical activity for durvalumab in combination with the anti-CTLA4 tremelimumab [118]. Durvalumab plus tremelimumab was recently granted orphan drug designation by the US FDA for patients with advanced HCC [119].
Transarterial chemoembolization (TACE) is a standard locoregional treatment for patients with intermediate-stage HCC, but many patients relapse within a year (e.g., based on a systematic review of efficacy across multiple studies of TACE using lipiodal-based regimens, the 1-year PFS rate was 40.6% and estimated 2- and 5-year OS rates were 51.8% and 32.4%, respectively [120]). Combination treatment with a PD-L1 inhibitor, atezolizumab and the VEGF inhibitor, bevacizumab, improved OS in advanced HCC compared with sorafenib [121]; these data provide a rationale for combining durvalumab with VEGF inhibition in locoregional HCC.
Two phase 3 trials of durvalumab in early-stage HCC are currently recruiting patients, EMERALD-1 and EMERALD-2 (Table 2). EMERALD-1 [122, 123] is a placebo-controlled, phase 3 trial of TACE in combination with durvalumab with or without bevacizumab, compared with TACE alone, in patients with locoregional HCC. EMERALD-2 [124, 125] is a placebo-controlled, phase 3 trial evaluating durvalumab with or without bevacizumab as adjuvant therapy in patients with HCC after curative resection or ablation who are at high risk of recurrence.
Other Cancers
Additional phase 3 trials are investigating durvalumab in patients with other early stage cancers, including unresectable esophageal squamous cell carcinoma (e.g., the placebo-controlled KUNLUN study [126], which is assessing durvalumab in combination with definitive CRT), resectable gastric and gastroesophageal junction cancer (e.g., a placebo-controlled trial of perioperative durvalumab in combination with FLOT [fluorouracil + leucovorin + oxaliplatin + docetaxel] CT [127]), and locally advanced cervical cancer (e.g., the placebo-controlled CALLA study [128, 129], which is assessing durvalumab with concurrent CRT, followed by durvalumab, versus concurrent CRT alone (Table 2)).
Conclusion
Patients diagnosed with early-stage cancers frequently relapse with locoregional or distant disease. The practice-changing survival benefit shown with durvalumab following CRT in the phase 3 PACIFIC trial of patients with stage III, unresectable NSCLC supports the strategy of using immunotherapy in a curative-intent paradigm. There may be inherent challenges associated with conducting a clinical development program in early-stage cancers, e.g., ensuring harmonization of locoregional treatments and coordinating among members of the multidisciplinary teams, which are integral to the patient journey. However, it is anticipated that the program for durvalumab will add considerably to our knowledge regarding its potential use (and immunotherapies, in general) in both resectable and unresectable early-stage disease. For example, it will address questions related to optimal sequencing or combination of durvalumab with other standard-of-care treatments (e.g., as adjuvant or neoadjuvant therapy), the optimal length of treatment with durvalumab, and the efficacy and safety of durvalumab in novel combinations with other agents.
In addition, the trials in the durvalumab development program are at the forefront of innovative, personalized cancer monitoring (e.g., via experimental use of ctDNA to monitor patients’ risk of relapse). These efforts, combined with other investigative trends, such as ‘stage shifting’ via population-scale early cancer detection, hold the promise to, once again, shift the treatment landscape in the curative-intent setting.
Finally, the extensive clinical development program for durvalumab is complemented by trials of other compounds in early-stage cancers, such as the recently reported phase 3 ADAURA trial, which assessed the EGFR inhibitor osimertinib as adjuvant treatment, showing a significantly prolonged DFS for patients with early-stage EGFR-mutant NSCLC [130]. The overall breadth and depth of AstraZeneca’s cancer trial portfolio have the potential for significant advances in the treatment of these early-stage tumors over the years to come.
References
Nixon N, Blais N, Ernst S, et al. Current landscape of immune therapy in treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25(5):e373–84.
Emens LA, Ascierto PA, Darcy PK, et al. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29.
Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30(8):1244–53.
Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–62.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82.
Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). In: Poster presented at the European Society for Medical Oncology Annual Meeting, Copenhagen; October 7–11, 2016.
Antonia SJ, Balmanoukian A, Brahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. 2019;14(10):1794–806.
Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
AstraZeneca. Imfinzi (durvalumab): U.S. prescribing information. https://www.imfinzi.com/. Accessed 4 Dec 2020.
Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29.
Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50.
Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–93.
Faivre-Finn C, Vicente D, Kurata T, et al. Brief report: Four-year survival with durvalumab after chemoradiotherapy in Stage III NSCLC – an update from the PACIFIC trial. J Thorac Oncol. Published online January 18, 2021.
Bristol Myers Squibb. Opdivo (nivolumab): U.S. prescribing information https://www.opdivo.com/. Accessed 11 Feb 2021.
Merck Sharp & Dohme Corp. Keytruda (pembrolizumab): U.S. prescribing information. https://www.keytruda.com/. Accessed 11 Feb 2021.
Robinson AG, Young K, Balchin K, Owen T, Ashworth A. Reasons for palliative treatments in stage III non-small-cell lung cancer: What contribution is made by time-dependent changes in tumour or patient status? Curr Oncol. 2015;22(6):399–404.
Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e341S-e368S.
American Society of Clinical Oncology. Bladder Cancer: Treatments by Stage. 5/2019. https://www.cancer.net/cancer-types/bladder-cancer/treatments-stage. Accessed 4 Dec 2020.
Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–21.
Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford). 2005;7(1):42–9.
Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219–27.
Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–9.
Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–55.
Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: The realities, challenges, and opportunities for new therapies. CA Cancer J Clin. 2018;68(6):488–505.
Sargos P, Baumann BC, Eapen L, et al. Risk factors for loco-regional recurrence after radical cystectomy of muscle-invasive bladder cancer: A systematic-review and framework for adjuvant radiotherapy. Cancer Treat Rev. 2018;70:88–97.
American Cancer Society. Cancer Facts and Figures 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html. Accessed 4 Dec 2020.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85.
Benzaquen J, Boutros J, Marquette C, Delingette H, Hofman P. Lung cancer screening, towards a multidimensional approach: Why and how? Cancers (Basel). 2019;11(2):212.
NCCN Guidelines. Non-small cell lung cancer. V3.2020; February 11, 2020. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 4 Dec 2020.
NCCN Guidelines. Small cell lung cancer. V3.2020; February 5, 2020. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 4 Dec 2020.
Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2017/ (based on November 2019 SEER data submission). Accessed 4 Dec 2020.
The Lung Ambition Alliance. https://www.lungambitionalliance.com/. Accessed 4 Dec 2020.
Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745–59.
Wolf Y, Bartok O, Patkar S, et al. UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell. 2019;179(1):219–35.
Andor N, Graham TA, Jansen M, et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2016;22(1):105–13.
Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–21.
Miao D, Margolis CA, Vokes NI, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–81.
Morris LGT, Riaz N, Desrichard A, et al. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7(9):10051–63.
Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–52.
Bakos O, Lawson C, Rouleau S, Tai LH. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018;6(1):86.
Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10.
Sun Z, Mao A, Wang Y, et al. Treatment with anti-programmed cell death 1 (PD-1) antibody restored postoperative CD8+ T cell dysfunction by surgical stress. Biomed Pharmacother. 2017;89:1235–41.
Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45(5):1470–6.
Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95.
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68.
Funaki S, Shintani Y, Kawamura T, Kanzaki R, Minami M, Okumura M. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep. 2017;38(4):2277–84.
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65.
Wang Y, Deng W, Li N, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185.
Horn L, Sandler AB, Putnam JB, Johnson DJ. The rationale for adjuvant chemotherapy in stage I non-small cell lung cancer. J Thorac Oncol. 2007;2:377–83.
Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9.
Artal Cortés Á, Calera Urquizu L, Hernando CJ. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res. 2015;4(2):191–7.
Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168.
Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8:1–20.
Bradley JD, Hu C, Komaki RU, et al. Long-term results of NRG oncology RTOG 0617: standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non–small-cell lung cancer. J Clin Oncol. 2020;38:706–14.
Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSGLU05-04. J Clin Oncol. 2015;33:2660–6.
Skrzypski M, Jassem J. Consolidation systemic treatment after radiochemotherapy for unresectable stage III non-small cell lung cancer. Cancer Treat Rev. 2018;66:114–21.
Tsujino K, Kurata T, Yamamoto S, et al. Is consolidation chemotherapy after concurrent chemo radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol. 2013;8:1181–9.
Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–6.
Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III nonsmall- cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–57660.
Chacon JA, Schutsky K, Powell DJ. The impact of chemotherapy, radiation and epigenetic modifiers in cancer cell expression of immune inhibitory and stimulatory molecules and anti-tumor efficacy. Vaccines (Basel). 2016;4:E43.
Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–80.
Neoadjuvant durvalumab alone or in combination with novel agents in resectable non-small cell lung cancer. ClinicalTrials.gov identifier: NCT03794544. https://clinicaltrials.gov/ct2/show/NCT03794544. Updated October 5, 2020. Accessed 18 Dec 2020.
Garcia-Campelo R, Forde P, Weder W, et al. NeoCOAST: neoadjuvant durvalumab alone or with novel agents for resectable, early-stage (I–IIIA non-small cell lung cancer. In: Poster presented at the IASLC 2019 World Conference on Lung Cancer (WCLC), Barcelona, 7–10 September 2019.
A Study of Neoadjuvant/Adjuvant Durvalumab for the Treatment of Patients With Resectable Non-small Cell Lung Cancer. ClinicalTrials.gov identifier: NCT03800134. https://clinicaltrials.gov/ct2/show/NCT03800134. Updated December 17, 2020. Accessed 18 Dec 2020.
Heymach JV, Taube JM, Mitusdomi T, et al. The AEGEAN phase 3 trial of neoadjuvant/adjuvant durvalumab in patients with resectable stage II/III NSCLC. In: Poster presented at the IASLC 2019 World Conference on Lung Cancer (WCLC), Barcelona, 7–10 September 2019.
Double blind placebo controlled study of adjuvant MEDI4736 in completely resected NSCLC. ClinicalTrials.gov identifier: NCT02273375. https://clinicaltrials.gov/ct2/show/NCT02273375. Updated April 3, 2020. Accessed 18 Dec 2020.
Phase III study to determine the efficacy of durvalumab in combination with chemotherapy in completely resected stage II-III non-small cell lung cancer (NSCLC) (MERMAID-1). ClinicalTrials.gov identifier: NCT04385368. https://clinicaltrials.gov/ct2/show/NCT04385368. Updated December 1, 2020. Accessed 18 Dec 2020.
Phase III study to determine efficacy of durvalumab in stage II-III non-small cell lung cancer (NSCLC) after curative intent therapy. (MERMAID-2). ClinicalTrials.gov identifier: NCT04642469. https://clinicaltrials.gov/ct2/show/NCT04642469. Updated November 24, 2020. Accessed 18 Dec 2020.
Durvalumab vs placebo with stereotactic body radiation therapy in early stage unresected non-small cell lung cancer patients (PACIFIC-4). ClinicalTrials.gov identifier: NCT03833154. https://clinicaltrials.gov/ct2/show/NCT03833154. Updated December 22, 2020. Accessed 11 Jan 2021.
Robinson C, Hu C, Machtay M, et al. PACIFIC-4/RTOG 3515: phase III study of durvalumab following SBRT for unresected stage I/II, lymph-node negative NSCLC. In: Poster presented at the IASLC 2019 World Conference on Lung Cancer (WCLC), Barcelona, 7–10 September 2019.
A study of durvalumab as consolidation therapy in non-small cell lung cancer patients (PACIFIC-5). ClinicalTrials.gov identifier: NCT03706690. https://clinicaltrials.gov/ct2/show/NCT03706690. Updated November 18, 2020. Accessed 18 Dec 2020.
Wu Y-L, Wang L, Sendur MAN, Kim Y-C, et al. PACIFIC-5: phase 3 study of durvalumab after either concurrent or sequential chemoradiotherapy (CRT) in patients with stage III NSCLC. In: Poster presented at the European School of Medical Oncology (ESMO) Asia Congress, Singapore, 22–24 November 2019.
A study to determine safety of durvalumab after sequential chemo radiation in patients with unresectable stage III non-small cell lung cancer. ClinicalTrials.gov identifier: NCT03693300. https://clinicaltrials.gov/ct2/show/NCT03693300. Updated December 14, 2020. Accessed 18 Dec 2020.
Garassino M, Faivre-Finn C, Mazieres J, et al. PACIFIC-6: A Phase 2 study of durvalumab following sequential chemoradiotherapy in patients with Stage III, unresectable NSCLC. In: Poster presented at the IASLC 2019 World Conference on Lung Cancer (WCLC), Barcelona, 7–10 September 2019.
Durvalumab alone or in combination with novel agents in subjects with NSCLC (COAST). ClinicalTrials.gov identifier: NCT03822351. https://clinicaltrials.gov/ct2/show/NCT03822351. Updated November 5, 2020. Accessed 18 Dec 2020.
Herbst RS, Barlesi F, Paz-Ares L, et al. COAST: Durvalumab alone or with novel agents for locally advanced, unresectable, Stage III non-small cell lung cancer. In: Poster presented at the IASLC 2019 World Conference on Lung Cancer (WCLC), Barcelona, 7–10 September 2019.
Study of durvalumab given with chemoradiation therapy in patients with unresectable non-small cell lung cancer. ClinicalTrials.gov identifier: NCT03519971. https://clinicaltrials.gov/ct2/show/NCT03519971. Updated October 28, 2020. Accessed 18 Dec 2020.
Bradley JD, Nishio M, Okamoto I, et al. PACIFIC-2: phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J Clin Oncol. 2019;37(15_suppl):TPS8573.
Study of durvalumab following radiation therapy in patients with stage 3 unresectable NSCLC ineligible for chemotherapy (DUART). ClinicalTrials.gov identifier: NCT04249362. https://clinicaltrials.gov/ct2/show/NCT04249362. Updated September 25, 2020. Accessed 18 Dec 2020.
Study of durvalumab + tremelimumab, durvalumab, and placebo in limited stage small-cell lung cancer in patients who have not progressed following concurrent chemoradiation therapy (ADRIATIC). ClinicalTrials.gov identifier: NCT03703297. https://clinicaltrials.gov/ct2/show/NCT03703297. Updated November 18, 2020. Accessed 18 Dec 2020.
Senan S, Okamoto I, Lee GW, et al. Design and rationale for a phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: the ADRIATIC study. Clin Lung Cancer. 2020;21(2):e84–8.
Lavin Y, Kobayashi S, Leader A, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169(4):750-765.e17.
Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–86 (published correction appears in N Engl J Med. 2018 Nov 29;379(22):2185).
Rusch VW, Chaft JE, Johnson B, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): initial results from a multicenter study (LCMC3). J Clin Oncol. 2018;36(15_suppl):8541.
Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemo-immunotherapy for the treatment of stage IIIA resectable non-small-cell lung cancer (NSCLC): a phase II multicenter exploratory study—final data of patients who underwent surgical assessment. J Clin Oncol. 2019;37(15_suppl):8509.
Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018;15(9):577–86.
Shu CA, Grigg C, Chiuzan C, et al. Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(15_suppl):8532.
Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non-small cell lung cancer. JAMA Oncol. 2018;4(9):1287–8.
Cheema PK, Rothenstein J, Melosky B, Brade A, Hirsh V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol. 2019;26(1):37–42.
Samstein R, Rimner A, Barker CA, et al. Combined immune checkpoint blockade and radiation therapy: timing and dose fractionation associated with greatest survival duration among over 750 treated patients. Int J Radiat Oncol Biol Phys. 2018;99:S129–30.
Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20(1):98–102.
Gadgeel SM. The optimal chemotherapy for stage III non-small cell lung cancer patients. Curr Oncol Rep. 2011;13(4):272–9.
Mitchell P, Thatcher N, Socinski MA, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. 2015;26:1134–42.
Liang J, Bi N, Wu S, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28:777–83.
Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6_suppl):vi99–105.
Oronsky B, Reid TR, Oronsky A, Carter CA. What’s new in SCLC? A review. Neoplasia. 2017;19(10):842–7.
Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–25.
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75.
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66.
Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–2.
Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67.
Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99.
Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20.
Massard C, Si H, Zhang Q, Higgs B, Raja R, Abdullah SE, Gupta A, Li W, van der Heijden M. Tumour mutation burden (TMB), PD-L1, IFN-γ signaling identify subgroups of patients (pts) who benefit from durvalumab (D, anti-PDL1) or D and tremelimumab (T, anti-CTLA4) treatment in urothelial bladder cancer (UC). Ann Oncol. 2019;30(5):V508.
Hashizume A, Umemoto S, Yokose T, et al. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget. 2018;9(75):34066–78.
Durvalumab + gemcitabine/cisplatin (neoadjuvant treatment) and durvalumab (adjuvant treatment) in patients with MIBC (NIAGARA). ClinicalTrials.gov identifier: NCT03732677. https://clinicaltrials.gov/ct2/show/NCT03732677. Updated December 17, 2020. Accessed 18 Dec 2020.
Powles T, Meeks JJ, Galsky MD, et al. A phase III, randomized, open label, multicenter, global study of efficacy and safety of durvalumab in combination with gemcitabine+cisplatin (G+C) for neoadjuvant treatment followed by durvalumab alone for adjuvant treatment in muscle-invasive bladder cancer (MIBC) (NIAGARA). J Clin Oncol. 2019;37(15_suppl):TPS4592.
Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii40–8.
NCCN Guidelines. Bladder cancer. V5.2020; May 12, 2020. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 4 Dec 2020.
Assessment of efficacy and safety of durvalumab plus BCG compared to the standard therapy with BCG in non-muscle invasive bladder cancer (POTOMAC). ClinicalTrials.gov identifier: NCT03528694. https://clinicaltrials.gov/ct2/show/NCT03528694. Updated October 5, 2020. Accessed 18 Dec 2020.
De Santis M, Abdrashitov R, Hegele A, et al. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus calmette-guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J Clin Oncol. 2019;37(7_suppl):TPS00.
Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18(2):291–7.
Kelley RK, Sangro B, Harris WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol. 2020;38(15_suppl):4508.
AstraZeneca news release. Imfinzi and tremelimumab granted Orphan Drug Designation in the US for liver cancer. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2020/imfinzi-and-tremelimumab-granted-orphan-drug-designation-in-the-us-for-liver-cancer-20012020.html. Accessed 2 Aug 2020.
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–16.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
A global study to evaluate transarterial chemoembolization (TACE) in combination with durvalumab and bevacizumab therapy in patients with locoregional hepatocellular carcinoma (EMERALD-1). ClinicalTrials.gov identifier: NCT03778957. https://clinicaltrials.gov/ct2/show/NCT03778957. Updated December 14, 2020. Accessed 18 Dec 2020.
Sangro B, Kudo M, Qin S, et al. A phase 3 study of transarterial chemoembolization combined with durvalumab followed by durvalumab ± bevacizumab in patients with locoregional HCC: EMERALD-1. In: Poster presented at the EASL Liver Cancer Summit 2020; Prague, Czech Republic; February 6–8, 2020.
Assess efficacy and safety of durvalumab alone or combined with bevacizumab in high risk of recurrence HCC patients after curative treatment (EMERALD-2). ClinicalTrials.gov identifier: NCT03847428. https://clinicaltrials.gov/ct2/show/NCT03847428. Updated December 16, 2020. Accessed 18 Dec 2020.
Knox J, Cheng A, Cleary S, et al. A phase 3 study of durvalumab with or without bevacizumab as adjuvant therapy in patients with hepatocellular carcinoma (HCC) who are at high risk of recurrence after curative hepatic resection. Ann Oncol. 2019;30(suppl 4):P187.
Study of durvalumab versus placebo in combination with definitive chemoradiation therapy in patient with ESCC (KUNLUN). ClinicalTrials.gov identifier: NCT04550260. https://clinicaltrials.gov/ct2/show/NCT04550260. Updated December 17, 2020. Accessed 18 December 2020.
Assessing durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer. ClinicalTrials.gov identifier: NCT04592913. https://clinicaltrials.gov/ct2/show/NCT04592913. Updated October 19, 2020. Accessed 18 December 2020.
Study of durvalumab with chemoradiotherapy for women with locally advanced cervical cancer (CALLA). ClinicalTrials.gov identifier: NCT03830866. https://clinicaltrials.gov/ct2/show/NCT03830866. Updated December 16, 2020. Accessed 18 December 2020.
Monk BJ, et al. CALLA: Efficacy and safety of durvalumab with and following concurrent chemoradiotherapy (CCRT) versus CCRT alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. J Clin Oncol. 2019;37(15_suppl):TPS5597.
Wu Y-L, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2020;383:1711–23.
Acknowledgements
Funding
AstraZeneca funded the journal’s Rapid Service and Open Access fees, in addition to medical writing support for the development of this manuscript.
Medical Writing Assistance
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Andrew Gannon, MS, MA, and (as contracted) Jean Scott, PhD, of Ashfield MedComms (New York, USA), an Ashfield Health company, and was funded by AstraZeneca.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Drs. Melillo, Chand, Yovine, Gupta and Massacesi are full-time employees of AstraZeneca with stock ownership.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Melillo, G., Chand, V., Yovine, A. et al. Curative-Intent Treatment with Durvalumab in Early-Stage Cancers. Adv Ther 38, 2759–2778 (2021). https://doi.org/10.1007/s12325-021-01675-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01675-0