Abstract
Asthma is a common, chronic inflammatory airway disease, characterised by unpredictable episodes of worsening symptoms, or exacerbations. Causes of asthma exacerbations include viral infections, exposure to allergen and air pollution, all of which increase the underlying inflammation that typifies asthma. Most (50–75%) patients are classed as having mild asthma, with symptoms that can be readily controlled with available inhaled medications. Paradoxically, for the past 30 years, the first treatment recommended in asthma management guidelines was short-acting β2-agonists (SABA), which not only have no anti-inflammatory properties but may, in fact, worsen inflammation. The Global Initiative for Asthma (GINA) 2019/2020 broke with this paradox by stating clearly that SABA should no longer be used alone as a reliever, for safety reasons. Instead, GINA now recommends an anti-inflammatory rescue/reliever approach for adult and adolescent patients, based on the combination of an inhaled corticosteroid with a rapid onset β2-agonist such as formoterol. This commentary highlights the fact that even patients with well-controlled mild asthma are at risk of severe, potentially life-threatening exacerbations, similar to those in patients with moderate or severe asthma, and therefore ‘mild asthma’, is a misnomer. The commentary describes the case history of a patient with mild asthma to illustrate how increasing use of SABA alone can worsen and prolong exacerbations when they occur. The author goes on to describe how the management of this patient’s exacerbation could have been improved, and provides up-to-date advice on broader aspects of the management of mild asthma and exacerbations, supported by the recent changes to the GINA recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Asthma affects an estimated 339 million people worldwide and, although the majority of those affected (50–75%) are classified as having ‘mild’ asthma, they are still at risk of having life-threatening flare-ups or exacerbations. |
Asthma exacerbations are responsible for a significant percentage of the total costs of asthma management world wide |
What was learned from the study? |
This Commentary presents current knowledge about asthma, illustrated by a description of a severe asthma exacerbation in a patient with mild asthma from a clinician who is an expert in the management of asthma. |
Recommendations on how the treatment of this patient’s exacerbation could have been improved are made and up-to-date advice is provided on the management of asthma and asthma exacerbations, supported by the recent changes to Global Initiative for Asthma (GINA) recommendations. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13303265.
Introduction
Asthma is an inflammatory disease of the airways, characterized by fluctuations of underlying inflammation giving rise to variable symptoms and decreases in lung function [1, 2]. Globally, asthma affects 339 million people, but the prevalence in different regions varies up to 13-fold, ranging from 2.8% to 37.6% in children aged 6–7 years, from 0.8% to 32.6% in adolescents aged 13–14 years, and from 0.2% to 21.0% in adults [3,4,5].
Most patients (50–75%) in the primary care setting are considered to have ‘mild’ asthma [6, 7], which traditionally involved treatment with regular inhaled corticosteroids (ICS, ‘preventer’ or maintenance) and as-needed short-acting β2-agonist bronchodilators (SABA, ‘reliever’ or ‘rescue’) for symptom relief [8, 9]. For patients with mild asthma, these medications, when taken correctly, can provide good symptom control, improve quality of life and enable the patients to lead a normal active life. Regrettably, however, many patients with mild asthma are only prescribed SABA, which do not address the inflammatory component of asthma. And, even when prescribed ICS, many mild asthma patients do not take them as directed, but use them intermittently when they have symptoms, or not at all. And ‘mild’ asthma is a misnomer— these patients can still experience severe exacerbations that may prove fatal, even if they seem to be well-controlled and adherent to maintenance therapy [6]. A recent systematic review found that up to 22% of patients with mild asthma were hospitalised for asthma or had experienced a severe exacerbation in the previous year, while severe exacerbation rates in patients taking only short-acting β2-agonist therapy ranged from 0.20 to 2.88 per year [7]. Patients with moderate or severe asthma are usually treated with ICS–long-acting β2-agonist (LABA) combinations, but may also need other medications to control or reduce their symptoms and reduce the risk of exacerbations.
The severity of asthma exacerbations in patients with ‘mild’ asthma can range from symptom worsenings that interrupt daily life and work productivity to severe and life-threatening episodes and even death. Exacerbation rates are lower in patients with mild asthma than in more severe disease, but in one survey 30–52% of exacerbations requiring emergency care occurred in patients who, in the previous 3 months, had symptoms occurring less than weekly or that were only triggered by exercise [6]. Significantly more absence from work or school, visits to doctors, attendance at emergency departments, and hospitalisations are reported by patients with mild asthma than by healthy matched controls [10]. Two large, recent studies showed that 18.8–22.0% of patients with mild asthma reported having had at least one severe exacerbation in the previous year [11, 12].
The National Review of Asthma Deaths in the UK showed that of 155 asthma deaths where severity could be estimated, 14 (9%) were being treated for mild asthma and 76 (49%) for moderate asthma [13]. A Canadian study estimated that mild asthma was responsible for 67% of total asthma patient-years, but for 14% of the total direct costs of asthma [14]. A very few patients experience sudden-onset asthma attacks that can prove fatal within an hour of onset, the majority have slower-onset asthma attacks that cause progressive difficulty in breathing and can prove fatal within hours to days [13, 15, 16]. Both of these scenarios are more frequent in patients with poorly controlled or uncontrolled asthma but can occur in patients with asthma of any severity [17].
The problem of reliance on SABA alone, without the concomitant use of ICS, when a patient has worsening symptoms, has become better understood in recent years. The Global Initiative for Asthma (GINA) report on asthma management now recommends ICS/formoterol as the preferred reliever medication for adults and adolescents across the spectrum of asthma severity, except in patients already taking an ICS/LABA combination that does not contain formoterol (Fig. 1) [1, 2]. SABA alone is no longer recommended as a reliever, not only because it can mask inflammation until a severe exacerbation occurs [4] but also because it can actually worsen inflammation in the absence of appropriate ICS therapy [18, 19]. SABA-only use happens more frequently than expected in practice, due to the low adherence to regular ICS therapy by patients with mild asthma. Recent studies have shown that approximately one-third of asthma patients in European and Nordic countries use more than three SABA canisters a year, indicating high use, which was significantly associated with increased risk of exacerbations and asthma-related primary care and hospital consultations [20,21,22]. And, in a recent review of the burden of mild asthma, the rates of exacerbation in patients treated with SABA alone ranged from 0.20 to 2.88 per year [7]. Because asthma is recognized to be an inflammatory disease, recommending use of SABA only as the treatment for mild asthma is a paradox in previous guidelines [23], because SABA have no anti-inflammatory properties [23, 24]. The new recommendation of ICS/formoterol as a reliever is a significant paradigm change in asthma management.
This Commentary describes a severe asthma exacerbation and the care that was provided to manage it, with a retrospective analysis of the care and a proposal for how care for the patient could have been optimized, including an alternative approach that simplifies the GINA step care recommendations.
This article does not contain data from any studies with human participants or animals performed by the author.
How an Asthma Exacerbation Unfolded…
The patient whose case is described here had been diagnosed as having mild asthma and been under the care of his physician for many years. With his permission, I am sharing his story with you, which will, I hope, illustrate issues in patients with physician-defined mild asthma. He had been well controlled on low-dose ICS alone, and was currently on beclometasone dipropionate (Qvar®, Teva, 100 µg bid). He used a SABA reliever (Ventolin MDI, GSK, salbutamol 100 µg) only occasionally, within guideline recommendations, for relief of symptoms and also prior to exercise. He had an asthma action plan and attended annual appointments at his family doctor’s practice.
The patient’s exacerbation experience started when he and his wife caught a winter-type ‘cold’. After a few days, his wife felt better but he was increasingly struggling to breathe. Although he continued to use his ICS twice daily, he started to use his SABA reliever more often, which did give him some short-lived relief of his symptoms. Within a few days, however, colleagues at work remarked how ill he seemed. He was struggling with talking at length and had to stop to rest halfway up a flight of stairs.
Subsequently, he had a rapid further deterioration and was taken for an urgent appointment with his doctor. His peak flow was 260 L/min, half of his previous average (520 L/min), but his oxygen saturation was within normal limits. He was prescribed oral steroids (OCS) and antibiotics and told his symptoms would improve in 24 to 48 h. [Case Note: Patient was prescribed prednisolone 30 mg/day and amoxicillin 500 mg capsules three times daily for 7 days]. Two days after finishing the medication, his condition again deteriorated, so another emergency visit to the family doctor was arranged. On examining him, the doctor said she could not hear a wheeze. However, he was unable to speak for more than 20 or 30 s without needing to rest. His peak flow had improved to 400 L/min, but was still much lower than it had been. Oxygen saturation was still normal and the doctor said “You can manage to speak a sentence, but you look very anxious. Is anything worrying you?” [Case Note: Patient history includes generalized anxiety disorder].
The doctor appeared to have mistaken his anxiety as the cause of his symptoms, rather than a result of them. However, she did send the patient to the emergency department at a local hospital. With a normal electrocardiogram, chest x-ray and complete blood count, he was treated with another course of OCS and different antibiotics. [Case Note: Patient was again prescribed prednisolone 30 mg/day × 7 days plus clarithromycin 500 mg twice daily x 7 days] It was not until 2 days after finishing the second course of medication that his breathing began to improve, and it subsequently took another 4 weeks to fully recover (Fig. 2).
When prescribing him the second course of OCS, the doctor had mentioned the possibility of changing his regular medication, but, despite having had this severe exacerbation, no follow-up appointment was arranged and his medication remained unchanged.
Analysis of an Exacerbation
This patient had clearly had a severe asthma exacerbation—according to ATS/ERS criteria [25]—triggered by a winter cold. Viral infections, such as respiratory syncytial virus (RSV) and human rhinovirus (HRV) infection, have long been recognized as one of the most common causes of asthma exacerbations [26, 27]. This was a pre-Covid-19 history, and now this would also be a concern, as a Covid infection could also lead to asthma-like symptoms, such as cough, although these would likely be associated with fever and muscle aches. Although vaccines for RSV, rhinovirus and Covid-19 are not currently available, primary care practitioners should endeavour to ensure their asthma patients are up to date with other vaccinations, particularly influenza and pneumococcal [28, 29].
A prescription for oral steroids at the first emergency consultation seems appropriate given the circumstances. However, a follow-up appointment should have been made for a few days/a week later to assess the patient’s status following treatment. This appointment would also be used to consider strategies to prevent further exacerbations, perhaps to alter his long-term inhaled therapy, either increasing the dose of ICS maintenance medication or replacing it with an ICS/LABA combination inhaler, reassess any triggers, review adherence, assess inhaler technique and review any currently untreated comorbidities [such as rhinitis, polyps, gastroesophageal reflux disease (GERD)and obstructive sleep apnoea].
The rationale for prescribing antibiotics is unclear from the patient’s history. There may have been suspicious chest sounds or a raised temperature remaining from the viral infection, but this is only speculation. Without clear evidence of a bacterial infection, there is little value in antibiotics for an asthma exacerbation.
But to go back one step—the patient was given advice during his annual reviews on increasing both maintenance and SABA reliever use when he recognized that symptoms were worsening. Instead, fairly typically, he seems to have only increased the SABA usage when his symptoms worsened so dramatically [30]. He had been given a personalized asthma action plan and that should have told him that he must quadruple his maintenance therapy when symptoms deteriorate and, if his SABA reliever was having little or no effect, then he should seek medical advice as soon as possible. However, many patients with asthma tolerate their symptoms [30] and wait a few days before implementing their action plan, sometimes because of pressure of work and/or the inconvenience and difficulty of arranging to have an appointment with a primary care practitioner.
When the reliever appeared not to be having any effect, his inhaler technique should have been checked by a knowledgeable person, such as an asthma nurse, pharmacist or physician, because poor inhaler technique has a direct, negative impact on treatment outcomes [31]. This patient did not appear to have had his inhaler technique assessed, even when he presented with the exacerbation, despite reporting that his reliever was not helping his symptoms.
At the second emergency visit, the suggestion that anxiety was this patient’s problem, rather than acute asthma worsening, seems a little unusual given the recent history and the low peak flow. Although anxiety can mimic asthma to an extent, it more often complicates it, and a ‘silent chest’ is a sign of very severe asthma [32], of which the doctor should have been aware. Fortunately, treatment with OCS was still provided. Again, however, a follow-up visit should have been arranged, to assess recovery, check inhaler technique, review the action plan, and possibly change the long-term medication or dose/regimen as indeed the doctor had mentioned.
Subsequent referral to an asthma specialist would also have been a reasonable option. This patient had a long history of well-controlled asthma on regular low-dose ICS with only occasional reliever use, so having such a severe exacerbation would have been sufficient cause to consider involving an expert for reassessment, especially because the clinician was unclear on what was happening. Frankly, even if the clinician suspected anxiety, a referral may have been reassuring to the patient. Referral to a specialist is appropriate when a primary care patient’s long-term condition worsens suddenly, or when a patient fails to respond to the standard GINA step care management (Fig. 1) or has poor asthma control despite showing good adherence and the correct inhalation technique [22].
Having an exacerbation requiring OCS is in itself a marker for review of the patient. The ‘Why’ this happened seems clear, a respiratory virus. The next question is ‘What’, as in what can we do to prevent this happening again? Spirometry is a more sensitive and accurate measure of lung function than peak flow, and a spirometry appointment could have been arranged, for at the very least 6 weeks after the exacerbation had resolved, to allow lung function to return to the patient’s normal values. The initial low peak flow rate on presentation could have been substantiated by poor spirometric values, which could have alerted the clinician to this really being asthma, despite the chest not sounding wheezy. Objective measurements usually drive better outcomes, although, in this case, the patient’s dyspnoea in the absence of wheeze were the driving factors that were overlooked when nothing ‘objective’ seemed to be out of place. Unfortunately, due to the current COVID pandemic, routine spirometry and even peak flow readings are now high-risk, aerosol-generating procedures and should be used with caution, perhaps even be avoided, particularly in a primary care setting.
Ideally, patients with asthma should be supported by regular assessment and a personalized asthma action plan from their primary care clinician or asthma nurse [1]. Unfortunately, patients, particularly those at either end of the asthma severity spectrum, may fail to attend asthma reviews. Patients with mild asthma may see no need to attend and those with more severe asthma may feel they are already optimally managed. Patients with poorly controlled asthma may be poorly adherent to treatment and reluctant to have this detected or challenged by their healthcare provider. Poor adherence to maintenance medication is all too common, particularly among patients with mild or intermittent symptoms and among adolescents [33, 34]. It may be intentional or unintentional, but in either case the result is poor asthma control leaving them at an increased risk of exacerbation [33]. Concern about side-effects of ICS, especially over long-term use, is another common factor in intentional non-adherence, and this should be addressed at every face-to-face interaction with a physician or asthma nurse. Regrettably, even today, after years of recommendations from asthma guidelines and reports, many patients do not receive a personalized asthma action plan that tells them what they should do if their asthma worsens [35, 36].
Opportunities for Improvement
Patients with mild asthma are at risk of severe exacerbations even after having been well-controlled for many years, as the patient in the case history clearly demonstrated [1, 6, 7]. It seems likely that the cause of the exacerbation in this case was the viral infection the patient was exposed to at home, leading to worsening of underlying inflammation of the airways and, in turn, decreasing lung function and symptoms. In vitro and in vivo studies show that HRV infection, arguably the most common cause of colds, induces the expression of a wide range of cytokines (IL-1β, IL-6, IL-11), growth factors (G-CSF, GM-CSF), and chemokines (CXCL8, CXCL5, CXCL10, RANTES) that may lead to the activation and recruitment of inflammatory cells to the airways [37, 38].
In this case, the viral infection-related increase in these pro-inflammatory cytokines and cells is likely to have been the cause of the severe exacerbation, and this may have been worsened if the patient was also atopic [39], something that isn’t clear from the history described. The patient’s atopic status should have been reviewed and recorded, either at initial diagnosis or during regular follow-up. Asthma is a chronic inflammatory disease, often involving Type 2 (Th2) inflammation and atopic/allergic asthma is the most common asthma phenotype. The underlying inflammation can vary in severity over time but it is usually present even in patients with mild asthma [6].
Measurement of fractional exhaled nitric oxide (FeNO) could also have indicated the level of inflammation in the patient’s lungs, and, like spirometry, this could also have confirmed that he was having an asthma exacerbation (rather than an anxiety attack) [9]. Low levels of FeNO (< 25 ppb for symptomatic adults with asthma, < 20 ppb for children < 12 years) indicate little or no inflammation, although this may, of course, be the result of effective treatment with ICS. In contrast, high levels (> 50 ppb for symptomatic adults, > 35 ppb for children) indicate the presence of inflammation that ICS will be highly effective against and can also confirm a suspected diagnosis of asthma [9, 40]. Although once the preserve of specialists, FeNO monitoring devices are increasingly affordable and portable, and many primary care practices have started to utilize them for routine evaluation, monitoring and diagnosis of their patients with asthma and even chronic obstructive pulmonary disease [41] (Table 1).
While a patient’s asthma is stable and well controlled, a blood eosinophil count can be a useful additional test that can be carried out in primary care. Patients with a high eosinophil count have an elevated risk of exacerbations and should be given an asthma action plan that reflects this increased risk. In a large UK study (n = 130,547), blood eosinophil count > 400/µL (versus ≤ 400/µL) increased the risk of having two or more exacerbations in the following year by more than 1.4-fold (p < 0.001) [42]. Sputum eosinophil counts are less available and more suited to specialist clinics, but management strategies using them have shown better outcomes with less total ICS used [43].
Oral steroids, on top of the patient’s ICS, should have been enough to bring the inflammation under control and rapidly bring the exacerbation to an end. The patient should have sought further medical advice when it became clear after a few days that the oral steroids were not sufficiently helping him. An appropriate follow-up would have also appreciated his lack of adequate response and the need for a more prolonged course of OCS and/or other changes in his inhaled medications to alleviate symptoms.
Subsequent management of the exacerbation highlights several opportunities for improvement. A follow-up appointment, soon after either emergency visit, would have revealed the continuing symptoms and enabled additional treatment to be given, either with a higher dose of OCS and/or the addition of a long-acting bronchodilator to the ICS, preferably in a single combination ICS/LABA inhaler. Other possible causes of his symptoms, such as pulmonary embolism or vocal cord dysfunction, especially with his history of generalized anxiety disorder, should also have been investigated and ruled out.
Another important aspect of asthma management that should have been considered is the patient’s inhaler technique [44]. This should always be checked because the effects of both the ICS and reliever would be negated by poor inhaler technique [31]. This could partly explain why the patient felt the reliever was not helping, despite taking it more often when his symptoms worsened. However, as SABA does not address the underlying flare of inflammation, and can even worsen it, reliever alone is insufficient to fully control symptoms during an exacerbation [1, 24].
The expiry date and dose counter on the inhaler, if present, should also be checked, because, for pressurised metered dose inhalers (pMDIs) without a dose counter, such as most salbutamol brands, patients can lose track of how many doses they have taken, particularly when they only use it occasionally. It is also possible that the patient may have ended up using an ‘empty’ pMDI, delivering only propellant, which would explain the lack of relief obtained. Dry powder inhalers and pMDIs with dose counters avoid this potential problem. Many inhalers need priming again, if not used for some time. Also, storing some dry powder inhalers for long periods in humid conditions, like those in a bathroom cupboard, can drastically reduce the dose delivered [45].
All asthma patients should be given a personalized asthma action plan, which should emphasize the need to increase the dose of maintenance therapy as well as the reliever, if symptoms worsen again in the future. An example of an action plan can be found at https://www.fpagc.com/tools-resources. At-risk patients should also be given a prescription for, or a supply of, oral steroids to use if this was ineffective.
Studies have shown that doubling the dose of maintenance ICS when symptoms worsen is not enough to prevent exacerbations, although quadrupling or quintupling it may have the desired effect [46, 47]. However, there appears to be a ‘window of opportunity’ in the 10 days or so leading up to an exacerbation during which timely additional ICS can help to prevent or ameliorate the exacerbation [30, 48,49,50]. Ensuring early usage of the action plan is imperative to taking advantage of the window of opportunity [30].
The New Treatment Paradigm?
In the case described earlier, the patient did not use his action plan correctly. He did not increase his ICS but did use his SABA reliever more. This had no effect on the increased inflammation, as his asthma action plan should have made clear. However, an alternative strategy to low-dose ICS plus SABA would be to utilise an anti-inflammatory reliever approach, as recommended since 2019 by the GINA asthma management report [1]. The GINA recommendation is partly based on evidence from the SYGMA 1 study in patients with mild asthma that showed that use of budesonide/formoterol as needed, as a reliever with anti-inflammatory activity, reduced asthma exacerbations by 64% in comparison to using as-needed SABA reliever alone [12]. In addition, the as-needed budesonide/formoterol approach was comparable with twice-daily low-dose budesonide maintenance therapy plus SABA in this respect, and as such could be an alternative treatment option to maintenance with low-dose ICS plus SABA [2, 11, 12].
When symptoms worsen, most patients instinctively increase their use of reliever [24, 30]. In the SYGMA studies, during this window of opportunity when symptoms first began to appear/worsen, the additional dose of ICS provided with every inhalation of the budesonide/formoterol reliever appears to have prevented many of these episodes of worsening asthma symptoms from developing into full-blown exacerbations [11, 12, 50]. In fact, use of budesonide/formoterol as needed was comparable with twice-daily low-dose budesonide maintenance therapy plus SABA in terms of preventing exacerbations in both SYGMA studies and achieved this with a much lower overall corticosteroid load [11, 12, 50].
These results have since been confirmed in the Novel START and PRACTICAL studies in which budesonide/formoterol was again used as an anti-inflammatory reliever in mild asthma, but in open-label, multicentre, randomised controlled trials [51, 52]. The 2020 update to the GINA asthma management report now recommends completely replacing SABA by use of ICS/formoterol as the preferred reliever for adults and adolescents across the spectrum of asthma severity, on the basis of safety, except in patients already taking an ICS/LABA combination that does not contain formoterol [2].
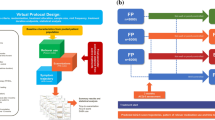
Two recent publications have proposed an even simpler version of step care management than the GINA recommendations across the asthma severity spectrum, for patients being treated with a budesonide/formoterol combination (Fig. 3) [53, 54]. For very mild asthma with infrequent symptoms, patients would take the combination only as needed. For mild to moderate asthma they would take either one or two inhalations twice daily, adding as-needed doses if they still have symptoms. For moderate to severe asthma, two doses twice daily would be the maintenance dose, again with additional doses for symptom relief as needed. In patients with moderate to severe asthma, this approach has already been proven more effective than higher-dose ICS plus SABA and conventional best practice in a range of studies [55, 56].
Proposal for new treatment paradigm based on budesonide/formoterol single inhaler combination. Reproduced with permission of the © ERS 2020: European Respiratory Journal 55 [1] 1901407; https://doi.org/10.1183/13993003.01407-2019 Published 9 January 2020
This new treatment paradigm would make step ups and step downs in therapy simpler, avoiding the need for patients to change inhaler or have more than one inhaler (probably involving different inhalation techniques), as the current ICS or ICS/LABA plus SABA regimen requires. Patients with the most severe asthma would continue with the budesonide/formoterol maintenance and anti-inflammatory reliever approach, but with the addition of whichever add-on therapy their specialist prescribes for them.
Conclusions
This Commentary along with the case study demonstrates that ‘mild’ asthma is a misnomer; patients with well-controlled mild asthma are also at risk of severe exacerbations, often triggered by viral infections like the common cold. This is something at-risk patients should be warned about at every asthma review. The myth that ‘mild asthma’ means easily treatable and non-life-threatening needs to be refuted, strongly.
The case described also highlights the limitations of SABA monotherapy as a reliever when asthma symptoms worsen due to an increase in the underlying inflammation. Although the patient was receiving maintenance low-dose ICS, the increased inflammation was not dealt with early enough in the exacerbation, due to the common situation of not actuating his action plan, and the ‘window of opportunity’ was missed. This case also highlights the need for better follow-up than the patient received or sought. Poor recognition of the exacerbation, inadequate patient education and an ignored personalised action plan together contributed to the prolonged and detrimental effects of the exacerbation.
The 2019 and 2020 GINA recommendations resolve a major paradox versus previous guidelines by recommending the use of an anti-inflammatory ICS/formoterol combination as the preferred reliever instead of SABA alone in adult and adolescent mild asthma (GINA Step 1) patients, or as an alternative treatment to low-dose ICS maintenance + as-needed SABA in GINA Step 2 patients [1, 2]. Low-dose ICS/formoterol is also the preferred reliever for patients prescribed budesonide/formoterol or beclometasone dipropionate/formoterol maintenance therapy in GINA Steps 3 to 5 patients but not for patients prescribed ICS/LABA combinations that do not contain formoterol [2].
References
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated 2019.
Global Initiative for Asthma (GINA). 2020 GINA Report, Global strategy for asthma management and prevention.
The Global Asthma Network. The Global Asthma Report 2018. Auckland, NZ.
Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–20.
Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18(11):1269–78.
Dusser D, Montani D, Chanez P, de Blic J, Delacourt C, Deschildre A, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604.
Fitzgerald JM, Barnes PJ, Chipps BE, Jenkins CR, O’Byrne PM, Pavord I, et al. The burden of exacerbations in mild asthma: a systematic review. ERJ Open. 2020;6(3):00359-2019.
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated 2018.
BTS/SIGN. British guideline on the management of asthma. London & Edinburgh: British Thoracic Society & Scottish Intercollegiate Guidelines Network; 2019.
Ding B, DiBonaventura M, Karlsson N, Ling X. A cross-sectional assessment of the prevalence and burden of mild asthma in urban China using the 2010, 2012, and 2013 China National Health and Wellness Surveys. J Asthma Allergy. 2017;545:632–43.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–87.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–76.
Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential enquiry report. London, UK; 2014.
Sadatsafavi M, Lynd L, Marra C, Carleton B, Tan WC, Sullivan S, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17(2):74–80.
Sur S, Hunt LW, Crotty TB, Gleich GJ. Sudden-onset fatal asthma. Mayo Clinic Proc. 1994;69:495–6.
James AL, Elliot JG, Abramson MJ, Walters EH. Time to death, airway wall inflammation and remodelling in fatal asthma. Eur Respir J. 2005;175(4):323–39.
Gullach AJ, Risgaard B, Hadberg Lynge T, Jabbari R, Glinge C, Haunsø S, et al. Sudden death in young persons with uncontrolled asthma—a nationwide cohort study in Denmark. BMC Pulm Med. 2015;15:35.
Gauvreau GM, Jordana M, Watson RM, Cockroft DW, O’Byrne PM. Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:1738–45.
Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990;265:3202-11.
Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β 2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 55(4):1901872.
Bloom CI, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk RJP, et al. Asthma-related health outcomes associated with short-acting β 2-agonist inhaler use: an observational UK study as part of the SABINA Global Program. Adv Ther. 2020;37(10):4190–208.
Larsson K, Kankaanranta H, Janson C, Lehtimäki L, Ställberg B, Løkke A, et al. Bringing asthma care into the twenty-first century. NPJ Prim Care Respir Med. 2020;30(1):25.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50(3):pii 1701103.
Bloom C, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk R, et al. Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: An observational UK study as part of the SABINA global program. Adv Ther. 2020;ePub ahead of print.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
Song DJ. Rhinovirus and childhood asthma: an update. Korean J Pediatr. 2016;59(11):432–9.
Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005;116:267–73.
Vasileiou E, Sheikh A, Butler C, El Ferkh K, von Wissmann B, McMenamin J, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(8):1388–95.
Kaplan A, Pierre Arsenault, Aw B, Brown V, Fox G, Grossman R, et al. Vaccine strategies for prevention of community-acquired pneumonia in Canada. Who would benefit most from pneumococcal immunization? Can Fam Physician. 2019;65(9):625–33.
Partridge M, van der Molen T, Myrseth S-E, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pum Med. 2006:13.
Price DB, Román-Rodríguez M, McQueen RB, Bosnic-Anticevich S, Carter V, Gruffydd-Jones K, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–81.
Hetzel M, Modell M. Asthma. Occas Pap R Coll Gen Pract. 1992;58:14–20.
Beasley R, Weatherall M, Shirtcliffe P, Hancox RJ. Combination corticosteroid/b-agonist inhaler as reliever therapy: a solution for intermittent and mild asthma? J Allergy Clin Immunol. 2014;133:39–41.
Kaplan A, Price D. Treatment adherence in adolescents with asthma. J Asthma Allergy. 2020;13:39–49.
Gupta S, Price C, Agarwal G, Chan D, Goel S, Boulet LP, et al. The electronic asthma management system (eAMS) improves primary care asthma management Eur Respir J. 2018;53(4):pii 1802241.
Kouri A, Kaplan A, Boulet LP, Gupta S. New evidence-based tool to guide the creation of asthma action plans for adults. Can Fam Physician. 2019;65:103–6.
Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34(2):192–203.
Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, D. P. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85-95.
Xatzipsalti M, Psarros F, Konstantinou G, Gaga M, Gourgiotis D, Saxoni-Papageorgiou P, et al. Modulation of the epithelial inflammatory response to rhinovirus in an atopic environment. Clin Exp Allergy. 2008;38(3):466–72.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Funston W, Higgins B. Improving the management of asthma in adults in primary care. The Practitioner. 2014;258(1776):15–9.
Price D, Wilson AM, Chisholm A, Rigazio A, A. B, Thomas M, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1-12.
Fleming L, Wilson N, Regamey N. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2012;67:193–8.
Bosnic-Anticevich S. Asthma management in primary care: caring, sharing and working together. Eur Respir J. 2016;47:1943–6.
Janson C, Lööf T, Telg G, Stratelis G, Nilsson F. Difference in resistance to humidity between commonly used dry powder inhalers: an in vitro study. npj Prim Care Respir Med. 2016;26:16053.
McKeever T, Mortimer K, Wilson A, Walker S, Brightling C, Skeggs A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med. 2018;378(10):902–10.
Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378(10):891–901.
van der Valk RJ, Malizia V, Antona R, Corsello G, La Grutta S. The value of FeNO measurement in childhood asthma: uncertainties and perspectives. Allergy. 2012;67:265–71.
Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O’Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160:594-99.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zheng J, Gustafson P, et al. Effect of a single day of increased as-needed budesonide–formoterol use on short-term risk of severe exacerbations in patients with mild asthma: a post hoc analysis of the SYGMA 1 study. Lancet Respir Med. 2020.
Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–28.
Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380(21):2020–30.
Beasley R, Braithwaite I, Semprini A, Kearns C, Weatherall M, Harrison TW, et al. ICS-formoterol reliever therapy stepwise treatment algorithm for adult asthma. Eur Respir J. 2020;55(1):1901407.
Bruce P, Hatter L, Beasley R. Anti-inflammatory reliever therapy in asthma: The evidence mounts but more is needed. Respirology. 2020;ePub ahead of print.
Jenkins C, Bateman ED, Sears MR, O’Byrne PM. What have we learnt about asthma control from trials of budesonide/formoterol as maintenance and reliever? Respirology. 2020;25(8):804–15.
Rogliani P, Ritondo BL, Ora J, Cazzola M, Calzetta L. SMART and as-needed therapies in mild to severe asthma: a network meta-analysis. Eur Respir J. 2020;ePub ahead of print.
Acknowledgements
Funding
Alan Kaplan received no funding for writing this commentary but the journal’s Rapid Service and Open Access Fees were funded by AstraZeneca.
Medical Writing and Editorial Assistance
The author would like to thank David Candlish of inScience Communications, Springer Healthcare Ltd, UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given his approval for this version to be published.
Disclosures
Alan Kaplan has participated in advisory boards or speaker bureaus for AstraZeneca, Behring, Covis, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Sanofi, Trudel, Behring, Teva, NovoNordisk, Merck Frosst and Pfizer.
Compliance with Ethics Guidelines
This article does not contain data from any studies with human participants or animals performed by the author.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during its development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kaplan, A. The Myth of Mild: Severe Exacerbations in Mild Asthma: An Underappreciated, but Preventable Problem. Adv Ther 38, 1369–1381 (2021). https://doi.org/10.1007/s12325-020-01598-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01598-2