Abstract
Introduction
Globally, individuals with asthma tend to overrely on short-acting β2-agonists (SABAs) and underuse inhaled corticosteroids, thereby undertreating the underlying inflammation. Such relief-seeking behavior has been reinforced by long-standing treatment guidelines, which until recently recommended SABA-only use for immediate symptom relief. We aimed to describe the current burden of SABA use among European individuals with asthma within the SABA use IN Asthma (SABINA) program.
Methods
Prescription and/or dispensing data during 2006–2017 from electronic medical records and/or national patient registries in the United Kingdom (UK), Germany, Italy, Spain, and Sweden were analyzed. Individuals aged at least 12 years old with a current asthma diagnosis and no other chronic respiratory conditions were included. Asthma treatment step and severity were based on treatment guidelines in use in each individual country. The proportion of individuals prescribed SABA was measured during a 12-month period. SABA overuse was defined as at least three SABA canisters per year.
Results
More than one million individuals with asthma were included across five European countries. Overall, the majority of individuals were over 45 years of age, except in Sweden (mean age 27.6 years) where individuals aged over 45 years were excluded to avoid a potential chronic obstructive pulmonary disease co-diagnosis. The study population was predominantly female (55–64%), except in the UK (46%). The prevalence of SABA overuse was 9% in Italy, 16% in Germany, 29% in Spain, 30% in Sweden, and 38% in the UK. In the UK, SABA overuse was greater in individuals with moderate-to-severe asthma versus individuals with mild asthma (58% versus 27%, respectively), while SABA overuse was similar in individuals with both mild (9–32%) and moderate-to-severe (8–31%) asthma in the other European countries.
Conclusions
The findings of this study from the SABINA program show that SABA overuse (at least three canisters per year) is common across Europe, despite the different healthcare and reimbursement policies of each country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Despite the availability of effective asthma treatments, some individuals are poorly controlled because of overreliance on short-acting β2-agonists (SABAs) and underuse of inhaled corticosteroids. |
As a result of growing evidence that SABA overreliance is associated with an increased risk of asthma-related exacerbations and mortality, a global view of SABA prescriptions is needed to better understand the public health burden of SABA overuse in asthma management. |
As part of the SABINA (SABA use IN Asthma) program, this study aimed to provide an overview of similarities and differences in SABA prescription trends across five European countries (UK, Germany, Italy, Spain, and Sweden) in over one million individuals. |
What was learned from the study? |
SABA overuse (≥ 3 canisters per year) occurred in approximately one-third of mild, moderate, and severe individuals with asthma across Europe, despite the different healthcare and reimbursement policies of each country. |
These findings indicate that there is a significant group of individuals who are not optimally treated according to current recommendations. |
Following the recent 2019 Global Initiative for Asthma (GINA) update, which no longer recommends treating adolescents and adults with as-needed SABA alone for symptom relief, changes in physician and patient behaviors towards SABA use, and updates to national healthcare policies, are required to ensure that individuals with asthma are not exposed to SABA alone in the treatment of their asthma. |
Introduction
Asthma is a chronic, heterogeneous, fluctuating, inflammatory disease of the airways that is estimated to affect 339 million people worldwide [1]. In Europe, over 8% of adults have asthma, with the highest prevalence found in the United Kingdom (UK) and Sweden [1,2,3]. Anti-inflammatory maintenance treatment with low-dose inhaled corticosteroids (ICS) is the cornerstone of asthma treatment [4]. As-needed short-acting β2-agonists (SABAs) have been traditionally prescribed for symptom relief, with or without daily maintenance treatment, depending on the level of asthma severity [5, 6]. However, evidence on the safety risks associated with high SABA use has grown substantially in the last few years [7,8,9,10]. In parallel, clinical trials have reported the superiority of anti-inflammatory reliever therapy with as-needed ICS–formoterol versus as-needed SABA in terms of symptom control and reduction in exacerbation risk [11,12,13,14,15]. Consequently, the 2019 Global Initiative for Asthma (GINA) recommendations have eliminated SABA monotherapy in step 1 and instead recommend as-needed low-dose ICS–formoterol as the preferred reliever in steps 1–2. In addition, in GINA steps 3–5, low-dose ICS–formoterol is the preferred reliever for patients prescribed ICS–formoterol maintenance and reliever therapy [16].
With this knowledge, SABA overreliance is now an even greater concern. However, it will be difficult to change this overreliance, linked to decades of patient behavior and guidelines recommending, until very recently, SABA use for immediate symptom relief and as the first treatment for newly diagnosed mild intermittent asthma [17, 18]. When symptoms worsen, most individuals with asthma overrely on their SABA inhaler for symptomatic relief, often at the expense of ICS maintenance therapy [19,20,21]. As SABAs have no anti-inflammatory effect [22, 23], they neither treat the underlying inflammation nor protect against exacerbations. The continued reliance on SABA relievers leaves individuals across all asthma severities at risk of preventable attacks whether adherent or not to their maintenance controller [11, 14]. Indeed, in the UK, SABA overuse and the relative underuse of ICS was highlighted as one of the underlying reasons for preventable asthma attacks and deaths [24].
There are limited data on SABA and ICS prescription trends in European countries, and a pan-European view of potential SABA overuse and relative ICS underuse is lacking. The SABA use IN Asthma (SABINA) program [25] was therefore initiated to describe the global extent of SABA and ICS use in asthma and its clinical consequences. For the purpose of this analysis, which was to understand the current state of asthma reliever prescriptions relative to recent treatment recommendations, we aimed to provide an overview of the similarities and differences in SABA prescription trends only, for individuals with asthma across European countries.
Methods
Study Design
The SABINA program encompasses three main pillars: SABINA I (a retrospective, observational database study with expanded objectives in the UK), SABINA II (a distributed harmonized set of multicountry retrospective observational database studies in Europe and Canada), and SABINA III (a prospectively collected multicountry cross-sectional study in 25 countries). Full details of the SABINA program are described elsewhere [25]. In this study, prescription data generated from the European arms of SABINA—SABINA I (UK) and SABINA II (Italy, Germany, Spain, and Sweden)—were analyzed. On the basis of data availability, individual country data were obtained from electronic medical records and/or national patient registries as shown in Table 1.
Patient Population
Individuals aged at least 12 years old with a current asthma diagnosis were included in the study. In Sweden, the study population included all individuals with asthma who collected at least two drugs for obstructive lung disease (ATC R03) from pharmacies in a 1-year period. In addition, the upper age limit of 45 years was applied in Sweden to ensure that individuals with chronic obstructive pulmonary disease (COPD) were excluded, a validated proxy for asthma [26]. The definition of current asthma varied across studies (Table 1). For instance, in most countries, current asthma was defined as an asthma diagnosis code within 1 or 3 years before the index date (date on which the individual first entered the study); however, in Sweden, it was defined as at least two prescriptions for a chronic obstructive lung disease medication within 12 months of study entry. All studies required individuals with asthma to have data for at least 12 months before and after study entry. Study periods varied between 2006 and 2018. However, all countries included recent data (2016–2018) on SABA use, while some countries, such as Sweden and the UK, included data from as early as 2006.
The studies performed as part of the SABINA program were each approved by the institutional review board of the ethics committee in their individual country and were conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Good Clinical Practice (GCP), and the applicable legislation on non-interventional studies and/or observational studies.
Study Measures
SABA Use
The percentage of individuals with asthma who were prescribed SABA was recorded during a 12-month period. SABA use was categorized by the number of canister prescriptions per year (0–2, 3–6, 7–12, or 13 and more). According to the guidelines, appropriate use of SABA is considered as fewer than three puffs per week, which is equivalent to fewer than 150 puffs/actuations per year or at most two prescribed canisters per year. For consistency across all countries in the SABINA program, one SABA canister was assumed to contain 150 inhalations [25]. However, in Germany and Spain, one canister was defined as containing 200 inhalations based on a preliminary analysis of the data, which showed that nearly all prescribed SABA canisters contained 200 doses. On the basis of this assumption and allowing for individuals to have multiple SABA inhalers at the same time, SABA overuse was defined as prescription/dispensing of at least three canisters per year.
Treatment Step and Asthma Severity
Individuals with asthma were categorized into treatment steps (1–5) and severity (mild, steps 1–2; moderate–severe, steps 3–5) by their ICS prescriptions (low, medium, or high) in the year prior to their index date on the basis of the 2016 British Thoracic Society (BTS) guidelines [6] (low-dose ICS, 400–799 µg beclometasone dipropionate [BDP] equivalent; medium-dose ICS, 800–1599 µg; high-dose ICS, at least 1600 µg) for the UK or 2018 GINA recommendations [5] (low-dose ICS, 200–500 µg BDP equivalent; medium-dose ICS, 500–1000 µg BDP equivalent; high-dose ICS, more than 1000 µg BDP equivalent) for the remaining countries.
Statistical Analysis
Baseline characteristics were described as mean (standard deviation, SD) for continuous variables and absolute and relative frequencies for categorical variables. Descriptive statistics were provided for the SABA prescription data.
Results
Patient Baseline Demographics and Clinical Characteristics
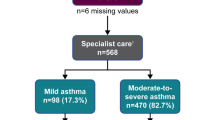
Overall, 1.06 million individuals with asthma were included across the countries. On average, most individuals were at least 45 years of age, except in Sweden where the mean age of individuals was 27.6 years (Table 2). Most individuals with asthma were female, except in the UK, where male individuals constituted the majority (54%). The severity of asthma (as determined by treatment step) varied across countries. In Germany and the UK, most of the study population included were treated as having mild asthma (60% and 65%, respectively). In Italy and Spain, most individuals had moderate-to-severe asthma (63% and 73%, respectively), while in Sweden, they were distributed almost equally across severities.
SABA Use
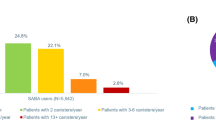
The characteristics of SABA use are summarized in Table 3 and Fig. 1. The mean number of annual SABA canisters used varied across countries. Overall, the prevalence of SABA overuse was 9% in Italy, 16% in Germany, 29% in Spain, 30% in Sweden, and 38% in the UK. In the UK, SABA overuse was greater in individuals with moderate-to-severe asthma versus mild asthma (58% versus 27%). Overall, SABA overuse was similar in individuals with mild (9–32%) and moderate-to-severe (8–31%) asthma in the other European countries.
SABA use in individuals with mild and moderate-to-severe asthma across European countries. In Germany, analysis was based on GP-treated individuals only (n = 29,636). Treatment steps were based on GINA 2018 for all countries, except the UK (BTS 2016). BTS British Thoracic Society, GINA Global Initiative for Asthma, GP general practitioner, SABA short-acting β2-agonist
Discussion
SABINA I and II assessed SABA prescription trends across European countries in over a million individuals with asthma. Overall, with the exception of Italy, SABA use was common across all asthma severities, and our data suggest that approximately one-third of individuals are overusing SABA.
Our findings are generally consistent with those of other studies in a European population using the same SABA canister cutoff, but different study designs. In the cross-sectional ASTHMAPOP survey among 15,587 adults in France, 28.3% of patients reported using at least three SABA canisters per year [27]. Similarly, a Polish study of pharmacy prescription records of 91,673 adult patients observed that 29–37% of patients with asthma were prescribed at least three SABA canisters per year [28]. Differences in national healthcare and medication reimbursement policies may impact medication prescribing practices and related clinical outcomes, and this needs to be taken into consideration for the present study findings. For instance, SABA is available without a prescription in Italy, Spain, and the UK (emergency access only), but not in Germany and Sweden. Notably, availability of SABA without a prescription has been linked to undertreatment of asthma (relative to the relevant guidelines recommendations) and infrequent consultations with physicians [29]. Moreover, it has been shown that without regular medical supervision, patients are more likely to overuse SABA [30]. Although SABA medication is not available without a prescription in many countries in the SABINA program, such findings have far-reaching global implications. Consequently, an improved understanding of this population of individuals with asthma who purchase SABA, especially their attitudes and beliefs about asthma and its treatment, is essential. A recent real-world, cross-sectional observational study in Australia, where SABA medication can be purchased from the community pharmacist without a prescription, identified a cohort of individuals with suboptimal asthma control, co-existing allergic rhinitis, and poor ICS adherence who were SABA overusers (used SABA more than twice a week in the past 4 weeks) [31]. Addressing such findings in primary care is critical to address the issue of SABA overuse. Because the availability of SABA without prescription was not taken into account in our analysis, actual SABA use may be even higher in countries that do not require a prescription to purchase SABA medication. This finding is particularly apparent in the assessment of SABA prescriptions in Italy in our study, where SABA overuse was less evident compared with other European countries. From initial market research, it is understood that a relatively large proportion of individuals with asthma obtain SABA inhalers without prescription, which could explain the low prevalence of SABA overuse in prescription databases. Further analyses are now planned to investigate this possibility in Italy.
Although similar SABA use trends were observed despite differences across national policies, data sources, and study designs, there were some differences in relation to SABA use and asthma severity. Among the five countries analyzed, the UK had the lowest percentage of individuals receiving treatment for moderate-to-severe asthma, and the highest average number of annual SABA prescriptions. This could indicate that individuals with uncontrolled asthma in the UK are more likely to be prescribed SABA rather than being reviewed and prescribed an increased dose of their maintenance therapy as recommended by guidelines [6]. Indeed, according to a recent survey among individuals with asthma in the UK, over 60% of respondents indicated that they did not receive basic asthma care, and approximately 20% of respondents did not receive an annual asthma review [32].
Exposure and adherence to maintenance ICS will likely impact SABA use, and it is, therefore, important to put these findings into the context of ICS exposure. SABA overuse was greater in individuals with moderate-to-severe asthma (up to 58%) compared with individuals with mild asthma, suggesting a greater degree of poor asthma control in these individuals despite receiving maintenance ICS to treat the underlying inflammation. Similar results were reported from the recent Polish pharmacy prescription study, in which patients on a higher treatment step received more SABA prescriptions compared with patients on lower treatment steps [28]. It is possible that low ICS adherence is a driving factor or that maintenance therapy exposure is simply an indication of disease severity and of an increased likelihood of the presence of symptoms that require SABA. More detailed analyses in the individual SABINA studies are underway to investigate the potential association of ICS and SABA use.
Overall, our findings indicate that there is considerable SABA use—and indeed SABA overuse—among individuals across Europe, which puts them at risk of adverse outcomes. Changes in physician and patient behaviors towards SABA use, active engagement in adapting 2019 GINA recommendations to local guidelines, and updates to national healthcare policies will be needed to ensure that individuals with asthma are not unnecessarily exposed to SABA alone in the treatment of their inflammatory disease. Physicians should encourage the appropriate use of ICS, eliminate SABA monotherapy as per evidence-based recommendations, challenge patient attitudes and practices around SABA use, and ensure that individuals understand that asthma is an inflammatory condition. Importantly, individuals should be trained on the appropriate use of therapy [33] and the technical use of inhalers [34]. Asthma education programs discouraging unregulated SABA use should be given due consideration. Education, together with regular medical reviews and written personal asthma action plans, is advocated in guidelines and has been shown to improve health outcomes for individuals with asthma [35]. Therefore, a drive to implement personal actions plans, describing how patients may recognize a deterioration in asthma control and what steps should be taken to re-establish control [36], could further assist in curbing SABA overuse. Other healthcare professionals, such as nurses and pharmacists, could also provide training and support to patients. Pharmacists can also play an integral role as they are in the unique position of being able to initiate conversations about SABA overuse and can spend time educating patients and answering any questions [31].
Potential limitations of this analysis relate to the observational nature of the included studies and the use of medical databases that were not necessarily designed for research purposes. However, the results were comparable to those from the UK arm of SABINA, which used research-quality data from the Clinical Practice Research Datalink [37]. This increases confidence in the robustness of the data across the rest of the countries. Additionally, the upper age limit of 45 years in Sweden may have led to more individuals with severe asthma being excluded from the analysis. However, the algorithm that was used to identify individuals with asthma based on pharmacy collection of drugs (ATC R03) for obstructive lung disease was shown to be a suitable proxy for asthma diagnosis in this age group in validation studies from Sweden [26]. This restriction was not necessary in other countries, where other validated algorithms were used (e.g., the UK) [38], and where sensitivity analyses were conducted to exclude individuals with a COPD co-diagnosis. Despite these differences, similar overall trends were seen across the countries, indicating that the age restriction applied to Sweden may have had limited impact on the results. Of note, dispensed or prescribed SABA may not always equal the medication taken and may lead to an overestimation of actual SABA overuse. Automatic repeat prescriptions, or simultaneous prescriptions of multiple SABA canisters, may result in individuals having more SABA inhalers in their possession, which they may not necessarily use. From clinical experience, we know that individuals with asthma typically have multiple SABA inhalers such that there is at least one inhaler in each of their surroundings (e.g., home, office, car). This is done so that individuals with asthma have immediate access to their reliever in the event of a sudden worsening in symptoms. Despite these limitations, the current manuscript assessed a large asthma population across five countries and provides important insights regarding SABA use and the extent of SABA overuse. The use of standardized thresholds for SABA overuse in the SABINA program enabled comparisons across countries, which were previously limited by the varying SABA overuse thresholds used across published studies [39,40,41]. The next steps for the program are to describe SABA use across additional countries, and to investigate the association of SABA use, maintenance ICS therapy, and clinical outcomes with healthcare resource use. Additionally, further context around patient’s quality of life and patient’s adherence behaviors will provide important insights into how to better interpret the program findings and how to potentially decrease SABA use in the future. More in-depth analyses should be performed in those countries that are involved in the SABINA program.
Conclusions
There is a growing body of evidence that high SABA use is associated with increased risk of adverse asthma-related outcomes. Individuals across all asthma severities remain at risk of exacerbations when they continue to overrely on SABA at the expense of ICS, leaving the underlying inflammation undertreated. Data from the SABINA program demonstrated that a considerable proportion of individuals with asthma across Europe are using at least three SABA canisters per year, indicating that there is a significant group of individuals who are currently not optimally treated according to the 2019 GINA recommendations. The combination of an ICS/fast-onset but long-acting β2-agonist will be an effective patient-friendly strategy for improving adherence to evidence-based guidelines. Further work to support changes to national treatment guidelines and, therefore, ensure successful implementation of the latest GINA recommendations is currently underway.
References
Global Asthma Report 2018. http://www.globalasthmareport.org. Accessed 27 Sept 2019.
Selroos O, Kupczyk M, Kuna P, et al. National and regional asthma programmes in Europe. Eur Respir Rev. 2015;24(137):474–83.
Gibson GJ, Loddenkemper R, Lundback B, Sibille Y. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J. 2013;42(3):559–63.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2019. https://ginasthma.org/gina-reports/. Accessed 27 Sept 2019.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. https://ginasthma.org/gina-reports/. Accessed 11 July 2019.
British Thoracic Society. Scottish Intercollegiate Guidelines Network: British guideline on the management of asthma. https://www.brit-thoracic.org.uk/document-library/guidelines/asthma/btssign-asthma-guideline-2016/. Accessed 21 July 2019.
Stanford RH, Shah MB, D’Souza AO, Dhamane AD, Schatz M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–7.
Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur Respir J. 1994;7(9):1602–9.
Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care. 1994;149(3):604–10.
Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020. https://doi.org/10.1183/13993003.01872-2019.
Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide–formoterol as needed for mild asthma. N Engl J Med. 2019;380(21):2020–30.
O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care. 2005;171(2):129–36.
Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–36.
O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide–formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–76.
Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–87.
Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J. 2019;53(6):1901046.
Beasley R, Bird G, Harper J, Weatherall M. The further paradoxes of asthma management: time for a new approach across the spectrum of asthma severity. Eur Respir J. 2018;52:1800694.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50(3):1701103.
FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–40.
Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
Sadatsafavi M, Tavakoli H, Lynd L, FitzGerald JM. Has asthma medication use caught up with the evidence? A 12-year population-based study of trends. Chest. 2017;151(3):612–8.
Hancox R, Aldridge R, Cowan J, et al. Tolerance to beta-agonists during acute bronchoconstriction. Eur Respir J. 1999;14(2):283–7.
Zhao H, Li R, Lv Y, et al. Albuterol inhalation increases FeNO level in steroid-naive asthmatics but not COPD patients with reversibility. Clin Respir J. 2017;11(3):328–36.
Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe. 2015;11(1):14–24.
Cabrera CS, Nan C, Lindarck N, Beekman M, Arnetorp S, van der Valk RJP. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting beta2-agonist use in asthma. Eur Respir J. 2019. https://doi.org/10.1183/13993003.01858-2019.
Örtqvist AK, Lundholm C, Wettermark B, Ludvigsson JF, Ye W, Almqvist C. Validation of asthma and eczema in population-based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf. 2013;22(8):850–60.
Raherison-Semjen C, Izadifar A, Russier M, et al. Late breaking abstract—asthma prevalence and management in adults in France in 2018: ASTHMAPOP survey. Eur Respir J. 2018;52(suppl 62):OA292.
Kupczyk M, Barg W, Bochenek G, et al. Late breaking abstract—overprescription of short-acting beta2-agonists in asthma management? Pharmacy reports from 91,673 patients in Poland. Eur Respir J. 2019;54(suppl 63):OA2107.
Gibson P, Henry D, Francis L, et al. Association between availability of non-prescription beta 2 agonist inhalers and undertreatment of asthma. BMJ. 1993;306(6891):1514–8.
Kuschner WG, Hankinson TC, Wong HH, Blanc PD. Nonprescription bronchodilator medication use in asthma. Chest. 1997;112(4):987–93.
Azzi EA, Kritikos V, Peters MJ, et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta2 agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9(8):e028995.
AsthmaUK. Falling through the gaps: why more people need basic asthma care. Annual Asthma Survey report 2017. https://www.asthma.org.uk/share/?rid=7044. Accessed 27 Sept 2019.
Breekveldt-Postma NS, Koerselman J, Erkens JA, van der Molen T, Lammers JW, Herings RM. Treatment with inhaled corticosteroids in asthma is too often discontinued. Pharmacoepidemiol Drug Saf. 2008;17(4):411–22.
Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the aerosol drug management improvement team. Respir Med. 2006;100(9):1479–94.
Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;1:Cd001117.
Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622–39.
Clinical Practice Research DataLink (CPRD). https://www.cprd.com/home. Accessed 17 Oct 2019.
Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD). BMJ Open. 2017;7(8):e017474.
Belhassen M, Nibber A, Van Ganse E, et al. Inappropriate asthma therapy—a tale of two countries: a parallel population-based cohort study. NPJ Prim Care Respir Med. 2016;26:16076.
Elkout H, Helms PJ, Simpson CR, McLay JS. Changes in primary care prescribing patterns for paediatric asthma: a prescribing database analysis. Arch Dis Child. 2012;97(6):521–5.
Tavakoli H, Mark FitzGerald J, Lynd LD, Sadatsafavi M. Predictors of inappropriate and excessive use of reliever medications in asthma: a 16-year population-based study. BMC Pulm Med. 2018;18(1):33.
Acknowledgements
We would like to acknowledge all the researchers involved in the SABINA program.
Funding
The SABINA program is funded by AstraZeneca. AstraZeneca also funded the journal’s Rapid Service Fees for publication of this manuscript and the open access fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by Urvashi Nikte, MDS and Michelle Rebello, PhD, of Cactus Communications (Mumbai, India), which was funded by AstraZeneca and conducted in accordance with the GPP3 guidelines (http://www.ismpp.org/gpp3).
Disclosures
Christer Janson has received payments for educational activities from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Teva, and has served on advisory boards arranged by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Teva. Andrew Menzies-Gow has attended advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, and Teva. He has received speaker fees from AstraZeneca, Novartis, Roche, and Teva. He has participated in research with AstraZeneca for which his institution has been remunerated and has attended international conferences with Teva. He has had consultancy agreements with AstraZeneca, Sanofi, and Vectura. Cassandra Nan is an employee of AstraZeneca and holds shares in AstraZeneca and GSK. Javier Nuevo is an employee of AstraZeneca. Alberto Papi reports grants, personal fees, non-financial support, and other from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, and GlaxoSmithKline; personal fees from Sanofi/Regeneron; personal fees and non-financial support from Zambon; grants, personal fees, non-financial support, and other from Teva; personal fees and non-financial support from Novartis; personal fees, non-financial support, and other from Mundipharma; personal fees and non-financial support from Almirall; grants, personal fees, and non-financial support from Menarini; and grants from Maugeri and Fondazione Chiesi Farmaceutici, outside the submitted work. Jennifer K Quint’s research group has received funds from The Health Foundation, MRC, British Lung Foundation, IQVIA, Chiesi, and Asthma UK outside the submitted work; grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Bayer, and Insmed. Santiago Quirce has been a consultant for ALK, AstraZeneca, GSK, Mundipharma, Novartis, Sanofi, and TEVA and received lecture fees from AstraZeneca, Chiesi, GSK, Leti, Mundipharma, and Novartis. Claus F Vogelmeier reports grants and personal fees from AstraZeneca, Grifols, GlaxoSmithKline, Novartis, and Boehringer Ingelheim and personal fees from CSL Behring, Chiesi Farmaceutici, MedUpdate, Menarini, and Nuvaira, outside the submitted work.
Compliance with Ethics Guidelines
The studies carried out under the SABINA program conformed with ethical principles that are consistent with the Declaration of Helsinki of 1964 and its later amendments, ICH GCP, GPP3, and the applicable legislation on non-interventional studies and/or observational studies.
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11590857.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Janson, C., Menzies-Gow, A., Nan, C. et al. SABINA: An Overview of Short-Acting β2-Agonist Use in Asthma in European Countries. Adv Ther 37, 1124–1135 (2020). https://doi.org/10.1007/s12325-020-01233-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01233-0