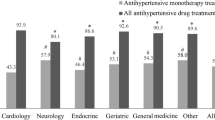
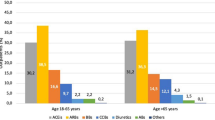
Abstract
Introduction
We evaluated the prevalence and control of dyslipidemia in a wide sample of patients referred to our ESH “Hypertension Excellence Centre” for high blood pressure (BP). Furthermore, we evaluated the role of adiposity on the serum lipid profile.
Methods
Observational study on 1219 consecutive outpatients with valid ambulatory BP monitoring (ABPM) referred for high BP. Patients with body mass index (BMI) ≥ 25 kg/m2 were defined as overweight/obese (OW/OB). Dyslipidemia and the control rates of low-density lipoprotein cholesterol (LDLc) were defined according to the 2016 ESC/EAS Guidelines.
Results
Mean age: 56.5 ± 13.7 years. Male prevalence: 55.6%. OW/OB patients were 70.2%. The prevalence of dyslipidemia was 91.1%. Lipid-lowering drugs were taken by 23.1% of patients. Patients with controlled LDLc comprised 28.5%, while BP was controlled in 41.6% of patients. Only 12.4% of patients had both 24-h BP and LDLc controlled at the same time. The higher the cardiovascular (CV) risk was, the lower was the rate of LDLc control (p < 0.001). Patients in secondary prevention had worse LDLc control than patients in primary prevention (OR 3.5 for uncontrolled LDLc, p < 0.001). OW/OB showed a more atherogenic lipid profile, characterized by lower high-density lipoprotein cholesterol (HDLc) (p < 0.001), higher non-HDLc (p = 0.006), higher triglycerides (p < 0.001), higher non-HDLc/HDLc (p < 0.001) and higher (non-HDLc + non-LDLc) (p < 0.001).
Conclusion
Dyslipidemia is still too often neglected in hypertensives, especially in patients at higher CV risk. OW/OB hypertensives have a “double-trouble” atherogenic lipid pattern likely driven by adiposity. We encourage a comprehensive evaluation of the lipid profile in all hypertensives, especially if they are OW/OB, to correctly assess their CV risk and improve their management.
Funding
Article processing charges funded by Servier SpA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is the major preventable cause of cardiovascular disease (CVD) and all-cause death worldwide [1]. It is often accompanied by several CV risk factors, such as dyslipidemia, which contributes to atherosclerosis in hypertensives [2]. The prevalence of hypercholesterolemia progressively increases from subjects with optimal to those with normal, high-normal and elevated blood pressure (BP) [3]. Non-pharmacologic interventions are fundamental as the first therapeutic approach [4,5,6,7], while statins represent the cornerstone of drug therapy for hypercholesterolemia. The benefit of adding a statin to anti-hypertensive treatment is well established in hypertensive patients with or without previous CV events [8]. Treating hypertension reduces CVD risk by only 20–25%, while data suggest that effective treatment of both hypertension and hypercholesterolemia would reduce this risk by > 50% [9]. Overweight and obesity are closely associated with high BP, and about 75% of essential hypertensives have been estimated to be overweight/obesity-dependent [10]. Higher body mass index (BMI) is associated with a worse BP profile and cardiac organ damage [11, 12]. At the same time, strong associations between adiposity and lipid profiles have been reported [13]. Obese patients with concomitant hypertension and dyslipidemia are less likely to achieve BP and lipid control [14]. A global CV risk assessment is fundamental for the correct management of patients in daily practice, and several international guidelines provide guidance to physicians for this purpose [15, 16]. Despite existing population strategies and the wide range of drugs available, the control rates of hypertension and dyslipidemia are still poor, especially in primary prevention [17]. The aim of our study was to evaluate the prevalence and control rates of dyslipidemia, according to the individual CV risk, in a wide sample of hypertensives referred to a “Hypertension Excellence Centre” of the European Society of Hypertension (ESH) for high BP. Then, we also evaluated the role of adiposity on the lipid profile in this population.
Methods
Study Design and Population
We conducted a retrospective observational study on 1219 consecutive outpatients referred to our “Hypertension Excellence Centre” of the ESH, from May 2013 to May 2018, for a first clinical evaluation of high BP. Most patients were referred to our Hypertension Centre by general practitioners and a minority by other specialists. Inclusion criteria were the following: age ≥ 18 years, no changes in drug therapy in the previous 3 months, valid 24-h ambulatory BP monitoring (ABPM) and a full lipid profile [total cholesterol (TC), high-density lipoprotein cholesterol (HDLc) and triglycerides (TG)] both performed within the previous month. This analysis focused on the parameters that patients had before attending the first clinical visit to our Centre. Patients referred to our Centre for reasons other than high BP (e.g., hypercholesterolemia) were not included in this study. All participants gave their informed written consent, and clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. This study was approved by the local institutional ethics committee (INRCA Ethics Committee).
Clinical Parameters
We considered the following clinical parameters: patient medical history, anthropometric measurements, laboratory tests, ABPM parameters and drug therapy. Among the anthropometric parameters, we evaluated BMI and waist circumference (WC) as indices of adiposity. In particular, BMI was defined as the body mass divided by the square of the body height and was expressed in units of kg/m2, and WC was measured to the nearest 0.1 cm at the midpoint between the lowest rib and the iliac crest with patients unclothed to the waist. Patients with BMI ≥ 25 kg/m2 were considered overweight/obese (OW/OB).
Fasting blood samples were collected in the morning in all patients. We evaluated the following laboratory parameters: TC, HDLc, TG, calculated low-density lipoprotein cholesterol (LDLc), non-HDLc, non-HDLc-to-HDLc ratio (non-HDLc/HDLc), (non-HDLc + non-LDLc), glycemia, creatinine and estimated glomerular filtration rate (eGFR). LDLc was calculated using a modified Friedewald method proposed by Martin et al. [18]. Non-HDLc was calculated by subtracting the HDLc from TC. (Non-HDLc + non-LDLc) was calculated by subtracting (HDLc + LDLc) from TC. We used this parameter as an estimate of the cholesterol contained in the very low-density lipoprotein and intermediate-density lipoprotein cholesterol. The eGFR was estimated using the CKD-EPI equation.
Minimum quality criteria for a satisfactory ABPM recording were based on recommendations by Omboni et al. [19]. Patients on anti-hypertensive therapy with mean 24-h BP < 130/80 mmHg were defined as controlled by therapy [20].
The Systemic Coronary Risk Estimation (SCORE) and its derived version validated also for older people (SCORE O.P.) were used to estimate the individual 10-year risk of fatal CVD [21, 22]. In particular, patients with a calculated SCORE ≥ 10% were defined at very high risk; patients with a calculated SCORE ≥ 5% and < 10% were defined at high risk; patients with a calculated SCORE ≥ 1% and < 5% were defined at moderate risk; patients with a calculated SCORE < 1 were defined at low risk.
Dyslipidemia was defined by the presence of at least one of the following findings: use of lipid-lowering drugs, TC > 190 mg/dl, HDLc < 40 mg/dl in males and < 48 mg/dl in females, TG > 150 mg/dl, LDLc levels above the targets recommended by the 2016 ESC/EAS Guidelines for the Management of Dyslipidemia, on the basis of the individual CV risk (115 mg/dl for patients at low-to-moderate risk; 100 mg/dl for patients at high risk; 70 mg/dl for patients at very high risk), non-HDLc ≥ 145 mg/dl in patients at low-to-moderate CV risk, ≥ 130 mg/dl in patients at high CV risk and ≥ 100 mg/dl in patients at very high CV risk [16]. Patients with overt CVD were defined at very high CV risk (secondary prevention). Patients taking rosuvastatin 20–40 mg or atorvastatin 40–80 mg were defined as being on high-intensity statin treatment. Diabetes mellitus was defined on the basis of documented medical history or the use of antidiabetic drugs. Metabolic syndrome was defined by the presence of three or more of the following five criteria: systolic BP > 130 mmHg or diastolic BP > 85 mmHg, fasting plasma glucose ≥ 100 mg/dl, WC ≥ 102 cm in males or ≥ 88 cm in females, fasting TG ≥ 150 mg/dl, fasting HDLc < 40 mg/dl in males or < 50 mg/dl in females [23]. Smoking status was ascertained during recruitment, and smoking habit was defined as current smoking or as previous smoking of at least 100 cigarettes in a lifetime. Chronic kidney disease (CKD) was defined as an eGFR < 60 ml/min/1.73 m2. Peripheral arterial disease (PAD) was defined based on the finding of carotid and/or lower limb arterial plaques at a previous Doppler ultrasound.
Statistical Analyses
Data were analyzed with the Statistical Package for Social Science version 13 (SPSS Inc. Chicago, IL, USA). p < 0.05 was defined as statistically significant. Normal continuous variables were expressed as mean ± standard deviation (SD). Skewed variables were expressed as median and interquartile range. Categorical variables were expressed as absolute number and percentage. Glycemia, TG, non-HDLc/HDLc and (non-HDLc + non-LDLc) were natural logarithmically transformed [ln(glycemia), ln(TG), ln(non-HDLc/HDLc), ln(non-HDLc + non-LDLc)] to normalize their distributions. The χ2 test was used to analyze the differences between categorical variables. The unpaired t test and Mann-Whitney U test were used to compare quantitative variables. Pearson correlation was used to analyze the relationship between continuous variables, and multiple linear regression was used for adjusting for covariates. Analyses regarding WC were performed only when available (391 patients). Analyses regarding the association between glycemia and anthropometric measurements were performed only in non-diabetic patients (1087 patients).
Results
General Characteristics
General characteristics of the study population according to sex are described in Table 1. Female patients were older and had higher prevalence of CKD than males, while male patients had higher BMI and higher prevalence of smoking and metabolic syndrome. Almost all patients had at least one criterion for dyslipidemia. Male patients had higher 24-h, daytime and nighttime BP values. Only 41.6% of patients had controlled BP, and female patients had a better BP control rate than male patients (47.5% vs. 36.7%; p < 0.001). The number of patients treated with anti-hypertensive drugs was 872 (71.5%): 72.9% of patients were taking angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (ACE-I/ARBs), 45.6% calcium channel blockers, 39.4% diuretics, 35.6% β-blockers, 6.3% α-blockers and 4.2% mineralocorticoid receptor antagonists. Among patients on anti-hypertensive therapy, 35.8% took only one anti-hypertensive drug, 32.7% took two anti-hypertensive drugs, 22.0% took three anti-hypertensive drugs, and 9.5% took more than three anti-hypertensive drugs. No difference in LDLc and non-HDLc was found between male and female patients. However, female patients had higher TC and HDLc, while male patients had higher TG levels. Lipid-lowering drugs were taken by 281 patients (23.1%), and only 19.2% of them were treated with high-intensity statins, without differences between male and female patients. The number of patients in secondary prevention was 298 (24.4% of the study population); they had higher prevalence of lipid-lowering drug treatment compared with patients in primary prevention (36.6% vs. 18.7%, p < 0.001). High-intensity statins were taken by only 25.9% of treated patients in secondary prevention. Statins were taken as follows: 37.0% atorvastatin, 34.5% simvastatin, 14.6% rosuvastatin, 5.7% pravastatin, 1.4% lovastatin and 0.7% fluvastatin, without any difference between male and female patients (all p > 0.05). Ezetimibe and fibrates were taken by 7.8% and 3.6% of treated patients, respectively. Prevalence of lipid-lowering therapy was higher in diabetic patients compared with the rest of the population (53.8% vs. 19.3%; p < 0.001), especially for atorvastatin (p < 0.001), simvastatin (p < 0.001), rosuvastatin (p = 0.002), ezetimibe (p = 0.001) and fibrates (p = 0.003).
General characteristics of the study population according to the presence of established CVD are also described in Table 1. Patients in secondary prevention were older and had higher prevalence of dyslipidemia, diabetes mellitus, CKD and PAD compared with patients in primary prevention. Moreover, they had lower diastolic ABP values, lower TC, LDLc, non-HDLc, higher glycemia and lower eGFR compared with patients in primary prevention.
Lipid Profile According to Adiposity
OW/OB patients were 70.2%, with male prevalence (61.3%). OW/OB patients had a higher risk of having diabetes mellitus [13.7% vs. 5.3%; p < 0.001; OR: 2.8 (95% CI 1.6–5.1)] and dyslipidemia [93.2% vs. 86.7%; p = 0.002; OR: 2.1 (95% CI 1.3–3.4)] than normal-weight patients. They had higher glycemia, higher non-HDLc, lower HDLc, higher TG, higher non-HDLc/HDLc and higher (non-HDLc + non-LDLc) levels compared with normal-weight patients (Fig. 1). Prevalence of lipid-lowering therapy (24.3% vs. 21.2%; p = 0.324) or types of statin used (all p = n.s. for each statin) did not differ between OW/OB and normal-weight patients. Correlations among the anthropometric measurements, glycemia and lipid profile according to sex are shown in Table 2. There were significant correlations among BMI and glycemia, TG, HDLc, non-HDLc, non-HDLc/HDLc and (non-HDLc + non-LDLc). Similar correlations were found with WC. The associations with BMI and WC remained significant even after adjusting for age, sex and lipid-lowering therapy. On the contrary, no associations emerged between BMI or WC and both TC and LDLc (see Table 2).
Evaluation of CV Risk and Dyslipidemia Control Rates
We estimated the individual CV risk as follows: 26.4% of patients had a very high CV risk, 9.2% had a high CV risk, 41.3% had a moderate CV risk, and 23.1% had a low CV risk. Only 28.5% of all patients had controlled LDLc. The higher the CV risk was, the higher was the prevalence of patients on lipid-lowering therapy (4.6% for low CV risk, 22.8% for moderate CV risk, 29.5% for high CV risk and 37.4% for very high CV risk, p < 0.001), but the lower was the rate of LDLc control (Fig. 2). In patients with no LDLc control, the respective mean distance to the LDLc target and the relative decrease required to achieve the target were the following: + 30.4 mg/dl (95% CI 26.9–33.9) for low-risk patients (relative decrease required = 20.9%); + 30.6 mg/dl (95% CI 28.0–33.2) for moderate-risk patients (relative decrease required = 21.0%); + 35.8 mg/dl (95% CI 30.6–41.1) for high-risk patients (relative decrease required = 26.4%); + 54.9 mg/dl (95% CI 50.9–59.0) for very-high-risk patients (relative decrease required = 44.0%). Patients in secondary prevention had lower rates of LDLc control than patients in primary prevention [12.8% vs. 33.7%; p < 0.001; OR 3.5 (95% CI 2.4–5.0)], despite better BP control (47.0% vs. 39.7%, p = 0.027). Patients with poor LDLc control tended to be older (57.0 ± 12.9 vs. 55.1 ± 15.5 years, p = 0.054) and had higher BMI (28.1 ± 4.6 vs. 27.3 ± 4.5 kg/m2, p = 0.039). As expected, patients treated with lipid-lowering therapy were more often controlled than untreated patients (40.9% vs. 24.8%, p < 0.001). The intensity of statin therapy did not affect the LDLc control rate (p = 0.307). The LDLc control rate was independent of sex (p = 0.660), smoking habit (p = 632), diabetes mellitus (p = 0.636), overweight/obesity (p = 0.101), CKD (p = 0.378) and PAD (p = 0.212). Regarding BP control, no associations were found between 24-h BP control and the several CV risk factors analyzed. Only 12.4% of patients had both 24-h BP and LDLc controlled at the same time, and patients in secondary prevention had lower control rates of these two CV risk factors taken together than patients in primary prevention [6.4% vs. 14.3%; p < 0.001; OR: 2.5 (95% CI 1.5–4.1)]. No differences in both 24-h BP and LDLc control rate emerged between patients with diabetes mellitus (p = 0.922), CKD (p = 0.565), PAD (p = 0.769) and their counterparts.
Discussion
Hypertension and dyslipidemia often coexist, and both contribute to atherosclerotic disease and its clinical consequences. Our data clearly show that dyslipidemia is highly prevalent but still neglected in hypertensives nowadays, and excessive adiposity plays a key role in the derangement of glucose and lipid metabolism, leading to a more atherogenic lipid profile. Physicians too often do not take into account the complexity of these patients, often focusing on the evaluation of a single major CV risk factor. On the contrary, controlling both hypertension and hypercholesterolemia should be a universal and mandatory goal for CVD prevention [24].
Less than half of the treated patients referred to us for hypertension by general practitioners had 24-h BP controlled in our study, in disagreement with a recent study based on office BP values that found higher BP control rates among Italian general practitioners [25]. This divergence could be explained by the better accuracy of the ABPM in identifying the real BP control in treated hypertensives [26].
Our work focused on dyslipidemia, and poor lipid control was even more prevalent in patients at higher CV risk, in whom the therapeutic LDLc target to be achieved should be lower. Indeed, a retrospective cross-sectional study on American Veterans showed that hypertensive patients with more CV risk factors had higher cholesterol levels, despite an apparently more intensive lipid-lowering treatment [27]. Similar data were reported in a European cohort on general practitioners’ patients, in which the LDLc target was achieved in only 61.3% of subjects, with rates decreasing as the CV risk increased [28]. In the EURIKA study [17], only 42% of treated dyslipidemic patients achieved their LDLc control, regardless of CV risk. Our data, although in a different population, show similar and not encouraging results 10 years later.
In patients at very high CV risk, high-intensity statin therapy is associated with improved survival and fewer major CV events compared with low- or moderate-intensity statin therapy, guided by the more potent LDL-lowering effects associated with positive effects on atherosclerotic plaque stabilization and even regression [29]. In agreement with previous studies [30], we found low LDLc control rates also in patients taking lipid-lowering drugs, highlighting the incorrect use of different molecules and dosages. In our real-life study, only few patients were taking high-intensity statins (19.2% among treated patients), even in secondary prevention (25.9% among treated patients). This could explain, at least in part, the lack of association between intensity of statin therapy and LDLc control, coupled with the poor lipid control rates found also in patients at higher CV risk. Previous studies on patients with coronary artery disease reported rates of statin use ranging from 70 to 78%, and a recent study on patients with symptomatic PAD showed that only a minority of patients (13.6%) were taking high-intensity statins [29]. The poor application of evidence-based guidelines, clinical inertia and low adherence/persistence to prescribed therapy [31] are likely to be key factors in determining the low rates of treatment and the poor control of dyslipidemia, especially in patients at higher CV risk, found in our study. Moreover, the LDLc control was independent of the presence/absence of several CV risk factors considered in our study. It is well known that hypertensives with concomitant diabetes mellitus or CKD are at even higher risk of CV organ damage and CVD [32]. In our study, diabetic patients had higher prevalence of lipid-lowering drug therapies; nevertheless, this was not associated with better LDLc control. A recent systematic review, conducted on patients at higher CV risk, reported similar findings: high prevalence of hypercholesterolemia (up to 84%) and poor lipid control rates, even in diabetics and patients with overt CVD [33]. General practitioners may not pay particular attention to dyslipidemia in these subjects if lipids are not increased to alarming levels. Therefore, LDLc may not always be accurately calculated, leading to underestimation of the CV risk and an under-prescription of lipid-lowering drugs. Furthermore, the optimal LDLc cutoff to reduce the CV risk may have to be even lower, given the recent results of PCSK9 inhibition with monoclonal antibodies [34].
Role of Overweight and Obesity on Lipid Profile
In our study, there was a high prevalence of OW/OB patients. The excessive adiposity, indicated by the high BMI values, was related to an atherogenic dyslipidemia, characterized by low HDLc, high non-HDLc and high TG. Moreover, in these OW/OB patients, the LDLc levels were often not at target according to their CV risk, revealing a real “double-trouble” lipid profile. Lipid-lowering therapy did not affect these findings, and the proportions of treated patients did not differ between normal-weight and OW/OB. This “double-trouble” lipid profile has been neglected as straightforward explanation of the high prevalence of vascular damage found in previous studies on hypertensives [35].
There is a strong relationship between hypertension and dyslipidemia, especially in OW/OB, in which excessive adiposity plays a key role in determining both high BP and a worse lipid profile, characteristics of metabolic syndrome [23]. Hypertension and atherogenic dyslipidemia find the same pathophysiologic bases in the excess of adipose tissue, through several neurohumoral and renal mechanisms [36,37,38].
Although the recommendations made by the major international guidelines are mainly focused on LDLc levels, recent studies also show the importance of the other circulating plasma lipids in determining CV risk. In particular, non-HDLc and non-HDLc/HDLc were found to be associated with CVD risk as much as LDLc in several populations. Especially in the presence of elevated triglycerides, it has been suggested that non-HDLc [the sum of LDL and very-low-density lipoprotein (VLDL) as well as VLDL remnant cholesterol] may correlate with CV risk better than LDLc [39, 40]. Atherogenic TG-rich remnant lipoproteins, characteristics of patient with central obesity and metabolic syndrome, are indeed more effectively accounted for by measuring non-HDLc [39]. Higher non-HDLc and TG are associated with greater coronary atheroma progression, irrespective of achieved LDLc levels [39]. On the other hand, the prognostic role of HDLc is currently most debated, and the relationship between HDLc and outcome is unlikely to be linear [41].
Study Limits
A strength of our study was the use of ABPM, which allowed greater accuracy in the assessment of BP control [42]. However, our study on a wide population of hypertensives in a real-life clinical setting has some limitations. First, our results are based on a selected population referred to our Hypertension Centre and may not be generalized to other populations. Low adherence to prescribed therapies may have played a role in determining our findings [8], but the design of our study and our available data did not permit accurately investigating this important aspect. Therefore, it was not possible to ascertain if the high rate of untreated dyslipidemia observed in our study was due to under-prescription by general practitioners and/or low adherence by patients. Moreover, results obtained from the analyses involving WC and PAD could have been affected by the limited availability of these data in our study population. Finally, the nature of the study did not allow us to assess the causality of the associations found.
Conclusions
Our study stressed the concept that dyslipidemia, if correctly evaluated using widespread, low-cost methodologies, is extremely prevalent among true hypertensives (documented by ABPM), so commonly seen in the daily clinical practice. We showed the magnitude of this crucial CV risk factor and its poor control rates in a wide population from a real-life clinical setting. Patients with hypertension often do not receive statin therapy and even fewer receive high-intensity statin therapy despite a higher CV risk. In addition, OW/OB hypertensives have a “double-trouble” atherogenic lipid pattern likely driven by adiposity. This is a very worrisome clinical picture that is still ignored or disregarded by most practicing physicians despite the previous knowledge on this topic. We encourage a comprehensive evaluation of the lipid profile in all hypertensives, especially if they are OW/OB, to correctly assess their CV risk and improve their management. Given the extremely low percentage of patients who had both BP and LDLc controlled at the same time, the use of fixed-dose combinations of multiple BP and lipid-lowering drugs may be helpful to improve the control rates of these two major concomitant, and often uncontrolled, CV risk factors.
References
Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–68.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38 (32):2459-2472.
Mancia G, Facchetti R, Bombelli M, et al. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertension. 2005;45(6):1072–7.
Bakker EA, Lee DC, Sui X, et al. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc. 2017;92(8):1214–22.
Bakker EA, Lee DC, Sui X, et al. Association of resistance exercise with the incidence of hypercholesterolemia in men. Mayo Clin Proc. 2018;93(4):419–28.
Sui X, Sarzynski MA, Lee DC, et al. Impact of changes in cardiorespiratory fitness on hypertension, dyslipidemia and survival: an overview of the epidemiological evidence. Prog Cardiovasc Dis. 2017;60(1):56–66.
Wu Y, Johnson BT, Acabchuk RL, et al. Yoga as antihypertensive lifestyle therapy: a systematic review and meta-analysis. Mayo Clin Proc. 2019;94(3):432–46.
Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94.
Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58.
Kotsis V, Jordan J, Micic D, et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. 2018;36(7):1427–40.
Sarzani R, Bordicchia M, Spannella F, et al. Hypertensive heart disease and obesity: a complex interaction between hemodynamic and not hemodynamic factors. High Blood Press Cardiovasc Prev. 2014;21(2):81–7.
Fedecostante M, Spannella F, Giulietti F, et al. Associations between body mass index, ambulatory blood pressure findings, and changes in cardiac structure: relevance of pulse and nighttime pressures. J Clin Hypertens (Greenwich). 2015;17(2):147–53.
Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–19.
Chopra I, Kamal KM, Candrilli SD, et al. Association between obesity and therapeutic goal attainment in patients with concomitant hypertension and dyslipidemia. Postgrad Med. 2014;126(1):66–77.
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81.
Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058.
Banegas JR, López-García E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32(17):2143–52.
Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–8.
Omboni S, Palatini P, Parati G. Working Group on Blood Pressure Monitoring of the Italian Society of Hypertension. Standards for ambulatory blood pressure monitoring clinical reporting in daily practice: recommendations from the Italian Society of Hypertension. Blood Press Monit. 2015; 20: 241–244.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
Cooney MT, Selmer R, Lindman A, et al. Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol. 2016;23(10):1093-103.
Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52.
Lewington S, Clarke R. Combined effects of systolic blood pressure and total cholesterol on cardiovascular disease risk. Circulation. 2005;112:3373–4.
Tocci G, Nati G, Cricelli C, et al. Prevalence and control of hypertension in the general practice in Italy: updated analysis of a large database. J Hum Hypertens. 2017;31(4):258–62.
Spannella F, Filipponi A, Giulietti F, et al. Prognostic role of masked and white-coat hypertension: 10-Year mortality in treated elderly hypertensives. J Hum Hypertens. 2018. https://doi.org/10.1038/s41371-018-0140-4. [Epub ahead of print].
Welch V, Tang SS. Treatment and control of BP and lipids in patients with hypertension and additional risk factors. Am J Cardiovasc Drugs. 2007;7(5): 381–9.
Di Martino M, Degli Esposti L, Filigheddu F, et al. Use of antihypertensive and lipid-lowering drugs: the management of cardiovascular risk in clinical practice. J Hum Hypertens. 2007;21(1):53–9.
Foley TR, Singh GD, Kokkinidis DG, et al. High-Intensity Statin Therapy Is Associated With Improved Survival in Patients With Peripheral Artery Disease. J Am Heart Assoc. 2017;6(7):e005699.
Poli A, Tragni E, Casula M, et al. How many patients need statin treatment in a low-cardiovascular-risk country? Low-density lipoprotein-cholesterol target and distance from target distribution in an Italian cohort. Nutr Metab Cardiovasc Dis. 2012;22(4):327–36.
Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133–8.
Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84.
de la Sierra A, Pintó X, Guijarro C, et al. Prevalence, treatment, and control of hypercholesterolemia in high cardiovascular risk patients: evidences from a systematic literature review in Spain. Adv Ther. 2015;32(10):944–61.
Sabatine MS, Giugliano RP. Low-density lipoprotein cholesterol treatment in the proprotein convertase subtilisin/kexin type 9 inhibitor era: getting back on target. JAMA Cardiol. 2017;2(9):935–6.
Zanchetti A, Bond MG, Hennig M, et al. Risk factors associated with alterations in carotid intima-media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. J Hypertens. 1998;16(7):949–61.
Sarzani R, Salvi F, Dessì-Fulgheri P, et al. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26(5):831–43.
Sarzani R, Spannella F, Giulietti, et al. Cardiac Natriuretic Peptides, Hypertension and Cardiovascular Risk. High Blood Press Cardiovasc Prev. 2017;24(2):115–26.
Spannella F, Giulietti F, Cocci G, et al. N-terminal pro B-Type natriuretic peptide is inversely correlated with low density lipoprotein cholesterol in the very elderly. Nutr Metab Cardiovasc Dis. 2018;28(6):629–35.
Puri R, Nissen SE, Shao M, et al. Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36(11):2220–8.
Eliasson B, Gudbjörnsdottir S, Zethelius B, et al. LDL-cholesterol versus non-HDL-to-HDL-cholesterol ratio and risk for coronary heart disease in type 2 diabetes. Eur J Prev Cardiol. 2014;21(11):1420–8.
Ko DT, Alter DA, Guo H, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68(19):2073–83.
Balietti P, Spannella F, Giulietti, et al. Ten-year changes in ambulatory blood pressure: the prognostic value of ambulatory pulse pressure. J Clin Hypertens (Greenwich). 2018; 20(9):1230–37.
Acknowledgements
We thank the participants of the study.
Funding
This research was funded by University “Politecnica delle Marche” (Ricerca di Ateneo to R. Sarzani; ID project: 15148). The unconditional support for the English check was provided and funded by Servier SpA. The unconditional support for the article processing and open access publication charges was provided and funded by Servier SpA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Francesco Spannella, Federico Giulietti, Chiara Di Pentima and Riccardo Sarzani have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the local institutional ethics committee (INRCA Ethics Committee), and informed consent was obtained from all individual participants included in the study.
Data Availability
The data set generated and analyzed during the current study is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7874354.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Spannella, F., Giulietti, F., Di Pentima, C. et al. Prevalence and Control of Dyslipidemia in Patients Referred for High Blood Pressure: The Disregarded “Double-Trouble” Lipid Profile in Overweight/Obese. Adv Ther 36, 1426–1437 (2019). https://doi.org/10.1007/s12325-019-00941-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00941-6