Abstract
Introduction
Bicalutamide (BIC), a non-steroidal anti-androgen, is FDA-indicated for use in combination with a luteinizing hormone-releasing hormone (LHRH) analog for treatment of Stage D2 metastatic carcinoma of the prostate. Lack of consensus exists regarding the clinical benefit of BIC use, either alone or combined use of BIC with an LHRH analog or antagonist (combined androgen blockade or CAB), versus treatment with androgen deprivation therapy (ADT) alone.
Methods
The SEER-Medicare database was used to identify prostate cancer patients aged ≥ 66 years diagnosed between 2007 and 2011 and who filled at least one prescription for BIC. Duration of BIC treatment was assessed in relation to ADT use; either alone (monotherapy), as part of CAB only, and as part of CAB followed by monotherapy. Additionally, we assessed use of BIC during or outside a potential testosterone flare prevention period (initiation within 2 months of an LHRH agonist).
Results
A total of 7521 prostate cancer patients who filled a prescription for BIC were identified. Eighteen percent of the cohort used BIC alone, over half the patients (54%) used BIC as part of CAB and 27% used BIC as part of CAB followed by monotherapy. Among men treated with BIC as part of CAB, 58% received BIC only within the potential flare period.
Conclusions
Although there is no FDA indication for BIC use as monotherapy, > 44% of patients in this study used BIC alone or as part of CAB followed by monotherapy. Further research is necessary to understand the outcomes of BIC utilization in these settings, particularly compared with newer second-generation anti-androgens.
Funding
Medivation LLC, a Pfizer company, and Astellas, Pharma, Inc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the third-leading cause of cancer death in men in the USA, with an estimated 29,430 deaths in 2018 [1]. Androgen receptor signaling is a dependent-molecular mechanism behind prostate cancer progression, and thus androgen deprivation therapy (ADT) is administered to men with advanced prostate cancer [2]. Surgical ADT, or bilateral orchiectomy, involves removal of the testes and spermatic cord [3] resulting in a more permanent testosterone depletion. Medical or chemical ADT involves chronic administration of luteinizing hormone-releasing hormone (LHRH) agonists or antagonists like Cerorelix, Ganirelix, Abarelix, or Degarelix [4]. During the first 1–3 weeks of LHRH-agonist therapy, the therapies may cause a temporary increase in testosterone, commonly referred to as a “flare” [5, 6]. Symptoms of a flare may include bone pain, uremia, ureteral obstruction, and development of neurologic sequelae, and the increase in testosterone may lead to a rapid progression of disease [7]. The use of medical or surgical ADT in conjunction with a non-steroidal anti-androgen is referred to as “combined androgen blockade” (CAB) and attempts to block androgens from all sources [6, 8]. The use of CAB compared with ADT alone has been controversial, with some meta-analyses of clinical trials reporting improvement in overall survival in patients treated with CAB and others reporting inconclusive results with high frequency of adverse health effects in CAB-treated patients [8, 9]. A 2007 RCT study by Akaza et al. showed that bicalutamide 80 mg offered a significant overall survival benefit compared with LHRH-agonist monotherapy without reducing tolerability in patients with locally advanced or metastatic prostate cancer [10].
Bicalutamide (BIC) is a non-steroidal, first-generation anti-androgen FDA-indicated for use in combination therapy with a LHRH analog approved for the treatment of Stage D2 metastatic carcinoma of the prostate. BIC blocks the effects of adrenal androgens at the androgen receptor to potentially prevent testosterone flare. A recent meta-analysis of both randomized clinical trials and observational studies demonstrated a non-statistically significant improvement in rates of disease flare among men taking an androgen suppressant and an anti-androgen compared with androgen suppression alone [7, 11]. Additionally, during and before this study period, no prospective randomized control trials have demonstrated a survival advantage with CAB over the serial use of a LHRH agonist and an anti-androgen [12]. Therefore, it is important to understand the treatment patterns and characteristics of BIC use within the prostate cancer population. The objectives of this research were to identify a cohort of BIC-treated elderly patients with prostate cancer, characterize them with respect to their disease severity, age, race, and presence of comorbidities, and describe the patterns of BIC use in relation to ADT.
Methods
Data Sources
The SEER-Medicare database links cancer registry information in selected US geographic areas with claims for covered health care services of Medicare beneficiaries. The SEER program represents approximately 28% of the US population, whereas Medicare covers health services for 97% of people aged 65 years and older. About 55% of SEER patients are ≥ age 65 and approximately 94% have been successfully linked with their Medicare claims [13,14,15]. Geographic areas selected for inclusion in SEER are based on adherence to operating and maintaining a high-quality, population-based cancer reporting system and specific patient populations of interest [16]. The SEER-Medicare database analytic variables include patient demographics at diagnosis (e.g., age and gender), tumor characteristics, Medicare enrollment information, International Classification of Diseases, Ninth Revision, Clinical Modification diagnoses and procedure codes, Healthcare Common Procedure Coding System and Current Procedural Terminology codes, and prescription claims data.
Study Design and Population
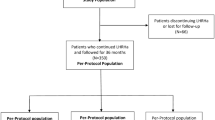
Incident prostate cancer cases diagnosed between January 1, 2007 and December 31, 2011 followed until December 31, 2013, with claims data reporting at least one filled BIC prescription any point after diagnosis were included in the study cohort (Fig. 1). All patients were required to be enrolled in Medicare parts A and B for the 12 months prior to their diagnosis and at least 2 years after diagnosis (or until death or the end of study observation). These restrictions ensured that a modified Charlson comorbidity scores could be calculated to capture comorbidity at diagnosis [17, 18] and that complete claims information would be available. Likewise, patients who were members of a Health Maintenance Organization were also excluded based upon same rational. Additional inclusion and exclusion information is detailed in Fig. 1. The Wayne State University Institutional Review Board determined that the study was Non-Human Participant Research (HPR# 2015-48). Thus, this article does not contain any studies with human participants or animals performed by any of the authors.
Statistical Analysis
The distribution of patient demographic and clinical characteristics is presented in Tables 1 and 2, respectively. Prostate cancer treatments administered as first- and second-line therapies were described as ADT use, including both surgical and chemical ADT, where surgical ADT was defined as bilateral orchiectomy, and chemical ADT was defined as the administration of Leuprolide Acetate, Goserelin Acetate, Triptorelin, Histrelin (LHRH agonists), or Degarelix (LHRH antagonist). As no formal time frame for CAB treatment has been established [19], in this study, CAB was defined as BIC use within 6 months of ADT based on clinical recommendation. A sensitivity analysis was performed using a 4-month window for CAB. Treatment patterns for BIC use in relation to ADT administration were divided into 4 categories: (1) BIC use alone as monotherapy (without ADT), (2) BIC as part of CAB (with ADT), (3) BIC as part of CAB followed by monotherapy, and (4) other (BIC use begun > 6 months before orchiectomy or chemical ADT and continued into CAB period).
Current National Comprehensive Cancer Network guidelines recommend that BIC precede or be co-administered with LHRH agonists for at least 7 days for testosterone flare prevention [19] for men with metastatic disease. In this study, the treatment for potential testosterone flare is defined as ≥ 1 prescription for BIC within 2 months before or after LHRH agonist therapy initiation. The 2-month window identifies patients who may have filled their prescriptions up to 35 days before LHRH agonist treatment initiation, but not initiated until 30 days prior [20,21,22,23,24,25,26,27]. To evaluate potential BIC used for testosterone flare only, the CAB category was stratified into BIC use only within ± 2 months of LHRH agonist start. These time periods are displayed in Fig. 2. The BIC treatment duration was calculated in terms of months from the first BIC claim to the last BIC claim, plus the number of days’ supply. The distribution of BIC and ADT use patterns were described overall and stratified by metastatic disease at diagnosis (M0 versus M1). In addition, the median, mode, and range of months of BIC use were calculated by treatment category. All analyses were completed using SAS v.9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
The majority of patients included in this study were non-Hispanic white (71%) and lived in a metropolitan area (81%) (Table 1). At the end of the study follow-up period (December 31, 2013), 13% of the cohort had died of prostate cancer and 14% had died from other causes (Table 1).
Most men (64%) had AJCC stage I–II cancer at diagnosis and 76% had no metastases at diagnosis. The Charlson Comorbidity Index score was 0–1 for 72% of participants. Chemical ADT was administered as primary treatment for nearly three-quarters of the cohort. Radiation was the primary second-line treatment used in 28% of the cohort, followed by chemical ADT, which was used by 12% of the cohort (Table 1).
Treatment Patterns and Duration
Table 2 includes the definitions used to classify BIC treatment pattern categories in relation to ADT as defined by BIC and ADT start and stop dates, as illustrated in Fig. 2. Eighteen percent of the cohort used BIC as monotherapy, with over half of patients (54%) using BIC as part of CAB and 27% using BIC as part of CAB followed by monotherapy. Among men treated with BIC as part of CAB, 58% (n = 2327) used BIC only within 2 months of LHRH agonist start, which may indicate use for testosterone flare only. A small number of patients (n = 122) began BIC treatment more than 6 months before bilateral orchiectomy or chemical ADT start and continued on therapy within the study’s CAB window. These patients had the longest median BIC treatment duration (29.7 months), followed by the patients treated with BIC as part of CAB followed by monotherapy (median 21.0 months of BIC therapy). Patients receiving BIC monotherapy persisted for a median of 4.4 months, while the majority of all BIC patients had only a median of 1 month of therapy with CAB.
Compared to men without metastatic prostate disease at diagnosis, more men diagnosed with metastatic prostate cancer used BIC as part of CAB followed by monotherapy (42 vs. 24%) and fewer used BIC as part of CAB (41 vs. 56%). Among metastatic patients who used BIC as part of CAB, only 35% used BIC within 2 months of LHRH agonist start, indicating a smaller proportion of metastatic prostate cancer patients using BIC during the ‘flare’ period than patients without metastatic disease at diagnosis (61%). Additional details about duration of BIC use can be found in Table 3.
Sensitivity Analysis
With respect to a sensitivity analysis, expanding the window between BIC prescription and initiation of LHRH agonist for CAB to a 4-month window, the proportion of patients using BIC monotherapy and CAB followed by monotherapy increased (19 and 32%, respectively), while the proportion of patients using BIC as part of CAB decreased to 47% (Supplemental Table 1). Among patients using BIC as part of CAB, a greater proportion (66%) used BIC within the potential testosterone flare prevention period. These patterns were also observed when the analyses were stratified by men diagnosed with and without metastases at diagnosis (49 and 34% used BIC as part of CAB, respectively).
Discussion
To our knowledge, this is the first study to determine the demographic and clinical characteristics of a cohort of elderly US prostate cancer patients using BIC and to compare various treatment patterns of BIC use alone, as part of CAB, or as part of CAB followed by monotherapy. Our study reported that greater than 44% of prostate cancer patients used BIC alone or as part of CAB followed by monotherapy, for which there is no FDA indication. Moreover, we found wide variation in the duration of BIC use, but the majority of utilization indicates relatively short durations of treatment in prostate cancer patients initially diagnosed with either M1 or M0 disease.
With low clinical benefit and weak clinical evidence strength in multiple prostate cancer settings, BIC has weak recommendation strength in several clinical guidelines [28,29,30]. A recent systematic review of clinical flare after LHRH agonist use identified a lack of historical data demonstrating disease progression during testosterone flare and hypothesized that anti-androgen use for flare prevention may be unnecessary [7]. In the largest randomized controlled trial to date using BIC monotherapy at high doses for early-stage prostate cancer, there were indications of a delay in disease progression, but no statistically significant improvement in overall survival compared with standard-of-care therapy [31]. Controlled clinical trial data to support BIC use in patients with castration-resistant prostate cancer are also sparse, with little clinical benefit reported when BIC was added to ongoing ADT [32,33,34]. Lastly, in a double-blind, placebo controlled, randomized clinical trial to examine the benefit of adding 150 mg of BIC to men undergoing radiation therapy with T2 and T3 disease observed improved overall survival, lower prostate cancer-specific mortality, and lower incidence of metastases [35]. The use of claims data to determine non-US-approved 150-mg BIC use coupled with radiation treatment is difficult due to reliance on claim dates. However, in this analysis, 21% of patients received only BIC while also undergoing radiation treatment.
In the recent TERRAIN and STRIVE randomized controlled trials that included castration-resistant prostate cancer patients, only 21–31% of those treated with BIC (while on ADT) had a decline in their PSA of at least 50% [2, 36]. In light of the newer and more efficacious anti-androgens available, monotherapy BIC and the serial use of ADT followed by BIC monotherapy observed in this cohort should warrant future observational or controlled studies for anti-androgen efficacy in these treatment settings. This is important, as anti-androgen withdrawal syndrome may occur after discontinuation of BIC, causing a decline in PSA that may lead to androgen receptor overexpression and induce disease progression [36]. In such cases, BIC can function as an androgen receptor partial agonist, which may induce disease progression [37, 38]. Enzalutamide, a potent second-generation oral androgen receptor inhibitor, was shown to have a statistically significant reduced risk of death as well as disease progression in two large randomized control trials evaluating treatment either before or after chemotherapy [39, 40]. Although a recent observational study of 47 metastatic castration-resistant prostate cancer patients treated with enzalutamide observed anti-androgen withdrawal syndrome in five patients (10.6%) after discontinuation of enzalutamide therapy [41], in contrast to BIC, enzalutamide does not have agonist activity for the wild-type androgen receptor [42, 43]. Furthermore, enzalutamide impairs nuclear translocation of the androgen receptor and DNA binding that can lead to reduced expression of androgen-dependent genes [42, 44].
There are multiple limitations associated with this study. As the SEER registry only records cancer stage at diagnosis, the post-diagnosis metastatic status of this cohort during BIC use was unknown and cannot readily be determined from administrative claims data. Thus, patients could not be accurately characterized as to whether BIC was used only in patients with metastatic disease or non-metastatic disease. Due to SEER-Medicare limitations, we were not able to perform the study on younger men; in addition, patient selection biases (e.g., preservation of sexual function) that were not assessed may have led to variability in observations. Moreover, we cannot associate utilization with clinical intent of the prescribing physician. Further, the STRIVE [36] and TERRAIN [2] studies published in 2015 and 2016 were after the time period that data were available for this analysis. This study does not reflect clinician behavior following the publication of these trials. Additionally, no formal recommendations have been made as to the duration of CAB treatment. Clinical trials of CAB have shown advantages of 3- and 6-month treatment duration [45]. A formalized recommendation for CAB treatment duration would guide both clinicians and researchers in their abilities to use and evaluate this therapy option.
Conclusions
Despite the absence of prospective randomized control trial during and before the study period, demonstrating an overall survival advantage with BIC and no FDA indication for BIC monotherapy, more than 44% of prostate cancer patients in this study used BIC alone or as part of CAB followed by monotherapy. Given the potential for adverse side effects after BIC treatment, and in light of other more efficacious second-generation anti-androgens currently available, future studies evaluating the risks, benefits, and outcomes associated with the BIC treatment patterns identified in this study are warranted.
Change history
16 August 2018
The original article can be found online.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–63.
Senagore AJ. The gale encyclopedia of surgery: G-O, vol. 2. Detroit, MI: Gale Group; 2004. pp. 1051–58.
Kreis W, Ahmann FR, Jordan VC, de Haan H, Scott M. Oestrogen pre-treatment abolishes luteinising hormone-releasing hormone testosterone stimulation. Br J Urol. 1988;62(4):352–4.
Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;3(Suppl 3):S10–4.
Labrie F. Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology. J Steroid Biochem Mol Biol. 2015;145:144–56.
Vis AN, van der Sluis TM, Al-Itejawi HH, van Moorselaar RJ, Meuleman EJ. Risk of disease flare with LHRH agonist therapy in men with prostate cancer: myth or fact? Urol Oncol. 2015;33(1):7–15.
Klotz L. Combined androgen blockade in prostate cancer: meta-analyses and associated issues. BJU Int. 2001;87(9):806–13.
Schmitt B, Wilt TJ, Schellhammer PF, DeMasi V, Sartor O, Crawford ED, et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology. 2001;57(4):727–32.
Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S, et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115(15):3437–45.
Maximum androgen blockade in advanced prostate cancer. an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355(9214):1491–8.
Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25(12):1596–605.
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked medicare-tumor registry database. Med Care. 1993;31(8):732–48.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3-18.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973–2014 varying), Nov 2016 Sub (1973–2014) [Internet]. 2017.
National Cancer Institute. SEER Registries: Population characteristics. 2011. https://seer.cancer.gov/registries/characteristics.html. Accessed 14 Aug 2017.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
National Comprehensive Cancer Network. NCCN clinical practice guideline in oncology: prostate cancer, 4th ed. 2011; PROS-F 1-4.
Appu S, Lawrentschuk N, Grills RJ, Neerhut G. Effectiveness of cyproterone acetate in achieving castration and preventing luteinizing hormone releasing hormone analogue induced testosterone surge in patients with prostate cancer. J Urol. 2005;174(1):140–2.
Kuhn JM, Billebaud T, Navratil H, Moulonguet A, Fiet J, Grise P, et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide). N Engl J Med. 1989;321(7):413–8.
Ferrari P, Castagnetti G, Ferrari G, Pollastri CA, Tavoni F, Dotti A. Combination treatment in M1 prostate cancer. Cancer. 1993;72(12 Suppl):3880–5.
Schulze H, Senge T. Influence of different types of antiandrogens on luteinizing hormone-releasing hormone analogue-induced testosterone surge in patients with metastatic carcinoma of the prostate. J Urol. 1990;144(4):934–41.
Kotake T, Usami M, Akaza H, Koiso K, Homma Y, Kawabe K, et al. Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Zoladex Study Group. Jpn J Clin Oncol. 1999;29(11):562–70.
Tsushima T, Nasu Y, Saika T, Maki Y, Noda M, Suyama B, et al. Optimal starting time for flutamide to prevent disease flare in prostate cancer patients treated with a gonadotropin-releasing hormone agonist. Urol Int. 2001;66(3):135–9.
Noguchi K, Uemura H, Harada M, Miura T, Moriyama M, Fukuoka H, et al. Inhibition of PSA flare in prostate cancer patients by administration of flutamide for 2 weeks before initiation of treatment with slow-releasing LH-RH agonist. Int J Clin Oncol. 2001;6(1):29–33.
Oh W, Landrum M, Lamont E. Does oral antiandrogen use before luteinizing hormone-releasing hormone therapy in patients with metastatic prostate cancer prevent clinical consequences of a testosterone flare? Urology. 2010;75:642–7.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59(4):572–83.
Saad F, Chi KN, Finelli A, Hotte SJ, Izawa J, Kapoor A, et al. The 2015 CUA-CUOG Guidelines for the management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J. 2015;9(3–4):90–6.
McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97(2):247–54.
Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–48.
Kucuk O, Fisher E, Moinpour CM, Coleman D, Hussain MH, Sartor AO, et al. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy failed: a Southwest Oncology Group study (SWOG 9235). Urology. 2001;58(1):53–8.
Manikandan R, Srirangam SJ, Pearson E, Brown SC, O’Reilly P, Collins GN. Diethylstilboestrol versus bicalutamide in hormone refractory prostate carcinoma: a prospective randomized trial. Urol Int. 2005;75(3):217–21.
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417–28.
Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE Trial. J Clin Oncol. 2016;34(18):2098–106.
Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81(2):242–51.
Paul R, Breul J. Antiandrogen withdrawal syndrome associated with prostate cancer therapies: incidence and clinical significance. Drug Saf. 2000;23(5):381–90.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Poole A, Gill D, Hahn AW, Johnson E, Carroll E, Boucher K, et al. Incidence and characterization of antiandrogen withdrawal syndrome after discontinuation of treatment with enzalutamide in castration-resistant prostate cancer. Clin Genitourin Cancer. 2017;16(1): e169–e72.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90.
von Klot CA, Kuczyk MA, Merseburger AS. No androgen withdrawal syndrome for enzalutamide: a report of disease dynamics in the postchemotherapy setting. Eur Urol. 2014;65(1):258–9.
Guerrero J, Alfaro IE, Gomez F, Protter AA, Bernales S. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate. 2013;73(12):1291–305.
Labrie F. GnRH agonists and the rapidly increasing use of combined androgen blockade in prostate cancer. Endocr Relat Cancer. 2014;21(4):R301–17.
Acknowledgements
We acknowledge the efforts of the Applied Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services Inc; and the Surveillance, Epidemiology and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding
This study was funded by Medivation LLC, a Pfizer company, and Astellas, Pharma, Inc., the co-developers of enzalutamide. The sponsors also funded the article processing charges. The sponsors and co-authors were responsible for study design, data analysis, decision to publish, and preparation of the manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship contributions
JLB-D: conception and design, interpretation of data, drafting of paper, revising paper critically for intellectual content, and final approval of published version. JJR: analysis and interpretation of data, drafting of paper, revising paper critically for intellectual content, and final approval of published version. LCB: interpretation of data, drafting of paper, revising paper critically for intellectual content, and final approval of published version. CG: drafting of paper, revising paper critically for intellectual content, and final approval of published version. JF: interpretation of data, drafting of paper, revising paper critically for intellectual content, and final approval of published version. NMS: revising paper critically for intellectual content, and final approval of published version. SCF: revising paper critically for intellectual content and final approval of published version. AB: revising paper critically for intellectual content and final approval of published version. EH: interpretation of data, revising paper critically for intellectual content, and final approval of published version. RGWQ: conception and design, revising paper critically for intellectual content, and final approval of published version.
Disclosures
Jennifer L. Beebe-Dimmer is an employee of Wayne State University, which received a grant from EpidStat for their contributions. Julie J. Ruterbusch is an employee of Wayne State University, which received a grant from EpidStat for their contributions. Lauren C. Bylsma is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Christina Gillezeau is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Jon Fryzek is an employee of EpidStat Institute, which received financial support from Medivation LLC, a Pfizer company, in connection with the development of the current study and this manuscript. Scott C. Flanders holds stock in Johnson & Johnson, Abbott Laboratories, and Abbvie. Ruben G. W. Quek received remuneration and funding from Pfizer for this work and holds stock in Pfizer and Amgen. Neil M. Schultz, Arie Barlev and Elisabeth Heath have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
The Wayne State University Institutional Review Board determined that the study was Non-Human Participant Research (HPR# 2015-48). Thus, this article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are not publicly available, as the data use agreement with the SEER-Medicare data stipulates that the data may not be used for purposes other than described in the original research proposal and the data may not be shared with anyone other than the approved collaborators listed on the research proposal.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6456149.
The original version of this article was revised due to retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Beebe-Dimmer, J.L., Ruterbusch, J.J., Bylsma, L.C. et al. Patterns of Bicalutamide Use in Prostate Cancer Treatment: A U.S. Real-World Analysis Using the SEER-Medicare Database. Adv Ther 35, 1438–1451 (2018). https://doi.org/10.1007/s12325-018-0738-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0738-5