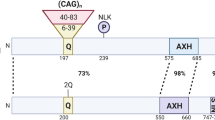
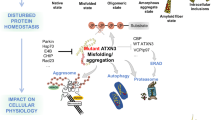
Abstract
Spinocerebellar ataxias (SCAs) represent a large group of hereditary degenerative diseases of the nervous system, in particular the cerebellum, and other systems that manifest with a variety of progressive motor, cognitive, and behavioral deficits with the leading symptom of cerebellar ataxia. SCAs often lead to severe impairments of the patient’s functioning, quality of life, and life expectancy. For SCAs, there are no proven effective pharmacotherapies that improve the symptoms or substantially delay disease progress, i.e., disease-modifying therapies. To study SCA pathogenesis and potential therapies, animal models have been widely used and are an essential part of pre-clinical research. They mainly include mice, but also other vertebrates and invertebrates. Each animal model has its strengths and weaknesses arising from model animal species, type of genetic manipulation, and similarity to human diseases. The types of murine and non-murine models of SCAs, their contribution to the investigation of SCA pathogenesis, pathological phenotype, and therapeutic approaches including their advantages and disadvantages are reviewed in this paper. There is a consensus among the panel of experts that (1) animal models represent valuable tools to improve our understanding of SCAs and discover and assess novel therapies for this group of neurological disorders characterized by diverse mechanisms and differential degenerative progressions, (2) thorough phenotypic assessment of individual animal models is required for studies addressing therapeutic approaches, (3) comparative studies are needed to bring pre-clinical research closer to clinical trials, and (4) mouse models complement cellular and invertebrate models which remain limited in terms of clinical translation for complex neurological disorders such as SCAs.

Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno-associated virus
- ADCA:
-
Autosomal dominant cerebellar ataxia
- ALS:
-
Amyotrophic lateral sclerosis
- ATXN:
-
Ataxin
- BBB:
-
Blood-brain barrier
- CIC:
-
Capicua
- CMA:
-
Chaperone-mediated autophagy
- CMV:
-
Cytomegalovirus
- CN:
-
Cerebellar nuclei
- CNS:
-
Central nervous system
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- DNMT1:
-
DNA methyltransferase 1 mutation
- Dox:
-
Doxycycline
- DRPLA:
-
Dentatorubral-pallidoluysian atrophy
- eIF2α:
-
Eukaryotic translation initiation factor 2 subunit 1
- FGF:
-
Fibroblast growth factor
- HSPs:
-
Hereditary spastic paraplegias
- IRES:
-
Internal ribosomal entry site
- KI:
-
Knock-in
- LAMP2A:
-
Lysosome-associated protein 2A
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- miR-RORα:
-
MicroRNA against RORα
- MSCV:
-
Murine stem cell virus
- NfL:
-
Neurofilament light chain
- NL/NP:
-
Neuronal loss/pathology
- NLS:
-
Nuclear localization signal
- PC:
-
Purkinje cell
- RORα:
-
Retinoid-related orphan receptor α
- ROS:
-
Reactive oxygen species
- SAGA:
-
Spt-Ada-GCN5 acetyltransferase
- SCA:
-
Spinocerebellar ataxia
- SN:
-
Substantia nigra
- STAU:
-
Staufen-1 protein
- Tg:
-
Transgenic
- TRE:
-
Tetracycline response element
References
Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs). Cerebellum. 2005;4:2–6. https://doi.org/10.1080/14734220510007914.
Mitoma H, Manto M. The physiological basis of therapies for cerebellar ataxias. Ther Adv Neurol Disord. 2016;9:396–413. https://doi.org/10.1177/1756285616648940.
Mitoma H, Manto M. The era of cerebellar therapy. Curr Neuropharmacol. 2019;17:3–6. https://doi.org/10.2174/1570159x1701181129111212.
Gandini J, Manto M, Bremova-Ertl T, Feil K, Strupp M. The neurological update: therapies for cerebellar ataxias in 2020. J Neurol. 2020;267:1211–20. https://doi.org/10.1007/s00415-020-09717-3.
Manto M, Marmolino D. Animal models of human cerebellar ataxias: a cornerstone for the therapies of the twenty-first century. Cerebellum. 2009;8:137–54. https://doi.org/10.1007/s12311-009-0127-3.
Cendelin J. From mice to men: lessons from mutant ataxic mice. Cerebellum Ataxias. 2014;1:4. https://doi.org/10.1186/2053-8871-1-4.
Sullivan R, Yau WY, O'Connor E, Houlden H. Spinocerebellar ataxia: an update. J Neurol. 2019;266:533–44. https://doi.org/10.1007/s00415-018-9076-4.
Schmahmann JD, Sherman JC. Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 1997;41:433–40.
Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174–83. https://doi.org/10.1159/000358801.
Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5:24. https://doi.org/10.1038/s41572-019-0074-3.
Coutelier M, Coarelli G, Monin ML, Konop J, Davoine CS, Tesson C, et al. A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain. 2017;140:1579–94. https://doi.org/10.1093/brain/awx081.
Galatolo D, Tessa A, Filla A, Santorelli FM. Clinical application of next generation sequencing in hereditary spinocerebellar ataxia: increasing the diagnostic yield and broadening the ataxia-spasticity spectrum. A retrospective analysis. Neurogenetics. 2018;19:1–8. https://doi.org/10.1007/s10048-017-0532-6.
Matilla-Dueñas A, Ashizawa T, Brice A, Magri S, McFarland KN, Pandolfo M, et al. Consensus paper: pathological mechanisms underlying neurodegeneration in spinocerebellar ataxias. Cerebellum. 2014;13:269–302. https://doi.org/10.1007/s12311-013-0539-y.
Ren H, Hao Z, Wang G. Autophagy and polyglutamine disease. Adv Exp Med Biol. 2020;1207:149–61. https://doi.org/10.1007/978-981-15-4272-5_9.
Neves-Carvalho A, Duarte-Silva S, Teixeira-Castro A, Maciel P. Polyglutamine spinocerebellar ataxias: emerging therapeutic targets. Expert Opin Ther Targets. 2020;24:1099–119. https://doi.org/10.1080/14728222.2020.1827394.
White M, Xia G, Gao R, Wakamiya M, Sarkar PS, McFarland K, et al. Transgenic mice with SCA10 pentanucleotide repeats show motor phenotype and susceptibility to seizure: a toxic RNA gain-of-function model. J Neurosci Res. 2012;90:706–14. https://doi.org/10.1002/jnr.22786.
Paul S, Dansithong W, Figueroa KP, Scoles DR, Pulst SM. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat Commun. 2018;9:3648. https://doi.org/10.1038/s41467-018-06041-3.
Matilla-Dueñas A, Sánchez I, Corral-Juan M, Dávalos A, Alvarez R, Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9:148–66. https://doi.org/10.1007/s12311-009-0144-2.
Du X, Wang J, Zhu H, Rinaldo L, Lamar KM, Palmenberg AC, et al. Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell. 2013;154:118–33. https://doi.org/10.1016/j.cell.2013.05.059.
Onofre I, Mendonça N, Lopes S, Nobre R, de Melo JB, Carreira IM, et al. Fibroblasts of Machado Joseph disease patients reveal autophagy impairment. Sci Rep. 2016;6:28220. https://doi.org/10.1038/srep28220.
Agudo-Canalejo J, Schultz SW, Chino H, Migliano SM, Saito C, Koyama-Honda I, et al. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature. 2021;591:142–6. https://doi.org/10.1038/s41586-020-2992-3.
Ashkenazi A, Bento CF, Ricketts T, Vicinanza M, Siddiqi F, Pavel M, et al. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature. 2017;545:108–11. https://doi.org/10.1038/nature22078.
Dagda RK, Merrill RA, Cribbs JT, Chen Y, Hell JW, Usachev YM, et al. The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem. 2008;283:36241–8. https://doi.org/10.1074/jbc.M800989200.
Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, et al. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42:313–21. https://doi.org/10.1038/ng.544.
Stucki DM, Ruegsegger C, Steiner S, Radecke J, Murphy MP, Zuber B, et al. Mitochondrial impairments contribute to spinocerebellar ataxia type 1 progression and can be ameliorated by the mitochondria-targeted antioxidant MitoQ. Free Radical Biology & Medicine. 2016;97:427–40. https://doi.org/10.1016/j.freeradbiomed.2016.07.005.
Cornelius N, Wardman JH, Hargreaves IP, Neergheen V, Bie AS, Tümer Z, et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fibroblasts: effect of coenzyme Q10 supplementation on these parameters. Mitochondrion. 2017;34:103–14. https://doi.org/10.1016/j.mito.2017.03.001.
Chou AH, Yeh TH, Kuo YL, Kao YC, Jou MJ, Hsu CY, et al. Polyglutamine-expanded ataxin-3 activates mitochondrial apoptotic pathway by upregulating Bax and downregulating Bcl-xL. Neurobiol Dis. 2006;21:333–45. https://doi.org/10.1016/j.nbd.2005.07.011.
Ward JM, Stoyas CA, Switonski PM, Ichou F, Fan W, Collins B, et al. Metabolic and organelle morphology defects in mice and human patients define spinocerebellar ataxia type 7 as a mitochondrial disease. Cell Rep. 2019;26:1189–202.e6. https://doi.org/10.1016/j.celrep.2019.01.028.
Fogel BL, Hanson SM, Becker EB. Do mutations in the murine ataxia gene TRPC3 cause cerebellar ataxia in humans? Mov Disord. 2015;30:284–6. https://doi.org/10.1002/mds.26096.
Scoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017;544:362–6. https://doi.org/10.1038/nature22044.
Ramachandran PS, Boudreau RL, Schaefer KA, La Spada AR, Davidson BL. Nonallele specific silencing of ataxin-7 improves disease phenotypes in a mouse model of SCA7. Mol Ther. 2014;22:1635–42. https://doi.org/10.1038/mt.2014.108.
Friedrich J, Kordasiewicz HB, O'Callaghan B, Handler HP, Wagener C, Duvick L, et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. 2018;3. https://doi.org/10.1172/jci.insight.123193.
McLoughlin HS, Moore LR, Chopra R, Komlo R, McKenzie M, Blumenstein KG, et al. Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice. Ann Neurol. 2018;84:64–77. https://doi.org/10.1002/ana.25264.
Lalonde R, Strazielle C. Motor performances of spontaneous and genetically modified mutants with cerebellar atrophy. Cerebellum. 2019;18:615–34. https://doi.org/10.1007/s12311-019-01017-5.
Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J Neurosci. 2004;24:8853–61. https://doi.org/10.1523/jneurosci.2978-04.2004.
Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron. 2002;34:905–19.
Lorenzetti D, Watase K, Xu B, Matzuk MM, Orr HT, Zoghbi HY. Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum Mol Genet. 2000;9:779–85. https://doi.org/10.1093/hmg/9.5.779.
Ramani B, Harris GM, Huang R, Seki T, Murphy GG, Costa Mdo C, et al. A knockin mouse model of spinocerebellar ataxia type 3 exhibits prominent aggregate pathology and aberrant splicing of the disease gene transcript. Hum Mol Genet. 2015;24:1211–24. https://doi.org/10.1093/hmg/ddu532.
Switonski PM, Szlachcic WJ, Krzyzosiak WJ, Figiel M. A new humanized ataxin-3 knock-in mouse model combines the genetic features, pathogenesis of neurons and glia and late disease onset of SCA3/MJD. Neurobiol Dis. 2015;73:174–88. https://doi.org/10.1016/j.nbd.2014.09.020.
Niewiadomska-Cimicka A, Doussau F, Perot JB, Roux MJ, Keime C, Hache A, et al. SCA7 mouse cerebellar pathology reveals preferential downregulation of key Purkinje cell-identity genes and shared disease signature with SCA1 and SCA2. J Neurosci. 2021;41:4910–36. https://doi.org/10.1523/jneurosci.1882-20.2021.
Takechi Y, Mieda T, Iizuka A, Toya S, Suto N, Takagishi K, et al. Impairment of spinal motor neurons in spinocerebellar ataxia type 1-knock-in mice. Neurosci Lett. 2013;535:67–72. https://doi.org/10.1016/j.neulet.2012.12.057.
Mieda T, Suto N, Iizuka A, Matsuura S, Iizuka H, Takagishi K, et al. Mesenchymal stem cells attenuate peripheral neuronal degeneration in spinocerebellar ataxia type 1 knockin mice. J Neurosci Res. 2016;94:246–52. https://doi.org/10.1002/jnr.23698.
Shuvaev AN, Hosoi N, Sato Y, Yanagihara D, Hirai H. Progressive impairment of cerebellar mGluR signalling and its therapeutic potential for cerebellar ataxia in spinocerebellar ataxia type 1 model mice. J Physiol. 2017;595:141–64. https://doi.org/10.1113/jp272950.
Shuvaev AN, Horiuchi H, Seki T, Goenawan H, Irie T, Iizuka A, et al. Mutant PKCγ in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J Neurosci. 2011;31:14324–34. https://doi.org/10.1523/jneurosci.5530-10.2011.
Alves S, Régulier E, Nascimento-Ferreira I, Hassig R, Dufour N, Koeppen A, et al. Striatal and nigral pathology in a lentiviral rat model of Machado-Joseph disease. Hum Mol Genet. 2008;17:2071–83. https://doi.org/10.1093/hmg/ddn106.
Nóbrega C, Nascimento-Ferreira I, Onofre I, Albuquerque D, Conceição M, Déglon N, et al. Overexpression of mutant ataxin-3 in mouse cerebellum induces ataxia and cerebellar neuropathology. Cerebellum. 2013;12:441–55. https://doi.org/10.1007/s12311-012-0432-0.
Alves S, Marais T, Biferi MG, Furling D, Marinello M, El Hachimi K, et al. Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins. Mol Neurodegener. 2016;11:58. https://doi.org/10.1186/s13024-016-0123-2.
Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34:204–9. https://doi.org/10.1038/nbt.3440.
Shinohara Y, Konno A, Nitta K, Matsuzaki Y, Yasui H, Suwa J, et al. Effects of neutralizing antibody production on AAV-PHP.B-mediated transduction of the mouse central nervous system. Mol Neurobiol. 2019;56:4203–14. https://doi.org/10.1007/s12035-018-1366-4.
Watanave M, Hoshino C, Konno A, Fukuzaki Y, Matsuzaki Y, Ishitani T, et al. Pharmacological enhancement of retinoid-related orphan receptor α function mitigates spinocerebellar ataxia type 3 pathology. Neurobiol Dis. 2019;121:263–73. https://doi.org/10.1016/j.nbd.2018.10.014.
Matsuzaki Y, Tanaka M, Hakoda S, Masuda T, Miyata R, Konno A, et al. Neurotropic properties of AAV-PHP.B are shared among diverse inbred strains of mice. Mol Ther. 2019;27:700–4. https://doi.org/10.1016/j.ymthe.2019.02.016.
Hordeaux J, Yuan Y, Clark PM, Wang Q, Martino RA, Sims JJ, et al. The GPI-linked protein LY6A drives AAV-PHP.B transport across the blood-brain barrier. Mol Ther. 2019;27:912–21. https://doi.org/10.1016/j.ymthe.2019.02.013.
Serra HG, Duvick L, Zu T, Carlson K, Stevens S, Jorgensen N, et al. RORalpha-mediated Purkinje cell development determines disease severity in adult SCA1 mice. Cell. 2006;127:697–708. https://doi.org/10.1016/j.cell.2006.09.036.
Konno A, Shuvaev AN, Miyake N, Miyake K, Iizuka A, Matsuura S, et al. Mutant ataxin-3 with an abnormally expanded polyglutamine chain disrupts dendritic development and metabotropic glutamate receptor signaling in mouse cerebellar Purkinje cells. Cerebellum. 2014;13:29–41. https://doi.org/10.1007/s12311-013-0516-5.
Hirai H, Kano M. Type 1 metabotropic glutamate receptor and its signaling molecules as therapeutic targets for the treatment of cerebellar disorders. Curr Opin Pharmacol. 2018;38:51–8. https://doi.org/10.1016/j.coph.2018.02.002.
Yasui H, Matsuzaki Y, Konno A, Hirai H. Global knockdown of retinoid-related orphan receptor α in mature Purkinje cells reveals aberrant cerebellar phenotypes of spinocerebellar ataxia. Neuroscience. 2020. https://doi.org/10.1016/j.neuroscience.2020.04.004.
Seki T, Yoshino KI, Tanaka S, Dohi E, Onji T, Yamamoto K, et al. Establishment of a novel fluorescence-based method to evaluate chaperone-mediated autophagy in a single neuron. PLoS One. 2012;7:e31232. https://doi.org/10.1371/journal.pone.0031232.
Seki T, Sato M, Kibe Y, Ohta T, Oshima M, Konno A, et al. Lysosomal dysfunction and early glial activation are involved in the pathogenesis of spinocerebellar ataxia type 21 caused by mutant transmembrane protein 240. Neurobiol Dis. 2018;120:34–50. https://doi.org/10.1016/j.nbd.2018.08.022.
Sato M, Ohta T, Morikawa Y, Konno A, Hirai H, Kurauchi Y, et al. Ataxic phenotype and neurodegeneration are triggered by the impairment of chaperone-mediated autophagy in cerebellar neurons. Neuropathol Appl Neurobiol. 2021;47:198–209. https://doi.org/10.1111/nan.12649.
Ma Y, Zhang L, Huang X. Genome modification by CRISPR/Cas9. Febs j. 2014;281:5186–93. https://doi.org/10.1111/febs.13110.
Fernandez-Funez P, Nino-Rosales ML, de Gouyon B, She WC, Luchak JM, Martinez P, et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–6. https://doi.org/10.1038/35040584.
Bakthavachalu B, Huelsmeier J, Sudhakaran IP, Hillebrand J, Singh A, Petrauskas A, et al. RNP-granule assembly via ataxin-2 disordered domains is required for long-term memory and neurodegeneration. Neuron. 2018;98:754–66.e4. https://doi.org/10.1016/j.neuron.2018.04.032.
Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell. 1998;93:939–49. https://doi.org/10.1016/s0092-8674(00)81200-3.
Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453:1107–11. https://doi.org/10.1038/nature06909.
Wu YL, Chang JC, Lin WY, Li CC, Hsieh M, Chen HW, et al. Treatment with caffeic acid and resveratrol alleviates oxidative stress induced neurotoxicity in cell and drosophila models of spinocerebellar ataxia type3. Sci Rep. 2017;7:11641. https://doi.org/10.1038/s41598-017-11839-0.
Wu YL, Chang JC, Lin WY, Li CC, Hsieh M, Chen HW, et al. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radical Biology & Medicine. 2018;115:309–17. https://doi.org/10.1016/j.freeradbiomed.2017.12.011.
Watchon M, Yuan KC, Mackovski N, Svahn AJ, Cole NJ, Goldsbury C, et al. Calpain inhibition is protective in Machado-Joseph disease zebrafish due to induction of autophagy. J Neurosci. 2017;37:7782–94. https://doi.org/10.1523/jneurosci.1142-17.2017.
Acosta JR, Watchon M, Yuan KC, Fifita JA, Svahn AJ, Don EK, et al. Neuronal cell culture from transgenic zebrafish models of neurodegenerative disease. Biol Open. 2018;7. https://doi.org/10.1242/bio.036475.
Christie NT, Lee AL, Fay HG, Gray AA, Kikis EA. Novel polyglutamine model uncouples proteotoxicity from aging. PLoS One. 2014;9:e96835. https://doi.org/10.1371/journal.pone.0096835.
Visentin C, Pellistri F, Natalello A, Vertemara J, Bonanomi M, Gatta E, et al. Epigallocatechin-3-gallate and related phenol compounds redirect the amyloidogenic aggregation pathway of ataxin-3 towards non-toxic aggregates and prevent toxicity in neural cells and Caenorhabditis elegans animal model. Hum Mol Genet. 2017;26:3271–84. https://doi.org/10.1093/hmg/ddx211.
Fardghassemi Y, Tauffenberger A, Gosselin S, Parker JA. Rescue of ATXN3 neuronal toxicity in Caenorhabditis elegans by chemical modification of endoplasmic reticulum stress. Dis Model Mech. 2017;10:1465–80. https://doi.org/10.1242/dmm.029736.
Teixeira-Castro A, Jalles A, Esteves S, Kang S, da Silva SL, Silva-Fernandes A, et al. Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain. 2015;138:3221–37. https://doi.org/10.1093/brain/awv262.
Tomioka I, Nagai Y, Seki K. Generation of Common Marmoset Model Lines of Spinocerebellar Ataxia Type 3. Front Neurosci. 2020;14:548002. https://doi.org/10.3389/fnins.2020.548002.
Tsou WL, Hosking RR, Burr AA, Sutton JR, Ouyang M, Du X, et al. DnaJ-1 and karyopherin α3 suppress degeneration in a new Drosophila model of spinocerebellar ataxia type 6. Hum Mol Genet. 2015;24:4385–96. https://doi.org/10.1093/hmg/ddv174.
Jackson SM, Whitworth AJ, Greene JC, Libby RT, Baccam SL, Pallanck LJ, et al. A SCA7 CAG/CTG repeat expansion is stable in Drosophila melanogaster despite modulation of genomic context and gene dosage. Gene. 2005;347:35–41. https://doi.org/10.1016/j.gene.2004.12.008.
Latouche M, Lasbleiz C, Martin E, Monnier V, Debeir T, Mouatt-Prigent A, et al. A conditional pan-neuronal Drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes. J Neurosci. 2007;27:2483–92. https://doi.org/10.1523/jneurosci.5453-06.2007.
Yanicostas C, Barbieri E, Hibi M, Brice A, Stevanin G, Soussi-Yanicostas N. Requirement for zebrafish ataxin-7 in differentiation of photoreceptors and cerebellar neurons. PLoS One. 2012;7:e50705. https://doi.org/10.1371/journal.pone.0050705.
Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–8. https://doi.org/10.1016/j.cub.2004.01.034.
Namikawa K, Dorigo A, Köster RW. Neurological disease modelling for spinocerebellar ataxia using zebrafish. J Exp Neurosci. 2019;13:1179069519880515. https://doi.org/10.1177/1179069519880515.
Namikawa K, Dorigo A, Zagrebelsky M, Russo G, Kirmann T, Fahr W, et al. Modeling neurodegenerative spinocerebellar ataxia type 13 in zebrafish using a Purkinje neuron specific tunable coexpression system. J Neurosci. 2019;39:3948–69. https://doi.org/10.1523/jneurosci.1862-18.2019.
Ren J, Jegga AG, Zhang M, Deng J, Liu J, Gordon CB, et al. A Drosophila model of the neurodegenerative disease SCA17 reveals a role of RBP-J/Su(H) in modulating the pathological outcome. Hum Mol Genet. 2011;20:3424–36. https://doi.org/10.1093/hmg/ddr251.
Kelp A, Koeppen AH, Petrasch-Parwez E, Calaminus C, Bauer C, Portal E, et al. A novel transgenic rat model for spinocerebellar ataxia type 17 recapitulates neuropathological changes and supplies in vivo imaging biomarkers. J Neurosci. 2013;33:9068–81. https://doi.org/10.1523/jneurosci.5622-12.2013.
Ishiguro T, Sato N, Ueyama M, Fujikake N, Sellier C, Kanegami A, et al. Regulatory role of RNA chaperone TDP-43 for RNA misfolding and repeat-associated translation in SCA31. Neuron. 2017;94:108–24.e7. https://doi.org/10.1016/j.neuron.2017.02.046.
Ishikawa K, Nagai Y. Molecular mechanisms and future therapeutics for spinocerebellar ataxia type 31 (SCA31). Neurotherapeutics. 2019;16:1106–14. https://doi.org/10.1007/s13311-019-00804-6.
Akita K, Arai S, Ohta T, Hanaya T, Fukuda S. Suppressed Nna1 gene expression in the brain of ataxic Syrian hamsters. J Neurogenet. 2007;21:19–29. https://doi.org/10.1080/01677060600843316.
Akita K, Arai S. The ataxic Syrian hamster: an animal model homologous to the pcd mutant mouse? Cerebellum. 2009;8:202–10. https://doi.org/10.1007/s12311-009-0113-9.
Veenstra GJ, Weeks DL, Wolffe AP. Distinct roles for TBP and TBP-like factor in early embryonic gene transcription in Xenopus. Science. 2000;290:2312–5. https://doi.org/10.1126/science.290.5500.2312.
Gazulla J, Tintoré MA. The P/Q-type voltage-dependent calcium channel as pharmacological target in spinocerebellar ataxia type 6: gabapentin and pregabalin may be of therapeutic benefit. Med Hypotheses. 2007;68:131–6. https://doi.org/10.1016/j.mehy.2006.06.014.
Koon AC, Chan HY. Drosophila melanogaster as a model organism to study RNA toxicity of repeat expansion-associated neurodegenerative and neuromuscular diseases. Front Cell Neurosci. 2017;11:70. https://doi.org/10.3389/fncel.2017.00070.
Johnson SL, Blount JR, Libohova K, Ranxhi B, Paulson HL, Tsou WL, et al. Differential toxicity of ataxin-3 isoforms in Drosophila models of spinocerebellar ataxia type 3. Neurobiol Dis. 2019;132:104535. https://doi.org/10.1016/j.nbd.2019.104535.
Wu S, Tan KJ, Govindarajan LN, Stewart JC, Gu L, Ho JWH, et al. Fully automated leg tracking of Drosophila neurodegeneration models reveals distinct conserved movement signatures. PLoS Biol. 2019;17:e3000346. https://doi.org/10.1371/journal.pbio.3000346.
Del Castillo U, Gnazzo MM, Sorensen Turpin CG, Nguyen KCQ, Semaya E, Lam Y, et al. Conserved role for ataxin-2 in mediating endoplasmic reticulum dynamics. Traffic. 2019;20:436–47. https://doi.org/10.1111/tra.12647.
Rodrigues AJ, Coppola G, Santos C, Costa Mdo C, Ailion M, Sequeiros J, et al. Functional genomics and biochemical characterization of the C. elegans orthologue of the Machado-Joseph disease protein ataxin-3. Faseb j. 2007;21:1126–36. https://doi.org/10.1096/fj.06-7002com.
Herzog LK, Kevei É, Marchante R, Böttcher C, Bindesbøll C, Lystad AH, et al. The Machado-Joseph disease deubiquitylase ataxin-3 interacts with LC3C/GABARAP and promotes autophagy. Aging Cell. 2020;19:e13051. https://doi.org/10.1111/acel.13051.
Matilla A, Roberson ED, Banfi S, Morales J, Armstrong DL, Burright EN, et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J Neurosci. 1998;18:5508–16. https://doi.org/10.1523/jneurosci.18-14-05508.1998.
Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 2010;6:e1001021. https://doi.org/10.1371/journal.pgen.1001021.
Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, Hill DE, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452:713–8. https://doi.org/10.1038/nature06731.
Klement IA, Skinner PJ, Kaytor MD, Yi H, Hersch SM, Clark HB, et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell. 1998;95:41–53. https://doi.org/10.1016/s0092-8674(00)81781-x.
Irwin S, Vandelft M, Pinchev D, Howell JL, Graczyk J, Orr HT, et al. RNA association and nucleocytoplasmic shuttling by ataxin-1. J Cell Sci. 2005;118:233–42. https://doi.org/10.1242/jcs.01611.
Zhang S, Williamson NA, Duvick L, Lee A, Orr HT, Korlin-Downs A, et al. The ataxin-1 interactome reveals direct connection with multiple disrupted nuclear transport pathways. Nat Commun. 2020;11:3343. https://doi.org/10.1038/s41467-020-17145-0.
Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, et al. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38:375–87. https://doi.org/10.1016/s0896-6273(03)00258-7.
Duvick L, Barnes J, Ebner B, Agrawal S, Andresen M, Lim J, et al. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67:929–35. https://doi.org/10.1016/j.neuron.2010.08.022.
Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, et al. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–68. https://doi.org/10.1016/s0092-8674(03)00349-0.
Lai S, O'Callaghan B, Zoghbi HY, Orr HT. 14-3-3 Binding to ataxin-1(ATXN1) regulates its dephosphorylation at Ser-776 and transport to the nucleus. J Biol Chem. 2011;286:34606–16. https://doi.org/10.1074/jbc.M111.238527.
Tsuda H, Jafar-Nejad H, Patel AJ, Sun Y, Chen HK, Rose MF, et al. The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins. Cell. 2005;122:633–44. https://doi.org/10.1016/j.cell.2005.06.012.
Fryer JD, Yu P, Kang H, Mandel-Brehm C, Carter AN, Crespo-Barreto J, et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science. 2011;334:690–3. https://doi.org/10.1126/science.1212673.
Gandelman M, Dansithong W, Figueroa KP, Paul S, Scoles DR, Pulst SM. Staufen 1 amplifies proapoptotic activation of the unfolded protein response. Cell Death Differ. 2020;27:2942–51. https://doi.org/10.1038/s41418-020-0553-9.
Neuenschwander AG, Thai KK, Figueroa KP, Pulst SM. Amyotrophic lateral sclerosis risk for spinocerebellar ataxia type 2 ATXN2 CAG repeat alleles: a meta-analysis. JAMA Neurol. 2014;71:1529–34. https://doi.org/10.1001/jamaneurol.2014.2082.
Scoles DR, Dansithong W, Pflieger LT, Paul S, Gandelman M, Figueroa KP, et al. ALS-associated genes in SCA2 mouse spinal cord transcriptomes. Hum Mol Genet. 2020;29:1658–72. https://doi.org/10.1093/hmg/ddaa072.
Canet-Pons J, Sen NE, Arsović A, Almaguer-Mederos LE, Halbach MV, Key J, et al. Atxn2-CAG100-KnockIn mouse spinal cord shows progressive TDP43 pathology associated with cholesterol biosynthesis suppression. Neurobiol Dis. 2021;152:105289. https://doi.org/10.1016/j.nbd.2021.105289.
Bäumer D, East SZ, Tseu B, Zeman A, Hilton D, Talbot K, et al. FTLD-ALS of TDP-43 type and SCA2 in a family with a full ataxin-2 polyglutamine expansion. Acta Neuropathol. 2014;128:597–604. https://doi.org/10.1007/s00401-014-1277-z.
Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–75. https://doi.org/10.1038/nature09320.
Toyoshima Y, Tanaka H, Shimohata M, Kimura K, Morita T, Kakita A, et al. Spinocerebellar ataxia type 2 (SCA2) is associated with TDP-43 pathology. Acta Neuropathol. 2011;122:375–8. https://doi.org/10.1007/s00401-011-0862-7.
Sen NE, Canet-Pons J, Halbach MV, Arsovic A, Pilatus U, Chae WH, et al. Generation of an Atxn2-CAG100 knock-in mouse reveals N-acetylaspartate production deficit due to early Nat8l dysregulation. Neurobiol Dis. 2019;132:104559. https://doi.org/10.1016/j.nbd.2019.104559.
ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04494256. Accessed 26 October 2020.
Meera P, Pulst S, Otis T. A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2. Elife. 2017;6. https://doi.org/10.7554/eLife.26377.
Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–62. https://doi.org/10.1523/jneurosci.0660-09.2009.
Schmitt I, Linden M, Khazneh H, Evert BO, Breuer P, Klockgether T, et al. Inactivation of the mouse Atxn3 (ataxin-3) gene increases protein ubiquitination. Biochem Biophys Res Commun. 2007;362:734–9. https://doi.org/10.1016/j.bbrc.2007.08.062.
Niewiadomska-Cimicka A, Hache A, Trottier Y. Gene deregulation and underlying mechanisms in spinocerebellar ataxias with polyglutamine expansion. Front Neurosci. 2020;14:571. https://doi.org/10.3389/fnins.2020.00571.
McLoughlin HS, Moore LR, Paulson HL. Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol Dis. 2020;134:104635. https://doi.org/10.1016/j.nbd.2019.104635.
Ramani B, Panwar B, Moore LR, Wang B, Huang R, Guan Y, et al. Comparison of spinocerebellar ataxia type 3 mouse models identifies early gain-of-function, cell-autonomous transcriptional changes in oligodendrocytes. Hum Mol Genet. 2017;26:3362–74. https://doi.org/10.1093/hmg/ddx224.
Bichelmeier U, Schmidt T, Hübener J, Boy J, Rüttiger L, Häbig K, et al. Nuclear localization of ataxin-3 is required for the manifestation of symptoms in SCA3: in vivo evidence. J Neurosci. 2007;27:7418–28. https://doi.org/10.1523/jneurosci.4540-06.2007.
Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–9. https://doi.org/10.1038/ng0197-62.
Saegusa H, Wakamori M, Matsuda Y, Wang J, Mori Y, Zong S, et al. Properties of human Cav2.1 channel with a spinocerebellar ataxia type 6 mutation expressed in Purkinje cells. Mol Cell Neurosci. 2007:34:261-70. doi 10.1016/j.mcn.2006.11.006
Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, Hu Y, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci U S A. 2008;105:11987–92. https://doi.org/10.1073/pnas.0804350105.
Mark MD, Krause M, Boele HJ, Kruse W, Pollok S, Kuner T, et al. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J Neurosci. 2015;35:8882–95. https://doi.org/10.1523/jneurosci.0891-15.2015.
Miyazaki Y, Du X, Muramatsu S, Gomez CM. An miRNA-mediated therapy for SCA6 blocks IRES-driven translation of the CACNA1A second cistron. Sci Transl Med. 2016;8:347ra94. https://doi.org/10.1126/scitranslmed.aaf5660.
Yoo SY, Pennesi ME, Weeber EJ, Xu B, Atkinson R, Chen S, et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron. 2003;37:383–401. https://doi.org/10.1016/s0896-6273(02)01190-x.
Yvert G, Lindenberg KS, Devys D, Helmlinger D, Landwehrmeyer GB, Mandel JL. SCA7 mouse models show selective stabilization of mutant ataxin-7 and similar cellular responses in different neuronal cell types. Hum Mol Genet. 2001;10:1679–92. https://doi.org/10.1093/hmg/10.16.1679.
Guyenet SJ, Mookerjee SS, Lin A, Custer SK, Chen SF, Sopher BL, et al. Proteolytic cleavage of ataxin-7 promotes SCA7 retinal degeneration and neurological dysfunction. Hum Mol Genet. 2015;24:3908–17. https://doi.org/10.1093/hmg/ddv121.
Chou AH, Chen CY, Chen SY, Chen WJ, Chen YL, Weng YS, et al. Polyglutamine-expanded ataxin-7 causes cerebellar dysfunction by inducing transcriptional dysregulation. Neurochem Int. 2010;56:329–39. https://doi.org/10.1016/j.neuint.2009.11.003.
Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–48. https://doi.org/10.1016/0092-8674(95)90273-2.
Cvetanovic M, Ingram M, Orr H, Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–99. https://doi.org/10.1016/j.neuroscience.2015.01.003.
Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–95. https://doi.org/10.1523/jneurosci.17-19-07385.1997.
Asher M, Rosa JG, Rainwater O, Duvick L, Bennyworth M, Lai RY, et al. Cerebellar contribution to the cognitive alterations in SCA1: evidence from mouse models. Hum Mol Genet. 2020;29:117–31. https://doi.org/10.1093/hmg/ddz265.
Tichanek F, Salomova M, Jedlicka J, Kuncova J, Pitule P, Macanova T, et al. Hippocampal mitochondrial dysfunction and psychiatric-relevant behavioral deficits in spinocerebellar ataxia 1 mouse model. Sci Rep. 2020;10:5418. https://doi.org/10.1038/s41598-020-62308-0.
Orengo JP, van der Heijden ME, Hao S, Tang J, Orr HT, Zoghbi HY. Motor neuron degeneration correlates with respiratory dysfunction in SCA1. Dis Model Mech. 2018;11. https://doi.org/10.1242/dmm.032623.
Suh J, Romano DM, Nitschke L, Herrick SP, DiMarzio BA, Dzhala V, et al. Loss of ataxin-1 potentiates Alzheimer’s pathogenesis by elevating cerebral BACE1 transcription. Cell. 2019;178:1159–75.e17. https://doi.org/10.1016/j.cell.2019.07.043.
Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17:1445–7. https://doi.org/10.1038/nm.2494.
Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4:e182. https://doi.org/10.1371/journal.pmed.0040182.
Dansithong W, Paul S, Figueroa KP, Rinehart MD, Wiest S, Pflieger LT, et al. Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model. PLoS Genet. 2015;11:e1005182. https://doi.org/10.1371/journal.pgen.1005182.
Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet. 2013;22:271–83. https://doi.org/10.1093/hmg/dds427.
Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26:44–50. https://doi.org/10.1038/79162.
Aguiar J, Fernández J, Aguilar A, Mendoza Y, Vázquez M, Suárez J, et al. Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specific Purkinje cell degeneration in transgenic mice. Neurosci Lett. 2006;392:202–6. https://doi.org/10.1016/j.neulet.2005.09.020.
Damrath E, Heck MV, Gispert S, Azizov M, Nowock J, Seifried C, et al. ATXN2-CAG42 sequesters PABPC1 into insolubility and induces FBXW8 in cerebellum of old ataxic knock-in mice. PLoS Genet. 2012;8:e1002920. https://doi.org/10.1371/journal.pgen.1002920.
Cemal CK, Carroll CJ, Lawrence L, Lowrie MB, Ruddle P, Al-Mahdawi S, et al. YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum Mol Genet. 2002;11:1075–94. https://doi.org/10.1093/hmg/11.9.1075.
Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28:12713–24. https://doi.org/10.1523/jneurosci.3909-08.2008.
Goti D, Katzen SM, Mez J, Kurtis N, Kiluk J, Ben-Haïem L, et al. A mutant ataxin-3 putative-cleavage fragment in brains of Machado-Joseph disease patients and transgenic mice is cytotoxic above a critical concentration. J Neurosci. 2004;24:10266–79. https://doi.org/10.1523/jneurosci.2734-04.2004.
Chou AH, Yeh TH, Ouyang P, Chen YL, Chen SY, Wang HL. Polyglutamine-expanded ataxin-3 causes cerebellar dysfunction of SCA3 transgenic mice by inducing transcriptional dysregulation. Neurobiol Dis. 2008;31:89–101. https://doi.org/10.1016/j.nbd.2008.03.011.
Boy J, Schmidt T, Wolburg H, Mack A, Nuber S, Bottcher M, et al. Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum Mol Genet. 2009;18:4282–95. https://doi.org/10.1093/hmg/ddp381.
Boy J, Schmidt T, Schumann U, Grasshoff U, Unser S, Holzmann C, et al. A transgenic mouse model of spinocerebellar ataxia type 3 resembling late disease onset and gender-specific instability of CAG repeats. Neurobiol Dis. 2010;37:284–93. https://doi.org/10.1016/j.nbd.2009.08.002.
Silva-Fernandes A, Costa Mdo C, Duarte-Silva S, Oliveira P, Botelho CM, Martins L, et al. Motor uncoordination and neuropathology in a transgenic mouse model of Machado-Joseph disease lacking intranuclear inclusions and ataxin-3 cleavage products. Neurobiol Dis. 2010;40:163–76. https://doi.org/10.1016/j.nbd.2010.05.021.
Haas E, Incebacak RD, Hentrich T, Maringer Y, Schmidt T, Zimmermann F, et al. A novel Ataxin-3 knock-in mouse model mimics the human SCA3 disease phenotype including neuropathological, behavioral, and transcriptional abnormalities. bioRxiv. 2020:2020.02.28.968024. doi https://doi.org/10.1101/2020.02.28.968024
Perkins EM, Clarkson YL, Sabatier N, Longhurst DM, Millward CP, Jack J, et al. Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J Neurosci. 2010;30:4857–67. https://doi.org/10.1523/jneurosci.6065-09.2010.
Armbrust KR, Wang X, Hathorn TJ, Cramer SW, Chen G, Zu T, et al. Mutant β-III spectrin causes mGluR1α mislocalization and functional deficits in a mouse model of spinocerebellar ataxia type 5. J Neurosci. 2014;34:9891–904. https://doi.org/10.1523/jneurosci.0876-14.2014.
Jayabal S, Ljungberg L, Erwes T, Cormier A, Quilez S, El Jaouhari S, et al. Rapid onset of motor deficits in a mouse model of spinocerebellar ataxia type 6 precedes late cerebellar degeneration. eNeuro. 2015;2. https://doi.org/10.1523/eneuro.0094-15.2015.
Unno T, Wakamori M, Koike M, Uchiyama Y, Ishikawa K, Kubota H, et al. Development of Purkinje cell degeneration in a knockin mouse model reveals lysosomal involvement in the pathogenesis of SCA6. Proc Natl Acad Sci U S A. 2012;109:17693–8. https://doi.org/10.1073/pnas.1212786109.
Garden GA, Libby RT, Fu YH, Kinoshita Y, Huang J, Possin DE, et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous Purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci. 2002;22:4897–905. https://doi.org/10.1523/jneurosci.22-12-04897.2002.
La Spada AR, Fu YH, Sopher BL, Libby RT, Wang X, Li LY, et al. Polyglutamine-expanded ataxin-7 antagonizes CRX function and induces cone-rod dystrophy in a mouse model of SCA7. Neuron. 2001;31:913–27. https://doi.org/10.1016/s0896-6273(01)00422-6.
Fusco AF, Pucci L, McCall AL, Dhindsa J, Kahn A, Switonski P, et al. Respiratory dysfunction in a mouse model of spinocerebellar ataxia 7. FASEB J. 2020;34:1. https://doi.org/10.1096/fasebj.2020.34.s1.05924.
Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38:758–69. https://doi.org/10.1038/ng1827.
Ho CS, Grange RW, Joho RH. Pleiotropic effects of a disrupted K+ channel gene: reduced body weight, impaired motor skill and muscle contraction, but no seizures. Proc Natl Acad Sci U S A. 1997;94:1533–8. https://doi.org/10.1073/pnas.94.4.1533.
Espinosa F, McMahon A, Chan E, Wang S, Ho CS, Heintz N, et al. Alcohol hypersensitivity, increased locomotion, and spontaneous myoclonus in mice lacking the potassium channels Kv3.1 and Kv3.3. J Neurosci. 2001;21:6657–65. https://doi.org/10.1523/jneurosci.21-17-06657.2001.
Joho RH, Street C, Matsushita S, Knöpfel T. Behavioral motor dysfunction in Kv3-type potassium channel-deficient mice. Genes Brain Behav. 2006;5:472–82. https://doi.org/10.1111/j.1601-183X.2005.00184.x.
Zhang Y, Snider A, Willard L, Takemoto DJ, Lin D. Loss of Purkinje cells in the PKCgamma H101Y transgenic mouse. Biochem Biophys Res Commun. 2009;378:524–8. https://doi.org/10.1016/j.bbrc.2008.11.082.
Ji J, Hassler ML, Shimobayashi E, Paka N, Streit R, Kapfhammer JP. Increased protein kinase C gamma activity induces Purkinje cell pathology in a mouse model of spinocerebellar ataxia 14. Neurobiol Dis. 2014;70:1–11. https://doi.org/10.1016/j.nbd.2014.06.002.
Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–71. https://doi.org/10.1038/379168a0.
Street VA, Bosma MM, Demas VP, Regan MR, Lin DD, Robinson LC, et al. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17:635–45. https://doi.org/10.1523/jneurosci.17-02-00635.1997.
van de Leemput J, Chandran J, Knight MA, Holtzclaw LA, Scholz S, Cookson MR, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. https://doi.org/10.1371/journal.pgen.0030108.
Friedman MJ, Shah AG, Fang ZH, Ward EG, Warren ST, Li S, et al. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nat Neurosci. 2007;10:1519–28. https://doi.org/10.1038/nn2011.
Portal E, Riess O, Nguyen HP. Automated home cage assessment shows behavioral changes in a transgenic mouse model of spinocerebellar ataxia type 17. Behav Brain Res. 2013;250:157–65. https://doi.org/10.1016/j.bbr.2013.04.042.
Chang YC, Lin CY, Hsu CM, Lin HC, Chen YH, Lee-Chen GJ, et al. Neuroprotective effects of granulocyte-colony stimulating factor in a novel transgenic mouse model of SCA17. J Neurochem. 2011;118:288–303. https://doi.org/10.1111/j.1471-4159.2011.07304.x.
Huang S, Ling JJ, Yang S, Li XJ, Li S. Neuronal expression of TATA box-binding protein containing expanded polyglutamine in knock-in mice reduces chaperone protein response by impairing the function of nuclear factor-Y transcription factor. Brain. 2011;134:1943–58. https://doi.org/10.1093/brain/awr146.
Yang S, Huang S, Gaertig MA, Li XJ, Li S. Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron. 2014;81:349–65. https://doi.org/10.1016/j.neuron.2013.12.002.
Huang S, Yang S, Guo J, Yan S, Gaertig MA, Li S, et al. Large polyglutamine repeats cause muscle degeneration in SCA17 mice. Cell Rep. 2015;13:196–208. https://doi.org/10.1016/j.celrep.2015.08.060.
Smeets CJ, Jezierska J, Watanabe H, Duarri A, Fokkens MR, Meijer M, et al. Elevated mutant dynorphin A causes Purkinje cell loss and motor dysfunction in spinocerebellar ataxia type 23. Brain. 2015;138:2537–52. https://doi.org/10.1093/brain/awv195.
Wang Q, Bardgett ME, Wong M, Wozniak DF, Lou J, McNeil BD, et al. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron. 2002;35:25–38. https://doi.org/10.1016/s0896-6273(02)00744-4.
Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM. Impaired spatial learning and defective theta burst induced LTP in mice lacking fibroblast growth factor 14. Neurobiol Dis. 2007;26:14–26. https://doi.org/10.1016/j.nbd.2006.11.014.
Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G. Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci. 2009;29:9244–54. https://doi.org/10.1523/jneurosci.1532-09.2009.
Maltecca F, Aghaie A, Schroeder DG, Cassina L, Taylor BA, Phillips SJ, et al. The mitochondrial protease AFG3L2 is essential for axonal development. J Neurosci. 2008;28:2827–36. https://doi.org/10.1523/jneurosci.4677-07.2008.
Hashiguchi S, Doi H, Kunii M, Nakamura Y, Shimuta M, Suzuki E, et al. Ataxic phenotype with altered Ca(V)3.1 channel property in a mouse model for spinocerebellar ataxia 42. Neurobiol Dis. 2019;130:104516. https://doi.org/10.1016/j.nbd.2019.104516.
Cook AA, Fields E, Watt AJ. Losing the beat: contribution of Purkinje cell firing dysfunction to disease, and its reversal. Neuroscience. 2020. https://doi.org/10.1016/j.neuroscience.2020.06.008.
Cui Y, Yang S, Li XJ, Li S. Genetically modified rodent models of SCA17. J Neurosci Res. 2017;95:1540–7. https://doi.org/10.1002/jnr.23984.
Colomer Gould VF. Mouse models of spinocerebellar ataxia type 3 (Machado-Joseph disease). Neurotherapeutics. 2012;9:285–96. https://doi.org/10.1007/s13311-012-0117-x.
Alves-Cruzeiro JM, Mendonça L, Pereira de Almeida L, Nóbrega C. Motor dysfunctions and neuropathology in mouse models of spinocerebellar ataxia type 2: a comprehensive review. Front Neurosci. 2016;10:572. https://doi.org/10.3389/fnins.2016.00572.
Bouskila M, Esoof N, Gay L, Fang EH, Deak M, Begley MJ, et al. TTBK2 kinase substrate specificity and the impact of spinocerebellar-ataxia-causing mutations on expression, activity, localization and development. Biochem J. 2011;437:157–67. https://doi.org/10.1042/bj20110276.
Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci. 2008;28:4640–8. https://doi.org/10.1523/jneurosci.5486-07.2008.
Matsuura T, Yamagata T, Burgess DL, Rasmussen A, Grewal RP, Watase K, et al. Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nat Genet. 2000;26:191–4. https://doi.org/10.1038/79911.
Diallo A, Jacobi H, Cook A, Labrum R, Durr A, Brice A, et al. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): a longitudinal cohort study. Lancet Neurol. 2018;17:327–34. https://doi.org/10.1016/s1474-4422(18)30042-5.
McMurtray AM, Clark DG, Flood MK, Perlman S, Mendez MF. Depressive and memory symptoms as presenting features of spinocerebellar ataxia. Journal of Neuropsychiatry & Clinical Neurosciences. 2006;18:420–2. https://doi.org/10.1176/jnp.2006.18.3.420.
Fancellu R, Paridi D, Tomasello C, Panzeri M, Castaldo A, Genitrini S, et al. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J Neurol. 2013;260:3134–43. https://doi.org/10.1007/s00415-013-7138-1.
Lo RY, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez C, et al. Depression and clinical progression in spinocerebellar ataxias. Parkinsonism Relat Disord. 2016;22:87–92. https://doi.org/10.1016/j.parkreldis.2015.11.021.
Asher M, Johnson A, Zecevic B, Pease D, Cvetanovic M. Ataxin-1 regulates proliferation of hippocampal neural precursors. Neuroscience. 2016;322:54–65. https://doi.org/10.1016/j.neuroscience.2016.02.011.
Cvetanovic M, Hu YS, Opal P. Mutant ataxin-1 inhibits neural progenitor cell proliferation in SCA1. Cerebellum. 2017;16:340–7. https://doi.org/10.1007/s12311-016-0794-9.
Hatanaka Y, Watase K, Wada K, Nagai Y. Abnormalities in synaptic dynamics during development in a mouse model of spinocerebellar ataxia type 1. Sci Rep. 2015;5:16102. https://doi.org/10.1038/srep16102.
Paucar M, Lundin J, Alshammari T, Bergendal Å, Lindefeldt M, Alshammari M, et al. Broader phenotypic traits and widespread brain hypometabolism in spinocerebellar ataxia 27. J Intern Med. 2020;288:103–15. https://doi.org/10.1111/joim.13052.
Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J Rare Dis. 2016;11:82. https://doi.org/10.1186/s13023-016-0447-6.
Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, et al. Consensus Paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15:369–91. https://doi.org/10.1007/s12311-015-0687-3.
Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77. https://doi.org/10.1007/s12311-013-0511-x.
Amokrane N, Viswanathan A, Freedman S, Yang CY, Desai NA, Pan MK, et al. Impulsivity in cerebellar ataxias: testing the cerebellar reward hypothesis in humans. Mov Disord. 2020;35:1491–3. https://doi.org/10.1002/mds.28121.
Cendelin J, Tichanek F. Cerebellar degeneration averts blindness-induced despaired behavior during spatial task in mice. Neurosci Lett. 2020;722:134854. https://doi.org/10.1016/j.neulet.2020.134854.
Tuma J, Kolinko Y, Vozeh F, Cendelin J. Mutation-related differences in exploratory, spatial, and depressive-like behavior in pcd and Lurcher cerebellar mutant mice. Front Behav Neurosci. 2015;9:116. https://doi.org/10.3389/fnbeh.2015.00116.
Asher M, Rosa JG, Cvetanovic M. Mood alterations in mouse models of spinocerebellar ataxia type 1. Sci Rep. 2021;11:713. https://doi.org/10.1038/s41598-020-80664-9.
Argyropoulos GPD, van Dun K, Adamaszek M, Leggio M, Manto M, Masciullo M, et al. The cerebellar cognitive affective/Schmahmann syndrome: a task force paper. Cerebellum. 2020;19:102–25. https://doi.org/10.1007/s12311-019-01068-8.
Yamamoto M, Kim M, Imai H, Itakura Y, Ohtsuki G. Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Rep. 2019;28:2923–38.e8. https://doi.org/10.1016/j.celrep.2019.07.078.
Perez-Lloret S, van de Warrenburg B, Rossi M, Rodríguez-Blázquez C, Zesiewicz T, Saute JAM, et al. Assessment of ataxia rating scales and cerebellar functional tests: critique and recommendations. Mov Disord. 2021;36:283–97. https://doi.org/10.1002/mds.28313.
Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24:1820–8. https://doi.org/10.1002/mds.22681.
Schmitz-Hübsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008;71:982–9. https://doi.org/10.1212/01.wnl.0000325057.33666.72.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20. https://doi.org/10.1212/01.wnl.0000219042.60538.92.
Kieling C, Rieder CR, Silva AC, Saute JA, Cecchin CR, Monte TL, et al. A neurological examination score for the assessment of spinocerebellar ataxia 3 (SCA3). Eur J Neurol. 2008;15:371–6. https://doi.org/10.1111/j.1468-1331.2008.02078.x.
Assadi M, Leone P, Veloski JJ, Schwartzman RJ, Janson CG, Campellone JV. Validating an ataxia functional composite scale in spinocerebellar ataxia. J Neurol Sci. 2008;268:136–9. https://doi.org/10.1016/j.jns.2007.11.016.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. https://doi.org/10.1016/s0022-510x(96)00231-6.
Zesiewicz TA, Wilmot G, Kuo SH, Perlman S, Greenstein PE, Ying SH, et al. Comprehensive systematic review summary: treatment of cerebellar motor dysfunction and ataxia: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:464–71. https://doi.org/10.1212/wnl.0000000000005055.
Manto M, Gandini J, Feil K, Strupp M. Cerebellar ataxias: an update. Curr Opin Neurol. 2020;33:150–60. https://doi.org/10.1097/wco.0000000000000774.
Feil K, Adrion C, Teufel J, Bösch S, Claassen J, Giordano I, et al. Effects of acetyl-DL-leucine on cerebellar ataxia (ALCAT trial): study protocol for a multicenter, multinational, randomized, double-blind, placebo-controlled, crossover phase III trial. BMC Neurol. 2017;17:7. https://doi.org/10.1186/s12883-016-0786-x.
Fields T, Patterson M, Bremova-Ertl T, Belcher G, Billington I, Churchill GC, et al. A master protocol to investigate a novel therapy acetyl-L-leucine for three ultra-rare neurodegenerative diseases: Niemann-Pick type C, the GM2 gangliosidoses, and ataxia telangiectasia. Trials. 2021;22:84. https://doi.org/10.1186/s13063-020-05009-3.
Grobe-Einsler M, Vogt IR, Schaprian T, Hurlemann R, Klockgether T, Kaut O. Effects of rivastigmine on patients with spinocerebellar ataxia type 3: a case series of five patients. Neurodegener Dis. 2020;20:104–9. https://doi.org/10.1159/000510057.
Bremova-Ertl T, Platt F, Strupp M. Sandhoff disease: improvement of gait by acetyl-DL-leucine: a case report. Neuropediatrics. 2020;51:450–2. https://doi.org/10.1055/s-0040-1715486.
Ilg W, Synofzik M, Brötz D, Burkard S, Giese MA, Schöls L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73:1823–30. https://doi.org/10.1212/WNL.0b013e3181c33adf.
Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabilitation & Neural Repair. 2012;26:515–22. https://doi.org/10.1177/1545968311425918.
Chuang CS, Chang JC, Soong BW, Chuang SF, Lin TT, Cheng WL, et al. Treadmill training increases the motor activity and neuron survival of the cerebellum in a mouse model of spinocerebellar ataxia type 1. Kaohsiung J Med Sci. 2019;35:679–85. https://doi.org/10.1002/kjm2.12106.
Salomova M, Tichanek F, Jelinkova D, Cendelin J. Forced activity and environmental enrichment mildly improve manifestation of rapid cerebellar degeneration in mice. Behav Brain Res. 2021;401:113060. https://doi.org/10.1016/j.bbr.2020.113060.
Romano S, Coarelli G, Marcotulli C, Leonardi L, Piccolo F, Spadaro M, et al. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:985–91. https://doi.org/10.1016/s1474-4422(15)00201-x.
Nag N, Tarlac V, Storey E. Assessing the efficacy of specific cerebellomodulatory drugs for use as therapy for spinocerebellar ataxia type 1. Cerebellum. 2013;12:74–82. https://doi.org/10.1007/s12311-012-0399-x.
Schmidt J, Schmidt T, Golla M, Lehmann L, Weber JJ, Hübener-Schmid J, et al. In vivo assessment of riluzole as a potential therapeutic drug for spinocerebellar ataxia type 3. J Neurochem. 2016;138:150–62. https://doi.org/10.1111/jnc.13606.
Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78:545–50. https://doi.org/10.1212/WNL.0b013e318247cc7a.
Connolly BS, Prashanth LK, Shah BB, Marras C, Lang AE. A randomized trial of varenicline (chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;79:2218. https://doi.org/10.1212/WNL.0b013e318278a059.
Filla A, Sacca F, De Michele G. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78:1538. https://doi.org/10.1212/WNL.0b013e318257ea5d.
Mendonça N, França MC Jr, Gonçalves AF, Januário C. Clinical features of Machado-Joseph Disease. Adv Exp Med Biol. 2018;1049:255–73. https://doi.org/10.1007/978-3-319-71779-1_13.
Wecker L, Engberg ME, Philpot RM, Lambert CS, Kang CW, Antilla JC, et al. Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology. 2013;73:75–86. https://doi.org/10.1016/j.neuropharm.2013.05.016.
ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01970098. Accessed 9 January 2021.
Nishizawa M, Onodera O, Hirakawa A, Shimizu Y, Yamada M. Effect of rovatirelin in patients with cerebellar ataxia: two randomised double-blind placebo-controlled phase 3 trials. J Neurol Neurosurg Psychiatry. 2020;91:254–62. https://doi.org/10.1136/jnnp-2019-322168.
Nakamura T, Honda M, Kimura S, Tanabe M, Oda S, Ono H. Taltirelin improves motor ataxia independently of monoamine levels in rolling mouse Nagoya, a model of spinocerebellar atrophy. Biol Pharm Bull. 2005;28:2244–7. https://doi.org/10.1248/bpb.28.2244.
Ijiro T, Yaguchi A, Yokoyama A, Abe Y, Kiguchi S. Ameliorating effect of rovatirelin on the ataxia in rolling mouse Nagoya. Eur J Pharmacol. 2020;882:173271. https://doi.org/10.1016/j.ejphar.2020.173271.
Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–82. https://doi.org/10.1046/j.1471-4159.1999.720879.x.
Perroud B, Jafar-Nejad P, Wikoff WR, Gatchel JR, Wang L, Barupal DK, et al. Pharmacometabolomic signature of ataxia SCA1 mouse model and lithium effects. PLoS One. 2013;8:e70610. https://doi.org/10.1371/journal.pone.0070610.
Saute JA, de Castilhos RM, Monte TL, Schumacher-Schuh AF, Donis KC, D'Ávila R, et al. A randomized, phase 2 clinical trial of lithium carbonate in Machado-Joseph disease. Mov Disord. 2014;29:568–73. https://doi.org/10.1002/mds.25803.
Duarte-Silva S, Neves-Carvalho A, Soares-Cunha C, Teixeira-Castro A, Oliveira P, Silva-Fernandes A, et al. Lithium chloride therapy fails to improve motor function in a transgenic mouse model of Machado-Joseph disease. Cerebellum. 2014;13:713–27. https://doi.org/10.1007/s12311-014-0589-9.
Duarte-Silva S, Silva-Fernandes A, Neves-Carvalho A, Soares-Cunha C, Teixeira-Castro A, Maciel P. Combined therapy with m-TOR-dependent and -independent autophagy inducers causes neurotoxicity in a mouse model of Machado-Joseph disease. Neuroscience. 2016;313:162–73. https://doi.org/10.1016/j.neuroscience.2015.11.030.
Awaad Y, Sansaricq C, Moroney J, Fish I, Kyriakakos A, Snyderman SE. Baclofen in the treatment of polymyoclonus and ataxia in a patient with homocystinuria. J Child Neurol. 1995;10:294–6. https://doi.org/10.1177/088307389501000408.
Bushart DD, Chopra R, Singh V, Murphy GG, Wulff H, Shakkottai VG. Targeting potassium channels to treat cerebellar ataxia. Ann Clin Transl Neurol. 2018;5:297–314. https://doi.org/10.1002/acn3.527.
Bushart DD, Huang H, Man LJ, Morrison LM, Shakkottai VG. A chlorzoxazone-baclofen combination improves cerebellar impairment in spinocerebellar ataxia type 1. Mov Disord. 2020. https://doi.org/10.1002/mds.28355.
Chopra R, Bushart DD, Shakkottai VG. Dendritic potassium channel dysfunction may contribute to dendrite degeneration in spinocerebellar ataxia type 1. PLoS One. 2018;13:e0198040. https://doi.org/10.1371/journal.pone.0198040.
Ashizawa T, Öz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat Rev Neurol. 2018;14:590–605. https://doi.org/10.1038/s41582-018-0051-6.
Keiser MS, Boudreau RL, Davidson BL. Broad therapeutic benefit after RNAi expression vector delivery to deep cerebellar nuclei: implications for spinocerebellar ataxia type 1 therapy. Mol Ther. 2014;22:588–95. https://doi.org/10.1038/mt.2013.279.
Keiser MS, Kordower JH, Gonzalez-Alegre P, Davidson BL. Broad distribution of ataxin 1 silencing in rhesus cerebella for spinocerebellar ataxia type 1 therapy. Brain. 2015;138:3555–66. https://doi.org/10.1093/brain/awv292.
Costa Mdo C, Luna-Cancalon K, Fischer S, Ashraf NS, Ouyang M, Dharia RM, et al. Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease. Mol Ther. 2013;21:1898–908. https://doi.org/10.1038/mt.2013.144.
Salvi J, Bertaso F, Mausset-Bonnefont AL, Metz A, Lemmers C, Ango F, et al. RNAi silencing of P/Q-type calcium channels in Purkinje neurons of adult mouse leads to episodic ataxia type 2. Neurobiol Dis. 2014;68:47–56. https://doi.org/10.1016/j.nbd.2014.04.005.
Tsou WL, Soong BW, Paulson HL, Rodríguez-Lebrón E. Splice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6. Neurobiol Dis. 2011;43:533–42. https://doi.org/10.1016/j.nbd.2011.04.016.
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–20. https://doi.org/10.1038/nm1076.
Silva AC, Lobo DD, Martins IM, Lopes SM, Henriques C, Duarte SP, et al. Antisense oligonucleotide therapeutics in neurodegenerative diseases: the case of polyglutamine disorders. Brain. 2020;143:407–29. https://doi.org/10.1093/brain/awz328.
Scoles DR, Pulst SM. Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 2018;15:707–14. https://doi.org/10.1080/15476286.2018.1454812.
Niu C, Prakash TP, Kim A, Quach JL, Huryn LA, Yang Y, et al. Antisense oligonucleotides targeting mutant ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med. 2018;10. https://doi.org/10.1126/scitranslmed.aap8677.
Hosp F, Vossfeldt H, Heinig M, Vasiljevic D, Arumughan A, Wyler E, et al. Quantitative interaction proteomics of neurodegenerative disease proteins. Cell Rep. 2015;11:1134–46. https://doi.org/10.1016/j.celrep.2015.04.030.
Rousseaux MWC, Tschumperlin T, Lu HC, Lackey EP, Bondar VV, Wan YW, et al. ATXN1-CIC complex is the primary driver of cerebellar pathology in spinocerebellar ataxia type 1 through a gain-of-function mechanism. Neuron. 2018;97:1235–43.e5. https://doi.org/10.1016/j.neuron.2018.02.013.
Nóbrega C, Mendonça L, Marcelo A, Lamazière A, Tomé S, Despres G, et al. Restoring brain cholesterol turnover improves autophagy and has therapeutic potential in mouse models of spinocerebellar ataxia. Acta Neuropathol. 2019;138:837–58. https://doi.org/10.1007/s00401-019-02019-7.
Mookerjee S, Papanikolaou T, Guyenet SJ, Sampath V, Lin A, Vitelli C, et al. Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment. J Neurosci. 2009;29:15134–44. https://doi.org/10.1523/jneurosci.4720-09.2009.
Djajadikerta A, Keshri S, Pavel M, Prestil R, Ryan L, Rubinsztein DC. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J Mol Biol. 2020;432:2799–821. https://doi.org/10.1016/j.jmb.2019.12.035.
Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–17. https://doi.org/10.1093/hmg/11.9.1107.
Alves S, Cormier-Dequaire F, Marinello M, Marais T, Muriel MP, Beaumatin F, et al. The autophagy/lysosome pathway is impaired in SCA7 patients and SCA7 knock-in mice. Acta Neuropathol. 2014;128:705–22. https://doi.org/10.1007/s00401-014-1289-8.
Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum. 2012;11:630–9. https://doi.org/10.1007/s12311-010-0182-9.
Hekman KE, Gomez CM. The autosomal dominant spinocerebellar ataxias: emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J Neurol Neurosurg Psychiatry. 2015;86:554–61. https://doi.org/10.1136/jnnp-2014-308421.
Meera P, Pulst SM, Otis TS. Cellular and circuit mechanisms underlying spinocerebellar ataxias. J Physiol. 2016;594:4653–60. https://doi.org/10.1113/jp271897.
Bushart DD, Shakkottai VG. Ion channel dysfunction in cerebellar ataxia. Neurosci Lett. 2019;688:41–8. https://doi.org/10.1016/j.neulet.2018.02.005.
Edamakanti CR, Do J, Didonna A, Martina M, Opal P. Mutant ataxin1 disrupts cerebellar development in spinocerebellar ataxia type 1. J Clin Invest. 2018;128:2252–65. https://doi.org/10.1172/jci96765.
Figueroa KP, Minassian NA, Stevanin G, Waters M, Garibyan V, Forlani S, et al. KCNC3: phenotype, mutations, channel biophysics-a study of 260 familial ataxia patients. Hum Mutat. 2010;31:191–6. https://doi.org/10.1002/humu.21165.
Pulst SM, Otis TS. Repolarization matters: mutations in the Kv4.3 potassium channel cause SCA19/22. Ann Neurol. 2012;72:829–31. https://doi.org/10.1002/ana.23803.
Lee YC, Durr A, Majczenko K, Huang YH, Liu YC, Lien CC, et al. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann Neurol. 2012;72:859–69. https://doi.org/10.1002/ana.23701.
Hsieh JY, Ulrich BN, Issa FA, Lin MA, Brown B, Papazian DM. Infant and adult SCA13 mutations differentially affect Purkinje cell excitability, maturation, and viability in vivo. Elife. 2020;9. https://doi.org/10.7554/eLife.57358.
Dell'Orco JM, Wasserman AH, Chopra R, Ingram MA, Hu YS, Singh V, et al. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J Neurosci. 2015;35:11292–307. https://doi.org/10.1523/jneurosci.1357-15.2015.
Egorova PA, Zakharova OA, Vlasova OL, Bezprozvanny IB. In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol. 2016;115:2840–51. https://doi.org/10.1152/jn.00913.2015.
Kasumu AW, Liang X, Egorova P, Vorontsova D, Bezprozvanny I. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32:12786–96. https://doi.org/10.1523/jneurosci.1643-12.2012.
Ferro A, Carbone E, Zhang J, Marzouk E, Villegas M, Siegel A, et al. Short-term succinic acid treatment mitigates cerebellar mitochondrial OXPHOS dysfunction, neurodegeneration and ataxia in a Purkinje-specific spinocerebellar ataxia type 1 (SCA1) mouse model. PLoS One. 2017;12:e0188425. https://doi.org/10.1371/journal.pone.0188425.
Harmuth T, Prell-Schicker C, Weber JJ, Gellerich F, Funke C, Drießen S, et al. Mitochondrial morphology, function and homeostasis are impaired by expression of an N-terminal calpain cleavage fragment of ataxin-3. Front Mol Neurosci. 2018;11:368. https://doi.org/10.3389/fnmol.2018.00368.
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–16. https://doi.org/10.1038/nn.4222.
Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22:269–85. https://doi.org/10.1016/j.celrep.2017.12.039.
Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–11. https://doi.org/10.1038/nn1750.
Lattke M, Reichel SN, Magnutzki A, Abaei A, Rasche V, Walther P, et al. Transient IKK2 activation in astrocytes initiates selective non-cell-autonomous neurodegeneration. Mol Neurodegener. 2017;12:16. https://doi.org/10.1186/s13024-017-0157-0.
Aikawa T, Mogushi K, Iijima-Tsutsui K, Ishikawa K, Sakurai M, Tanaka H, et al. Loss of MyD88 alters neuroinflammatory response and attenuates early Purkinje cell loss in a spinocerebellar ataxia type 6 mouse model. Hum Mol Genet. 2015;24:4780–91. https://doi.org/10.1093/hmg/ddv202.
Kim JH, Lukowicz A, Qu W, Johnson A, Cvetanovic M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia. 2018;66:1972–87. https://doi.org/10.1002/glia.23451.
Qu W, Johnson A, Kim JH, Lukowicz A, Svedberg D, Cvetanovic M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J Neuroinflammation. 2017;14:107. https://doi.org/10.1186/s12974-017-0880-z.
Cendelin J, Buffo A, Hirai H, Magrassi L, Mitoma H, Sherrard R, et al. Task force paper on cerebellar transplantation: are we ready to treat cerebellar disorders with cell therapy? Cerebellum. 2019. https://doi.org/10.1007/s12311-018-0999-1.
Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, et al. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13:913–7. https://doi.org/10.3109/14653249.2011.579958.
Tsai YA, Liu RS, Lirng JF, Yang BH, Chang CH, Wang YC, et al. Treatment of spinocerebellar ataxia with mesenchymal stem cells: a phase I/IIa clinical study. Cell Transplant. 2017;26:503–12. https://doi.org/10.3727/096368916x694373.
Sotelo C, Alvarado-Mallart RM. Embryonic and adult neurons interact to allow Purkinje cell replacement in mutant cerebellum. Nature. 1987;327:421–3. https://doi.org/10.1038/327421a0.
Sotelo C, Alvarado-Mallart RM. Reconstruction of the defective cerebellar circuitry in adult Purkinje cell degeneration mutant mice by Purkinje cell replacement through transplantation of solid embryonic implants. Neuroscience. 1987;20:1–22.
Takayama H, Kohsaka S, Shinozaki T, Inoue H, Toya S, Ueda T, et al. Immunohistochemical studies on synapse formation by embryonic cerebellar tissue transplanted into the cerebellum of the weaver mutant mouse. Neurosci Lett. 1987;79:246–50.
Tomey DA, Heckroth JA. Transplantation of normal embryonic cerebellar cell suspensions into the cerebellum of lurcher mutant mice. Exp Neurol. 1993;122:165–70. https://doi.org/10.1006/exnr.1993.1117.
Triarhou LC, Zhang W, Lee WH. Graft-induced restoration of function in hereditary cerebellar ataxia. Neuroreport. 1995;6:1827–32.
Triarhou LC, Zhang W, Lee WH. Amelioration of the behavioral phenotype in genetically ataxic mice through bilateral intracerebellar grafting of fetal Purkinje cells. Cell Transplant. 1996;5:269–77.
Li J, Imitola J, Snyder EY, Sidman RL. Neural stem cells rescue nervous Purkinje neurons by restoring molecular homeostasis of tissue plasminogen activator and downstream targets. J Neurosci. 2006;26:7839–48. https://doi.org/10.1523/jneurosci.1624-06.2006.
Kaemmerer WF, Low WC. Cerebellar allografts survive and transiently alleviate ataxia in a transgenic model of spinocerebellar ataxia type-1. Exp Neurol. 1999;158:301–11. https://doi.org/10.1006/exnr.1999.7099.
Chang YK, Chen MH, Chiang YH, Chen YF, Ma WH, Tseng CY, et al. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci. 2011;18:54. https://doi.org/10.1186/1423-0127-18-54.
Matsuura S, Shuvaev AN, Iizuka A, Nakamura K, Hirai H. Mesenchymal stem cells ameliorate cerebellar pathology in a mouse model of spinocerebellar ataxia type 1. Cerebellum. 2014;13:323–30. https://doi.org/10.1007/s12311-013-0536-1.
Mendonca LS, Nobrega C, Hirai H, Kaspar BK, Pereira de Almeida L. Transplantation of cerebellar neural stem cells improves motor coordination and neuropathology in Machado-Joseph disease mice. Brain. 2015;138:320–35. https://doi.org/10.1093/brain/awu352.
Purkartova Z, Tuma J, Pesta M, Kulda V, Hajkova L, Sebesta O, et al. Morphological analysis of embryonic cerebellar grafts in SCA2 mice. Neurosci Lett. 2014;558:154–8. https://doi.org/10.1016/j.neulet.2013.11.020.
Cendelin J, Mitoma H, Manto M. Neurotransplantation therapy and cerebellar reserve. CNS Neurol Disord Drug Targets. 2018;17:172–83. https://doi.org/10.2174/1871527316666170810114559.
Li T, Liu Y, Yu L, Lao J, Zhang M, Jin J, et al. Human umbilical cord mesenchymal stem cells protect against SCA3 by modulating the level of 70 kD heat shock protein. Cell Mol Neurobiol. 2018;38:641–55. https://doi.org/10.1007/s10571-017-0513-1.
Jones J, Jaramillo-Merchan J, Bueno C, Pastor D, Viso-Leon M, Martinez S. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis. 2010;40:415–23. https://doi.org/10.1016/j.nbd.2010.07.001.
Chen KA, Cruz PE, Lanuto DJ, Flotte TR, Borchelt DR, Srivastava A, et al. Cellular fusion for gene delivery to SCA1 affected Purkinje neurons. Mol Cell Neurosci. 2011;47:61–70. https://doi.org/10.1016/j.mcn.2011.03.003.
Huda F, Fan Y, Suzuki M, Konno A, Matsuzaki Y, Takahashi N, et al. Fusion of human fetal mesenchymal stem cells with “degenerating” cerebellar neurons in spinocerebellar ataxia type 1 model mice. PLoS One. 2016;11:e0164202. https://doi.org/10.1371/journal.pone.0164202.
Chintawar S, Hourez R, Ravella A, Gall D, Orduz D, Rai M, et al. Grafting neural precursor cells promotes functional recovery in an SCA1 mouse model. J Neurosci. 2009;29:13126–35. https://doi.org/10.1523/jneurosci.0647-09.2009.
Hsieh J, Liu JW, Harn HJ, Hsueh KW, Rajamani K, Deng YC, et al. Human olfactory ensheathing cell transplantation improves motor function in a mouse model of type 3 spinocerebellar ataxia. Cell Transplant. 2017;26:1611–21. https://doi.org/10.1177/0963689717732578.
Babuska V, Houdek Z, Tuma J, Purkartova Z, Tumova J, Kralickova M, et al. Transplantation of embryonic cerebellar grafts improves gait parameters in ataxic Lurcher mice. Cerebellum. 2015;14:632–41. https://doi.org/10.1007/s12311-015-0656-x.
Fuca E, Guglielmotto M, Boda E, Rossi F, Leto K, Buffo A. Preventive motor training but not progenitor grafting ameliorates cerebellar ataxia and deregulated autophagy in tambaleante mice. Neurobiol Dis. 2017;102:49–59. https://doi.org/10.1016/j.nbd.2017.02.005.
Cendelin J, Purkartova Z, Kubik J, Ulbricht E, Tichanek F, Kolinko Y. Long-term development of embryonic cerebellar grafts in two strains of Lurcher mice. Cerebellum. 2018;17:428–37. https://doi.org/10.1007/s12311-018-0928-3.
Purkartova Z, Tichanek F, Kolinko Y, Cendelin J. Embryonic cerebellar graft morphology differs in two mouse models of cerebellar degeneration. Cerebellum. 2019;18:855–65. https://doi.org/10.1007/s12311-019-01067-9.
Houdek Z, Cendelin J, Kulda V, Babuska V, Cedikova M, Kralickova M, et al. Intracerebellar application of P19-derived neuroprogenitor and naive stem cells to Lurcher mutant and wild type B6CBA mice. Med Sci Monit. 2012;18:Br174–80.
Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976;73:208–12.
Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287:167–201.
Berezniuk I, Fricker LD. A defect in cytosolic carboxypeptidase 1 (Nna1) causes autophagy in Purkinje cell degeneration mouse brain. Autophagy. 2010;6:558–9. https://doi.org/10.4161/auto.6.4.11813.
Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–73. https://doi.org/10.1038/42009.
Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, et al. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480:543–6. https://doi.org/10.1038/nature10671.
Wong MMK, Hoekstra SD, Vowles J, Watson LM, Fuller G, Németh AH, et al. Neurodegeneration in SCA14 is associated with increased PKCγ kinase activity, mislocalization and aggregation. Acta Neuropathol Commun. 2018;6:99. https://doi.org/10.1186/s40478-018-0600-7.
Chuang CY, Yang CC, Soong BW, Yu CY, Chen SH, Huang HP, et al. Modeling spinocerebellar ataxias 2 and 3 with iPSCs reveals a role for glutamate in disease pathology. Sci Rep. 2019;9:1166. https://doi.org/10.1038/s41598-018-37774-2.
Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–67. https://doi.org/10.1080/14734220701490995.
Mariën P, Beaton A. The enigmatic linguistic cerebellum: clinical relevance and unanswered questions on nonmotor speech and language deficits in cerebellar disorders. Cerebellum Ataxias. 2014;1:12. https://doi.org/10.1186/2053-8871-1-12.
Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. https://doi.org/10.1007/s12311-014-0627-7.
Moro A, Moscovich M, Farah M, Camargo CHF, Teive HAG, Munhoz RP. Nonmotor symptoms in spinocerebellar ataxias (SCAs). Cerebellum Ataxias. 2019;6:12. https://doi.org/10.1186/s40673-019-0106-5.
Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JDE, et al. Consensus paper: cerebellum and social cognition. Cerebellum. 2020. https://doi.org/10.1007/s12311-020-01155-1.
Hilber P, Cendelin J, Le Gall A, Machado ML, Tuma J, Besnard S. Cooperation of the vestibular and cerebellar networks in anxiety disorders and depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2019;89:310–21. https://doi.org/10.1016/j.pnpbp.2018.10.004.
Tuma J, Kolinko Y, Jelinkova D, Hilber P, Cendelin J. Impaired spatial performance in cerebellar-deficient Lurcher mice is not associated with their abnormal stress response. Neurobiol Learn Mem. 2017;140:62–70. https://doi.org/10.1016/j.nlm.2017.02.009.
Lorivel T, Cendelin J, Hilber P. Familiarization effects on the behavioral disinhibition of the cerebellar Lurcher mutant mice: use of the innovative Dual Maze. Behav Brain Res. 2021;398:112972. https://doi.org/10.1016/j.bbr.2020.112972.
Ouyang S, Xie Y, Xiong Z, Yang Y, Xian Y, Ou Z, et al. CRISPR/Cas9-targeted deletion of polyglutamine in spinocerebellar ataxia type 3-derived induced pluripotent stem cells. Stem Cells Dev. 2018;27:756–70. https://doi.org/10.1089/scd.2017.0209.
Funding
The work on this article was supported by the following grants and projects: Charles University Research Fund (project number Q39 and project no. CZ.02.1.01/0.0/0.0/16_019/0000787) “Fighting INfectious Diseases,” awarded by the Ministry of Education, Youth and Sports of the Czech Republic, financed from the European Regional Development Fund (Jan Cendelin, Filip Tichanek, and Jan Tuma); R01 NS197387, R01NS109077, National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) (Marija Cvetanovic); Brain/MINDS from Japan Agency for Medical Research and development, AMED (Grant Number JP20dm0207057), KAKENHI (Grant Number 18H02521) (Hirokazu Hirai); grants R37NS033123, R21NSNS103009, and UO1NS103883 from the National Institutes of Health (USA) (Stefan M. Pulst); (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R21NS103009, R01NS097903, R37NS033123, and U01NS103883 (Mandi Gandelman)Gandelman); and National Institutes of Health/National Institute of Neurological Disorders and Stroke grants RO1-NS022920 and RO1-NS045667 (Harry T. Orr).
Author information
Authors and Affiliations
Contributions
The authors were responsible for drafting specific sections, and all of them revised and contributed to the entire article.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Conflict of Interest
M. Strupp is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology, and Section Editor of F1000. He has received speaker’s honoraria from Abbott, Actelion, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, MSD, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris. He is a shareholder and investor of IntraBio. He is the distributor of “M-glasses” and the “Positional vertigo” App. He acts as a consultant for Abbott, Actelion, Auris Medical, Heel, IntraBio, and Sensorion. M. Manto is Editor-in-Chief of The Cerebellum and Cerebellum and Ataxias. He has received royalties from Springer, Cambridge University Press, and Elsevier.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cendelin, J., Cvetanovic, M., Gandelman, M. et al. Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications. Cerebellum 21, 452–481 (2022). https://doi.org/10.1007/s12311-021-01311-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-021-01311-1