Abstract
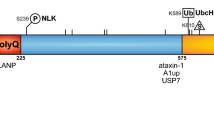
Spinocerebellar ataxia type 1 (SCA1) is one of nine polyglutamine (polyQ) diseases and is characterized as an adult late-onset, progressive, dominantly inherited genetic disease. SCA1 is caused by an increase in the number of CAG repeats in the ATXN1 gene leading to an expanded polyQ tract in the ATAXIN-1 protein. ATAXIN-1 is broadly expressed throughout the brain. However, until recently, SCA1 research has primarily centered on the cerebellum, given the characteristic cerebellar Purkinje cell loss observed in patients, as well as the progressive motor deficits, including gait and limb incoordination, that SCA1 patients present with. There are, however, also other symptoms such as respiratory problems, cognitive defects and memory impairment, anxiety, and depression observed in SCA1 patients and mouse models, which indicate that there are extra-cerebellar effects of SCA1 that cannot be explained solely through changes in the cerebellar region of the brain alone. The existing gap between human and mouse model studies of extra-cerebellar regions in SCA1 makes it difficult to answer many important questions in the field. This review will cover both the cerebellar and extra-cerebellar effects of SCA1 and highlight the need for further investigations into the impact of mutant ATXN1 expression in these regions. This review will also discuss implications of extra-cerebellar effects not only for SCA1 but other neurodegenerative diseases showing diverse pathology as well.



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- SCA1:
-
Spinocerebellar ataxia type 1
- CAG:
-
Cytosine-adenine-guanine
- polyQ:
-
Polyglutamine
- SCAs:
-
Spinocerebellar ataxias
- MRI:
-
Magnetic resonance imaging
- CNS:
-
Central nervous system
- CIC:
-
Capicua
- RBMs:
-
RNA binding motif proteins
- PC:
-
Purkinje cell
- CCAS:
-
Cerebellar cognitive affective syndrome
- WT:
-
Wild-type
- Tg:
-
Transgenic
- Pcp2:
-
Purkinje cell protein 2
- KI:
-
Knock-in
- PHQ:
-
Patient Health Questionnaire
- KO:
-
Knock-out
- ASO:
-
Antisense oligonucleotide
- CMAP:
-
Compound motor action potential
- SGL:
-
Subgranular layer
- NIs:
-
Nuclear inclusions
- CA1:
-
Cornu Ammonis 1
- CA2:
-
Cornu Ammonis 1
- GM:
-
Gray matter
- WM:
-
White matter
- SCA2:
-
Spinocerebellar ataxia type 2
- SCA3:
-
Spinocerebellar ataxia type 3
- HD:
-
Huntington’s disease
- TFs:
-
Transcription factors
- NLS:
-
Nuclear localization signal
- SAR:
-
Self-association region
References
Orr HT, Chung MYI, Banfi S, Kwiatkowski TJ, Servadio A, Beaudet AL et al (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4:221–226
Zoghbi HY, Orr HT (2009) Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, Spinocerebellar ataxia type 1. J Biol Chem 284:7425–7429
Ruano L, Melo C, Silva MC, Coutinho P (2014) The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology 42:174–183
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Spinocerebellar Ataxias 3:291–304
Ranum LPW, Chung MY, Banfi S, Bryer A, Schut LJ, Ramesar R et al (1994) Molecular and clinical correlations in spinocerebellar ataxia type I: Evidence for familial effects on the age at onset. Am J Hum Genet 55:244–252
Matilla T, Volpini V, Genis D, Rosell J, Corral J, Davalos A et al (1993) Presymptomatic analysis of spinocerebellar ataxia type 1 (SCA1) via the expansion of the SCA1 CAG-repeat in a large pedigree displaying anticipation and parental male bias. Hum Mol Genet 2:2123–2128
Globas C, du Montcel ST, Baliko L, Boesch S, Depondt C, DiDonato S et al (2008) Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord 23:2232–2238
Kameya T, Abe K, Aoki M, Sahara M, Tobita M, Konno H et al (1995) Analysis of spinocerebellar ataxia type 1 (sca1)-related cag trinucleotide expansion in japan. Neurology 45:1587–1594
Goldfarb LG, Vasconcelos O, Platonov FA, Lunkes A, Kipnis V, Kononova S et al (1996) Unstable triplet repeat and phenotypic variability of spinocerebellar ataxia type 1. Ann Neurol 39:500–506
Burright EN, Brent Clark H, Servadio A, Matilla T, Feddersen RM, Yunis WS et al (1995) SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82:937–948
Zoghbi HY, Orr HT (1995) Spinocerebellar ataxia type 1. Cell Biol 6:29–35
Reetz K, Costa AS, Mirzazade S, Lehmann A, Juzek A, Rakowicz M et al (2013) Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain 136:905–917
Koscik TR, Sloat L, van der Plas E, Joers JM, Deelchand DK, Lenglet C et al (2020) Brainstem and striatal volume changes are detectable in under 1 year and predict motor decline in spinocerebellar ataxia type 1. Brain Commun 2:1–13
Martins Junior CR, Martinez ARM, Vasconcelos IF, de Rezende TJR, Casseb RF, Pedroso JL et al (2018) Structural signature in SCA1: clinical correlates, determinants and natural history. J Neurol 265:2949–2959. https://doi.org/10.1007/s00415-018-9087-1
Servadio A, Koshy B, Armstrong D, Antalffy B, Orr HT, Zoghbi HY (1995) Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet 10:94–98
Paulson HL, Shakkottai VG, Clark HB, Orr HT (2017) Polyglutamine spinocerebellar ataxias-from genes to potential treatments. Nat Rev Neurosci 18:613–626. https://doi.org/10.1038/nrn.2017.92
Orr HT (2012) Cell biology of spinocerebellar ataxia. J Cell Biol 197:167–177
Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, Hill DE et al (2008) Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature 452:713–718
Lam YC, Bowman AB, Jafar-Nejad P, Lim J, Richman R, Fryer JD et al (2006) ATAXIN-1 interacts with the repressor capicua in its native complex to cause SCA1 neuropathology. Cell 127:1335–1347
Nitschke L, Coffin SL, Xhako E, El-Najjar DB, Orengo JP, Alcala E et al (2021) Modulation of ATXN1 S776 phosphorylation reveals the importance of allele-specific targeting in SCA1. JCI Insight [Internet] 6. https://insight.jci.org/articles/view/144955
Klement IA, Skinner PJ, Kaytor MD, Yi H, Hersch SM, Clark HB et al (1998) Ataxin-1 nuclear localization and aggregation: role in polyglutarnine- induced disease in SCA1 transgenic mice. Cell 95:41–53
Genis D, Matilla T, Volpini V, Rosell J, Dávalos A, Ferrer I et al (1995) Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology 45:24–30
Robitaille Y, Schut L, Kish SJ (1995) Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neuropathol 90:572–581
Gilman S, Sima AAF, Junck L, Kluin KJ, Koeppe RA, Lohman ME et al (1996) Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol 39:241–255. http://doi.wiley.com/10.1002/ana.410390214
Haines DE, Dietrichs E (2012) The cerebellum— structure and connections. Handb Clin Neurol. https://doi.org/10.1016/B978-0-444-51892-7.00001-2
Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K et al (2002) A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34:905–919
Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL (2005) The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493
Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I et al (2003) Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol 250:207–211
Fancellu R, Paridi D, Tomasello C, Panzeri M, Castaldo A, Genitrini S et al (2013) Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J Neurol 260:3134–3143
Ma J, Wu C, Lei J, Zhang X (2014) Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms. Int J Clin Exp Med 7:5765–5771
Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P (2016) A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J Rare Dis 11:1–9. https://doi.org/10.1186/s13023-016-0447-6
Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U et al (2010) Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum 9:433–442
Bürk K (2007) Cognition in hereditary ataxia. Cerebellum 6:280–286
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Asher M, Rosa JG, Rainwater O, Duvick L, Bennyworth M, Lai RY et al (2020) Cerebellar contribution to the cognitive alterations in SCA1: evidence from mouse models. Hum Mol Genet 29:117–131
Banfi S, Servadio A, Chung MY, Capozzoli F, Duvick LA, Elde R et al (1996) Cloning and developmental expression analysis of the murine homolog of the spinocerebellar ataxia type 1 gene (Sca1). Hum Mol Genet 5:33–40
Orengo JP, Nitschke L, van der Heijden ME, Ciaburri NA, Orr HT, Zoghbi HY (2022) Reduction of mutant ATXN1 rescues premature death in a conditional SCA1 mouse model. JCI Insight
Luo L, Wang J, Lo RY, Figueroa KP, Pulst SM, Kuo PH et al (2017) The initial symptom and motor progression in spinocerebellar ataxias. Cerebellum 16:615–622
Rivaud-Pechoux S, Dürr A, Gaymard B, Cancel G, Ploner CJ, Agid Y et al (1998) Eye movement abnormalities correlate with genotype in autosomal dominant cerebellar ataxia type I. Ann Neurol 43:297–302
Diallo A, Jacobi H, Schmitz-Hübsch T, Cook A, Labrum R, Durr A et al (2017) Body Mass Index decline is related to spinocerebellar ataxia disease progression. Mov Disord Clin Pract 4:689–697
Abe K, Kameya T, Tobita M, Konno H, Itoyama Y (1996) Molecular and clinical analysis on muscle wasting in patients with spinocerebellar ataxia type 1. Muscle Nerve 19:900–902
Flurkey K, Currer J, Harrison DE (2006) The mouse in biomedical research. In: Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A (eds) Norm biol husbandry model, 2nd edn. Elsevier, Amsterdam, p 645
Orengo JP, van der Heijden ME, Hao S, Tang J, Orr HT, Zoghbi HY (2018) Motor neuron degeneration correlates with respiratory dysfunction in SCA1. Dis Model Mech. https://doi.org/10.1242/dmm.032623
Lo RY, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez C et al (2016) Depression and clinical progression in spinocerebellar ataxias. Park Relat Disord 22:87–92. https://doi.org/10.1016/j.parkreldis.2015.11.021
Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16:606–613
Schmitz-Hübsch T, Coudert M, Tezenas du Montcel S, Giunti P, Labrum R, Dürr A et al (2011) Depression comorbidity in spinocerebellar ataxia. Mov Disord 26:870–876
Bürk K, Bösch S, Globas C, Zühlke C, Daum I, Klockgether T et al (2001) Executive dysfunction in spinocerebellar ataxia type 1. Eur Neurol 46:43–48
Tichanek F, Salomova M, Jedlicka J, Kuncova J, Pitule P, Macanova T et al (2020) Hippocampal mitochondrial dysfunction and psychiatric-relevant behavioral deficits in spinocerebellar ataxia 1 mouse model. Sci Rep 10:1–14
Rüb U, Bürk K, Timmann D, den Dunnen W, Seidel K, Farrag K et al (2012) Spinocerebellar ataxia type 1 (SCA1): New pathoanatomical and clinico-pathological insights. Neuropathol Appl Neurobiol 38:665–680
Seidel K, Siswanto S, Brunt ERP, Den Dunnen W, Korf HW, Rüb U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124:1–21
Ramani B, Panwar B, Moore LR, Wang B, Huang R, Guan Y et al (2017) Comparison of spinocerebellar ataxia type 3 mouse models identifies early gain-of-function, cell-autonomous transcriptional changes in oligodendrocytes. Hum Mol Genet 26:3362–3374
Hekman KE, Gomez CM (2015) The autosomal dominant spinocerebellar ataxias: emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J Neurol Neurosurg Psychiatry 86:554–561. http://www.ncbi.nlm.nih.gov/pubmed/25136055
Maas RPPWM, Van Gaalen J, Klockgether T, Van De Warrenburg BPC (2015) The preclinical stage of spinocerebellar ataxias. Neurology 85:96–103
Cvetanovic M, Ingram M, Orr H, Opal P (2015) Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience 289:289–299. https://linkinghub.elsevier.com/retrieve/pii/S0306452215000159
Shiwaku H, Yoshimura N, Tamura T, Sone M, Ogishima S, Watase K et al (2010) Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cell-autonomous toxicity. EMBO J 29:2446–2460. https://doi.org/10.1038/emboj.2010.116
Shiwaku H, Yagishita S, Eishi Y, Okazawa H (2013) Bergmann glia are reduced in spinocerebellar ataxia type 1. NeuroReport 24:620–625
Guerrini L, Lolli F, Ginestroni A, Belli G, Della Nave R, Tessa C et al (2004) Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain 127:1785–1795
Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F et al (1996) Autosomal dominant cerebellar ataxia type I Clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain 119:1497–1505. https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/119.5.1497
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C et al (2010) Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage 49:158–168. https://doi.org/10.1016/j.neuroimage.2009.07.027
Voogd J, Glickstein M (1998) The anatomy of the cerebellum. Trends Neurosci 21:370–375
Deelchand DK, Joers JM, Ravishankar A, Lyu T, Emir UE, Hutter D et al (2019) Sensitivity of volumetric magnetic resonance imaging and magnetic resonance spectroscopy to progression of spinocerebellar ataxia type 1. Mov Disord Clin Pract 6:549–558
Rüb U, Bürk K, Schöls L, Brunt ER, De Vos RAI, Orozco Diaz G et al (2004) Damage to the reticulotegmental nucleus of the pons in spinocerebellar ataxia type 1, 2, and 3. Neurology 63:1258–1263
Adachi M, Kawanami T, Ohshima H, Hosoya T (2006) Characteristic signal changes in the pontine base on T2- and multishot diffusion-weighted images in spinocerebellar ataxia type 1. Neuroradiology 48:8–13
Driessen TM, Lee PJ, Lim J (2018) Molecular pathway analysis towards understanding tissue vulnerability in spinocerebellar ataxia type 1. Elife 7:1–32
Friedrich J, Kordasiewicz HB, O’Callaghan B, Handler HP, Wagener C, Duvick L et al (2018) Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI insight. https://doi.org/10.1172/jci.insight.123193
Lee W, Lavery L, Rousseaux MWC, Rutledge EB, Jang Y, Wan Y et al (2021) Dual targeting of brain region-specific kinases potentiates neurological rescue in Spinocerebellar ataxia type 1. EMBO J 40:1–22
Martins CR, Martinez ARM, de Rezende TJR, Branco LMT, Pedroso JL, Barsottini OGP et al (2017) Spinal cord damage in spinocerebellar ataxia type 1. Cerebellum 16:792–796
Takechi Y, Mieda T, Iizuka A, Toya S, Suto N, Takagishi K et al (2013) Impairment of spinal motor neurons in spinocerebellar ataxia type 1-knock-in mice. Neurosci Lett 535:67–72. https://doi.org/10.1016/j.neulet.2012.12.057
Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP et al (2012) Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma 29:2748–2760
Finsterer J, Grisold W (2015) Disorders of the lower cranial nerves. J Neurosci Rural Pract 6:377–391
Kawai Y, Suenaga M, Sobue GHW (2009) Cognitive impairment in spinocerebellar degeneration. Eur Neurol 61:257–268
Suh J, Romano DM, Nitschke L, Herrick SP, DiMarzio BA, Dzhala V et al (2019) Loss of ataxin-1 potentiates Alzheimer’s pathogenesis by elevating cerebral BACE1 transcription. Cell 178:1159-1175.e17. https://doi.org/10.1016/j.cell.2019.07.043
Spalding KL, Bergmann O, Alkass K, Bernard S, Huttner HB, Westerlund I et al (2013) Dynamics of hippocampal neurogenesis in adult humans. Cell 153:1219–1227
Bird CM, Burgess N (2008) The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci 9:182–194
Hatanaka Y, Watase K, Wada K, Nagai Y (2015) Abnormalities in synaptic dynamics during development in a mouse model of spinocerebellar ataxia type 1. Sci Rep 5:1–5
Cvetanovic M, Hu YS, Opal P (2017) Mutant ataxin-1 inhibits neural progenitor cell proliferation in SCA1. Cerebellum 16:340–347
Rakic P (1998) Young neurons for old brains? Nat Neurosci 1:645–647
Asher M, Johnson A, Zecevic B, Pease D, Cvetanovic M (2016) Ataxin-1 regulates proliferation of hippocampal neural precursors. Neuroscience 322:54–65. https://doi.org/10.1016/j.neuroscience.2016.02.011
Matute C, Ransom BR (2012) Roles of white matter in central nervous system pathophysiologies. ASN Neuro 4:89–101
Della NR, Ginestroni A, Tessa C, Salvatore E, De Grandis D, Plasmati R et al (2008) Brain white matter damage in SCA1 and SCA2 An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage 43:10–19
Nakayama T, Nakayama K, Takahashi Y, Ohkubo K, Tobe H, Soma M et al (2001) Case of spinocerebellar ataxia type 1 showing high intensity lesions in the frontal white matter on T2-weighted magnetic resonance images. Med Sci Monit 7:299–303
Pulst S, Nechiporuk A, Nechiporuk T, Gispert S, Chen X, Lopes-cendes I et al (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 14:269–276
Kawaguchi Y, Okamoto T, Taniwakil M, Aizawa M, Inoue M, Katayama S et al (1994) CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet 8:221–228
Paulson H (2012) Machado-Joseph disease/spinocerebellar ataxia type 3. Handb Clin Neurol 103:437–449
Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J (2017) Spinocerebellar ataxia type 2: clinicogenetic aspects, mechanistic insights, and management approaches. Front Neurol 8:472
Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE et al (1983) A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306:234–238
Bhide PG, Day M, Sapp E, Schwarz C, Sheth A, Kim J et al (1996) Expression of normal and mutant huntingtin in the developing brain. J Neurosci 16:5523–5535
Reiner A, Dragatsis I, Dietrich P (2011) Genetics and neuropathology of huntington’s disease. Int Rev Neurobiol 98:325–372
Lorenzetti D, Watase K, Xu B, Matzuk MM, Orr HT, Zoghbi HY (2000) Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum Mol Genet 9:779–785
Acknowledgements
We would like to thank all members of the Lim laboratory for providing valuable comments and feedback. The figures were created with BioRender.com.
Funding
This work was supported by National Institute of Health grants AG076154 (J.L.), AG074609 (J.L.), AG066447 (J.L.), NS083706 (J.L.), NS088321 (J.L.), MH119803 (J.L.), T32 NS007224 (K.L.), and the Lo Graduate Fellowship for Excellence in Stem Cell Research (K.L.).
Author information
Authors and Affiliations
Contributions
VO and JL developed the concept. VO, NG, KL, FH, and JL wrote the manuscript. All authors reviewed and edited the manuscript and provided comments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olmos, V., Gogia, N., Luttik, K. et al. The extra-cerebellar effects of spinocerebellar ataxia type 1 (SCA1): looking beyond the cerebellum. Cell. Mol. Life Sci. 79, 404 (2022). https://doi.org/10.1007/s00018-022-04419-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04419-7