Abstract
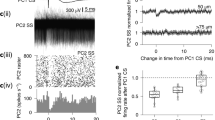
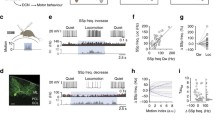
Small conductance Ca2+-activated potassium (SK) current provides an important modulator of excitatory synaptic transmission, which undergoes plastic regulation via multiple mechanisms. We examined whether inhibitory input processing is also dependent on SK current in the cerebellar nuclei (CN) where inhibition provides the only route of information transfer from the cerebellar cortical Purkinje cells. We employed dynamic clamping in conjunction with computer simulations to address this question. We found that SK current plays a critical role in the inhibitory synaptic control of spiking output. Specifically, regulation of SK current density resulted in a gain control of spiking output, such that low SK current promoted large output signaling for large inhibitory cell input fluctuations due to Purkinje cell synchronization. In contrast, smaller nonsynchronized Purkinje cell input fluctuations were not amplified. Regulation of SK density in the CN therefore would likely lead to important consequences for the transmission of synchronized Purkinje cell activity to the motor system.







Similar content being viewed by others
References
Palkovits M, Mezey E, Hamori J, Szentagothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and synapses. Exp Brain Res. 1977;28:189–209.
Jaeger D. Mini-review: synaptic integration in the cerebellar nuclei—perspectives from dynamic clamp and computer simulation studies. Cerebellum. 2011;10:659–66.
Walter JT, Khodakhah K. The linear computational algorithm of cerebellar Purkinje cells. J Neurosci. 2006;26:12861–72.
Walter JT, Khodakhah K. The advantages of linear information processing for cerebellar computation. Proc Natl Acad Sci U S A. 2009;106:4471–6.
Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci. 2000;20:3006–16.
De Zeeuw CI, Hoebeek FE, Bosman LWJ, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12:327–44.
Shin SL, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol. 2006;96:3485–91.
Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:129–47.
Uusisaari M, Obata K, Knopfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–11.
Molineux ML, Mehaffey WH, Tadayonnejad R, Anderson D, Tennent AF, Turner RW. Ionic factors governing rebound burst phenotype in rat deep cerebellar neurons. J Neurophysiol. 2008;100:2684–701.
Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, et al. Specific t-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci U S A. 2006;103:5555–60.
Alvina K, Ellis-Davies G, Khodakhah K. T-type calcium channels mediate rebound firing in intact deep cerebellar neurons. Neuroscience. 2009;158:635–41.
Sangrey T, Jaeger D. Multiple components of rebound spiking in deep cerebellar nucleus neurons. Eur J Neurosci. 2010;32:1646–57.
Hoebeek FE, Witter L, Ruigrok TJ, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci U S A. 2010;107:8410–5.
Bengtsson F, Ekerot CF, Jorntell H. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PLoS One. 2011;6:e18822.
Lang EJ, Sugihara I, Welsh JP, Llinas R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–39.
Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Hausser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 2009;29:8005–15.
Ozden I, Sullivan MR, Lee HM, Wang SSH. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar purkinje neurons. J Neurosci. 2009;29:10463–73.
Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–709.
Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–16.
Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal ca1 pyramidal neurons. J Neurosci. 1998;18:7613–24.
Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci. 2004;24:9921–32.
Wolfart J, Roeper J. Selective coupling of t-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–13.
Deister CA, Chan CS, Surmeier DJ, Wilson CJ. Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons. J Neurosci. 2009;29:8452–61.
Canavier CC, Landry RS. An increase in AMPA and a decrease in SK conductance increase burst firing by different mechanisms in a model of a dopamine neuron in vivo. J Neurophysiol. 2006;96:2549–63.
Gauck V, Jaeger D. The contribution of NMDA and AMPA conductances to the control of spiking in neurons of the deep cerebellar nuclei. J Neurosci. 2003;23:8109–18.
Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–6.
Pugh JR, Raman IM. GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys J. 2005;88:1740–54.
Anchisi D, Scelfo B, Tempia F. Postsynaptic currents in deep cerebellar nuclei. J Neurophysiol. 2001;85:323–31.
Steuber V, Schultheiss NW, Silver RA, De Schutter E, Jaeger D. Determinants of synaptic integration and heterogeneity in rebound firing explored with data driven models of deep cerebellar nucleus cells. J Comput Neurosci. 2011;30:633–58.
Steuber V, De Schutter E, Jaeger D. Passive models of neurons in the deep cerebellar nuclei: the effect of reconstruction errors. Neurocomputing. 2004;58–60:563–8.
Jahnsen H. Extracellular activation and membrane conductances of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:149–68.
Llinas R, Muhlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–58.
Bower J, Beeman D. The book of genesis. New York: Springer; 1997.
Lin RJ, Jaeger D. Using computer simulations to determine the limitations of dynamic clamp stimuli applied at the soma in mimicking distributed conductance sources. J Neurophysiol. 2011;105:2610–24.
Feng S, Jaeger D. The role of SK calcium-dependent potassium currents in regulating the activity of deep cerebellar nucleus neurons: a dynamic clamp study. Cerebellum. 2008;7:542–6.
Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–503.
Nedergaard S, Flatman JA, Engberg I. Nifedipine-conotoxin-sensitive and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra-pars-compacta neurons. J Physiol Lond. 1993;466:727–47.
De Waele C, Serafin M, Khateb A, Yabe T, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea-pig—apamin-induced rhythmic burst firing—an in-vitro and in-vivo study. Exp Brain Res. 1993;95:213–22.
McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, et al. Ca(v)3 t-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–94.
Alvina K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by n-type calcium channels in juvenile rats. J Physiol Lond. 2008;586:2523–38.
Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:936–47.
Maylie J, Adelman JP. Cholinergic signaling through synaptic SK channels: it's a protein kinase but which one? Neuron. 2010;68:809–11.
Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to p/q-type calcium channels in cerebellar purkinje neurons. J Neurosci. 2004;24:8818–22.
Hosy E, Piochon C, Teuling E, Rinaldo L, Hansel C. SK2 channel expression and function in cerebellar Purkinje cells. J Physiol Lond. 2011;589:3433–40.
Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30:13630–43.
Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–28.
Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, et al. Small conductance Ca2+-activated k+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–6.
Bond CT, Maylie J, Adelman JP. Sk channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–11.
De Schutter E, Steuber V. Patterns and pauses in Purkinje cell simple spike trains: experiments, modeling and theory. Neuroscience. 2009;162:816–26.
Destexhe A, Pare D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol. 1999;81:1531–47.
Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24.
Alvina K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–8.
Tadayonnejad R, Anderson D, Molineux ML, Mehaffey WH, Jayasuriya K, Turner RW. Rebound discharge in deep cerebellar nuclear neurons in vitro. Cerebellum. 2010;9:352–74.
Tadayonnejad R, Mehaffey WH, Anderson D, Turner RW. Reliability of triggering postinhibitory rebound bursts in deep cerebellar neurons. Channels (Austin). 2009;3:149–55.
Medina JF, Lisberger SG. Encoding and decoding of learned smooth-pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol. 2009;102:2039–54.
Cao Y, Maran SK, Dhamala M, Jaeger D, Heck DH. Behavior-related pauses in simple-spike activity of mouse Purkinje cells are linked to spike rate modulation. J Neurosci. 2012;32:8678–85.
Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–48.
Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–64.
Marder E, Prinz AA. Current compensation in neuronal homeostasis. Neuron. 2003;37:2–4.
Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92.
Pedroarena C. BK and KV3.1 potassium channels control different aspects of deep cerebellar nuclear neurons action potentials and spiking activity. Cerebellum. 2011;10:647–58.
Euler T, Denk W. Dendritic processing. Curr Opin Neurobiol. 2001;11:415–22.
Eilers J, Konnerth A. Dendritic signal integration [review] [55 refs]. Curr Opin Neurobiol. 1997;7:385–90.
Johnston D, Magee JC, Colbert CM, Christie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–86.
London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–32.
Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–69.
Hoebeek FE, Stahl JS, van Alphen AM, Schonewille M, Luo C, Rutteman M, et al. Increased noise level of Purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron. 2005;45:953–65.
Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, et al. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8A (Na(v)1.6) in cerebellar Purkinje neurons and granule cells. J Neurophysiol. 2006;96:785–93.
Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated k+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–8.
Cheron G, Sausbier M, Sausbier U, Neuhuber W, Ruth P, Dan B, Servais L. BK channels control cerebellar purkinje and golgi cell rhythmicity in vivo. Plos One. 2009;4:e7991.
Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset dystonia-parkinsonism. Nat Neurosci. 2011;14:357–65.
Jinnah HA, Hess EJ, LeDoux MS, Sharma N, Baxter MG, DeLong MR. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20:283–92.
LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145:457–67.
LeDoux MS. Animal models of dystonia: lessons from a mutant rat. Neurobiol Dis. 2011;42:152–61.
Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–97.
Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci. 2010;30:7258–68.
Acknowledgments
This work was supported by grants from the national Institute of Mental Health R01-MH065634 and National Institute of Neurological Disorder and Stroke R21 NS074296 to Dieter Jaeger
Conflict of Interest
The authors have no financial or personal relationships that might bias this work, such as consultancies, stock ownership, equity interests, or patent-licensing arrangements. The writing was entirely carried out by the authors without any additional help.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 107 kb)
Rights and permissions
About this article
Cite this article
Feng, S.S., Lin, R., Gauck, V. et al. Gain Control of Synaptic Response Function in Cerebellar Nuclear Neurons by a Calcium-Activated Potassium Conductance. Cerebellum 12, 692–706 (2013). https://doi.org/10.1007/s12311-013-0476-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0476-9