Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is a severe systemic inflammatory response syndrome caused by a potentially lethal tick-borne SFTS virus. A rapid and robust extrafollicular B-cell response to fatal or sublethal SFTS virus infection is known to generate a robust extrafollicular B-cell response that is skewed to lambda-monotypic B-blasts. We present a case of fatal SFTS virus infection-associated histiocytic necrotizing lymphadenitis showing an effaced nodal architecture due to florid lambda-monotypic B-blasts that can raise a diagnostic pitfall in hematopathology. We examined histologic and immunophenotypic features of inguinal lymph node tissue of a 49-year-old Korean woman, who presented with a high fever and painful left inguinal lymphadenopathy associated with fatal SFTS virus infection. The nodal architecture of the excised lymph node appeared to be diffusely effaced by expanded paracortical zones by an extensive proliferation of immunoblast-like large lymphoid cells along with minor foci of necrosis associated with infiltration of phagocytosing histiocytes, featuring the proliferative phase of Kikuchi-Fujimoto disease. Immunophenotypically, however, the proliferating cells consisted predominantly of lambda-monotypic B-blasts. This misleading aberrant B-cell response should be recognized by pathologists to avoid misinterpretation of the lambda-monotypic B-blast proliferation in SFTS viral infection-associated histiocytic necrotizing lymphadenitis as diffuse large B-cell lymphoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A florid extrafollicular proliferation of reactively transformed B-blasts such as B-immunoblasts and plasmablasts can be a diagnostic pitfall when it obliterates the landmarks of normal histology architecture of the affected lymph node [1]. Acute primary viral infection can be a clinical setting that can induce a rapid and strong, extrafollicular B-cell immune response that bypasses the germinal center reaction, resulting in a florid extrafollicular proliferation of B-immunoblasts that further differentiate into plasmablasts [2]. This is the case especially when these cells express monotypic immunoglobulin light chains [3, 4]. Such immunophenotypic aberrancy in actively proliferating nonneoplastic B-blasts has been reported to occur in response to fatal severe fever with thrombocytopenia syndrome (SFTS) virus infection [5].
SFTS is a potentially lethal tick-borne acute viral hemorrhagic fever caused by the SFTS virus (officially named as Dabie bandavirus) infection endemic in China, South Korea, and Japan [6]. SFTS virus is a single-stranded negative-sense RNA virus primarily targeting to disturb myeloid dendritic cells and secondarily targeting the immunoblasts or plasmablasts at the end stage of lethal infection [5, 7]. SFTS shows a broad spectrum of clinical manifestations ranging from acute self-limited febrile illness to a cytokine storm-associated systemic inflammatory response syndrome [8, 9], with a higher fatality in cases complicated with hemophagocytic lymphohistiocytosis (HLH) [10]. More than two-third of patients with SFTS virus infection develop painful lymphadenitis in the regional lymph nodes that drain the tick-bite sites [9]. It has been reported that the normal architecture of the affected lymph nodes is completely effaced by a distinctive histiocytic necrotizing inflammatory process characterized by the proliferation of immunoblast-like large atypical lymphocytes that are accompanied by infiltration of phagocytosing histiocytes [11,12,13,14].
We present a case of fatal SFTS virus infection-associated histiocytic necrotizing lymphadenitis that is characterized by extensive proliferation of B-blasts expressing lambda-monotypic immunoglobulin light chain. Pathologists need to recognize this potential pitfall and avoid mistaking the florid proliferation of lambda-monotypic B-blasts in fatal SFTS virus infection for diffuse large B-cell lymphoma (DLBCL).
Clinical history
A 49-year-old Korean woman on oral hypoglycemics for the past 8 years, residing in Incheon, South Korea, presented to the local clinic with a 3-day history of fever (body temperature, 38.4–39 °C) with chills and myalgia that developed about 10 days after hiking trip to a mountain in Chungcheongbuk-do in the central region of South Korea. Clinical work-up revealed she had significant leukopenia (white blood cell, 810 × 10−9 L), thrombocytopenia (34,000 × 10−9 L), and tender unilateral inguinal lymphadenopathies that measured 0.5 cm to 2.5 cm on the left side (Fig. 1). Her skin was free of insect bite sites. There was no tonsillar/adenoid enlargement. There was no other lymphadenopathy or organomegaly. She denied recent weight loss, arthritic symptoms, or skin rash. She did not recall any recent history of tick bites. As her clinical presentation was suggestive of impending hemophagocytic lymphohistiocytosis (HLH) associated with SFTS virus infection that is endemic in South Korea [15], she was immediately referred to the intensive care unit in the university hospital on day 4 of the illness. She was treated accordingly while waiting for the result of relevant diagnostic tests for zoonotic infections. All the potentially relevant tests including EBV serologic tests and quantitative PCR for EBV-DNA on peripheral blood were negative except for the real-time reverse transcription-polymerase chain reaction test for the detection of the SFTS virus genome in her serum, which came later positive. She developed disseminated intravascular coagulation (DIC) that was rapidly followed by hepatorenal failure and acute encephalopathy on day 6 of illness. On day 7 of the illness (hospital day 4), an inguinal lymph node was excised to exclude underlying malignancy. The marrow aspiration/trephine biopsy was performed on day 8 of the illness (hospital day 5). She died of refractory multi-organ failure on day 10 of the illness (on hospital day 7).
Materials and methods
The excised lymph node was serially sliced, fixed in 10% buffered formalin overnight, and processed to be embedded in paraffin according to conventional procedures. The bone marrow trephine biopsy sample collected from the posterior iliac crest was fixed in 10% buffered formalin overnight, decalcified in 10% EDTA overnight, and processed to be embedded. The hematoxylin and eosin (H&E)-stained histologic sections were microscopically examined. The histologic sections were analyzed retrospectively using immunohistochemistry for CD3, CD20, PAX5, CD30, CD10, BCL6, MUM1, myeloperoxidase (MPO), CD38, CD138, CD68, CD123, CD163, and Ki67 and in situ hybridization (ISH) staining using the probes for Epstein-Barr virus (EBV)-encoded RNA (EBER) and immunoglobulin light chain (kappa and lambda) mRNA.
Result
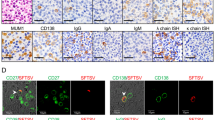
Histologic sections of the inguinal lymph node showed effacement of the nodal architecture of the lymph node by a monotonous population of immunoblast-like large atypical lymphoid cells that were accompanied by conspicuous phagocytosing histocytes with crescentic nuclei and apoptotic bodies in their cytoplasm (Fig. 2A–B). There were minor foci of discrete necrotic areas (Fig. 2C) and a few small EBV-positive bystander cells (Fig. 2D). A panel of immunohistochemistry and ISH stains revealed the presence of exuberant extrafollicular proliferation of immunoblast-like large B cells (Fig. 3A–D) that were monotypic for the immunoglobulin lambda light chain (Fig. 3E–F). Although they were diffusely positive for MUM1 (Fig. 3G), CD38 (data not shown), and CD79a (data not shown), a significant proportion of them appeared to have lost the expression of PAX5 (Fig. 3H). However, they were negative for CD138 (Fig. 3I) and CD30 (Fig. 3J). The staining for CD68 and MPO showed the presence of phagocytosing histiocytes (data not shown) that were negative for CD123 (Fig. 3K) but positive for CD163 (Fig. 3L). The bone marrow aspiration showed conspicuous hemophagocytosing histiocytes, which were also evident on the sections of the bone marrow biopsy (Fig. 4A). Immunohistochemical stains revealed mild interstitial infiltration of reactive small T cells (Fig. 4B) and a few sparse small aggregates of immunoblast-like large B cells (Fig. 4C–D). However, we have failed to demonstrate the light chain restriction in the B-immunoblasts because the bone marrow tissue sample was not sufficient (Fig. 4E–F).
Histopathologic findings of the inguinal lymph node. The nodal architecture is effaced by a monotonous population of immunoblast-like large atypical lymphoid cells (a and b) that are accompanied by conspicuous phagocytosing histocytes with crescentic nuclei and apoptotic bodies in their cytoplasm. There are minor patchy areas of necrosis (c). EBER in situ hybridizations stain shows only a few EBV-positive bystander cells (d)
Immunoarchitecture of the inguinal lymph node. Immunohistochemistry and ISH stains disclose effacement of the nodal architecture by the exuberant extrafollicular proliferation of immunoblast-like large B cells along with extensive depletion of lymphoid follicles (a–d). The immunoblast-like large B cells are monotypic for immunoglobulin lambda light chain (e and f). Although they are positive for MUM1, a significant proportion of them have lost the expression of PAX5 and still have not gained the expression of CD138 (i). They are negative for CD30 (j). The phagocytosing histiocytes are negative for CD123 (k) but positive for CD163 (l)
Histopathologic findings of bone marrow biopsy. The bone marrow is slightly hypercellular with a conspicuous infiltration of phagocytosing histiocytes (a). Immunohistochemistry reveals the presence of infiltration of reactive small T cells and a few sparse aggregates of immunoblast-like large B cells that are positive for CD20 and MUM1 (b–d). However, in situ hybridizations (ISH) using the probes for immunoglobulin kappa (e) and lambda (f) light chain mRNAs have failed to demonstrate the light chain restriction
Discussion
The pathognomonic histopathology of the lymphadenopathy associated with fatal SFTS virus infection is necrotizing lymphadenitis [14], in which the nodal architecture is completely effaced by a florid proliferation of antigen-activated B-immunoblasts that differentiate into plasmablasts [16]. Interestingly, the SFTS virus-infected B-blasts (B-immunoblasts and plasmablasts) seem to exhibit a bias towards the monotypic expression of the immunoglobulin lambda light chain [7, 17], which can pose a potential diagnostic pitfall in lymph node biopsy.
Patients with SFTS usually present with abrupt onset of flu-like nonspecific systemic symptoms occasionally accompanied by lymphadenopathy in the lymph nodes that drain the tick bite sites [18]. As SFTS can be suspected clinically based on clinical signs and symptoms and the definitive diagnosis can be established by the detection of the viral genome in the serum of suspected patients by quantitative real-time RT-PCR assay with very high specificity [6, 19], histologic evaluation of the affected lymph node is not necessary to make the diagnosis of SFTS. However, patients with SFTS virus infection can develop reactive lymphadenopathies that appear abnormally hypermetabolic when viewed with fludeoxyglucose-positron emission tomography, posing significant angst to both physicians and patients [20, 21].
Since Takahashi et al. [22] have described for the first time the histopathologic findings of lymphadenopathy associated with fatal SFTS virus infection, additional case reports have highlighted the pathognomonic histology of necrotizing lymphadenitis associated with fatal or sublethal SFTS virus infection (Table 1), which can mimic the necrotic phase of Kikuchi-Fujimoto disease. In contrast, the excisional biopsy of the inguinal lymph node in the present case showed features reminiscent of the proliferative phase of Kikuchi-Fujimoto disease, which histomorphologically can simulate infectious mononucleosis lymphadenitis [23, 24] or diffuse large cell lymphoma [10]. The present case was clinically diagnosed with SFTS and treated as such while waiting for the results of confirmatory laboratory tests that came later positive for SFTS virus infection. However, as the patient showed a rapid downhill course with the development of clinical signs and symptoms of impending multiorgan failure, excisional biopsy of the inguinal lymph node and bone marrow trephine biopsy were performed on day 7 and day 8 of the illness, respectively, as a routine clinical work-up to exclude the possibility of underlying malignant lymphoma.
We were able to exclude the possibility of proliferative phase Kikuchi-Fujimoto disease by demonstrating that most of the proliferating large lymphoid cells in the present case were CD20-positive B-blasts in contrast to the T-immunoblasts with cytotoxic T-cell immunophenotype in the proliferative phase of Kikuchi-Fujimoto disease [13, 25,26,27]. The negative result in serologic/molecular tests for acute EBV infection and EBER-ISH staining on the lymph node biopsy supported the exclusion of infectious mononucleosis lymphadenitis. Much to our surprise, however, the proliferating B-blasts in our case showed monotypic expression of immunoglobulin lambda light chain, posing a major concern in the histopathologic differential diagnosis. However, the clinical presentation with painful/tender and relatively small (≤ 2.5 cm) lymphadenopathies limited to the inguinal region in a patient with a recent history of a hiking trip to a mountainous area endemic to SFTS and a very short duration from symptom onset to death is unusual for DLBCL. Although we had no opportunity to perform PCR studies for rearrangement of light and heavy chains of immunoglobulin genes, it seems that the fatal SFTS virus infection can induce a florid polyclonal proliferation of B-immunoblasts or plasmablasts [28].
According to recently published case studies, the B-blasts expressing immunoglobulin lambda-light chain are the major target of fatal SFTS virus infection [17, 29]. They are also known to play a key role in carrying the viral infection to the peripheral organs [30]. Hence, the B-cell immune response to the SFTS virus appears to be biased towards the use of immunoglobulin lambda light chain, in a way similar to the B-cell immune response to HIV [31] or Kaposi sarcoma-associated herpesvirus [32]. It is also well known that early primary antiviral responses can induce prominent extrafollicular B-cell responses that bypass the ordinary germinal center responses [33, 34], which can cause exuberant extrafollicular proliferation of B-blasts (immunoblasts and plasmablasts) and the depletion of reactive lymphoid follicles. In the same manner, the early primary antiviral B-cell response to fatal SFTS virus infection seems to induce a rapid strong extrafollicular B response [33, 34]. As the B-blasts on different stages of differentiation/maturation can show highly variable expression of immunohistochemical markers such as leukocyte common antigen (CD45), CD20, PAX5, MUM1, CD138, and CD38 [34, 35], the rapidly proliferating B-blasts in our case may correspond to the reactively transformed B-immunoblasts that are on their way to further differentiation towards the early acute-phase plasmablasts (MUM1+, CD138−), but not yet to the later steady-state plasmablasts (MUM1+, CD138+) [34, 36].
As viral infection can skew immunoglobulin light chain repertoire leading to antigen-driven expansion of monotypic B-blasts [37] or elicit an immunoglobulin lambda light chain-biased response [38], we also speculate that the florid proliferation of CD20-positive lambda-monotypic large B-blasts in our case might be the morphologic correlate of this peculiar extrafollicular B-cell response to SFTS virus that bypasses the germinal center reaction, resulting in a florid extrafollicular expansion of B-blasts that are biased to immunoglobulin lambda-light chain [34, 36]. This might also account for the transient appearance of lambda-monotypic plasmablasts in the peripheral blood of patients with acute and severe SFTS virus infection [39]. Furthermore, a significant proportion of the proliferating immunoblast-like large B cells in our case appears to have lost the expression of PAX5 and instead gained the expression of MUM1 and CD38, but still not have gained the expression of CD138 which plays important roles in the selection of functionally more mature plasmablasts [40]. This also appears to prove an immunophenotypic correlate of the extrafollicular B-cell response to acute SFTS virus infection [35, 40].
In summary, to highlight the pitfall of lambda-monotypic B-cell proliferation in benign/reactive lymphadenopathy, we presented a case of fatal SFTS virus infection-associated histiocytic necrotizing lymphadenitis with Kikuchi-Fujimoto-like histology. Pathologists should be aware of this pitfall in SFTS viral infection that can mislead pathologists without the benefit of thorough information on the clinical and immunoarchitectural findings of the disease.
References
Tzankov A, Dirnhofer S (2018) A pattern-based approach to reactive lymphadenopathies. Semin Diagn Pathol 35:4–19. https://doi.org/10.1053/j.semdp.2017.05.002
Elsner RA, Shlomchik MJ (2020) Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53:1136–1150. https://doi.org/10.1016/j.immuni.2020.11.006
Evans MG, Crymes A, Crombie JL, Basu SS, Dillon DA, Wong WJ (2022) Monotypic plasmacytoid cells mimicking lymph node malignancy in the setting of COVID-19 recovery. Am J Hematol 97:666–667. https://doi.org/10.1002/ajh.26408
Kroft SH (2004) Monoclones, monotypes, and neoplasia pitfalls in lymphoma diagnosis. Am J Clin Pathol 121:457–459. https://doi.org/10.1309/RR8A-XUWM-9FYX-178A
Suzuki T, Sato Y, Sano K, Arashiro T, Katano H, Nakajima N, Shimojima M, Kataoka M, Takahashi K, Wada Y (2020) Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J Clin Invest 130:799–812. https://doi.org/10.1172/JCI129171
Casel MA, Park SJ, Choi YK (2021) Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med 53:713–722. https://doi.org/10.1038/s12276-021-00610-1
Yamaoka S, Weisend C, Ebihara H (2020) Identifying target cells for a tick-borne virus that causes fatal hemorrhagic fever. J Clin Invest 130:598–600. https://doi.org/10.1172/JCI134512
Deng B, Zhou B, Zhang S, Zhu Y, Han L, Geng Y, Jin Z, Liu H, Wang D, Zhao Y (2013) Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome Bunyavirus infection in Northeast China. PLoS ONE 8:e80802. https://doi.org/10.1371/journal.pone.0080802
Gai Z-T, Zhang Y, Liang M-F, Jin C, Zhang S, Zhu C-B, Li C, Li X-Y, Zhang Q-F, Bian P-F (2012) Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206:1095–1102. https://doi.org/10.1093/infdis/jis472
Jung IY, Ahn K, Kim J, Choi JY, Kim HY, Uh Y, Kim YK (2019) Higher fatality for severe fever with thrombocytopenia syndrome complicated by hemophagocytic lymphohistiocytosis. Yonsei Med J 60:592–596. https://doi.org/10.3349/ymj.2019.60.6.592
Hiraki T, Yoshimitsu M, Suzuki T, Goto Y, Higashi M, Yokoyama S, Tabuchi T, Futatsuki T, Nakamura K, Hasegawa H (2014) Two autopsy cases of severe fever with thrombocytopenia syndrome (SFTS) in Japan: a pathognomonic histological feature and unique complication of SFTS. Pathol Int 64:569–575. https://doi.org/10.1111/pin.12207
Sun J, Min Y-Q, Li Y, Sun X, Deng F, Wang H, Ning Y-J (2021) Animal model of severe fever with thrombocytopenia syndrome virus infection. Front Microbiol 12. https://doi.org/10.3389/fmicb.2017.00104
Bosch X, Guilabert A, Miquel R, Campo E (2004) Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol 122:141–152. https://doi.org/10.1309/YF081L4TKYWVYVPQ1
Saijo M (2022) Severe fever with thrombocytopenia syndrome, a viral hemorrhagic fever, endemic to Japan: achievements and directions to the future in the scientific and medical research. Jpn J Infect Dis 75:217–227. https://doi.org/10.7883/yoken.JJID.2021.851
Choi SJ, Park S-W, Bae I-G, Kim S-H, Ryu SY, Kim HA, Jang H-C, Hur J, Jun J-B, Jung Y (2016) Severe fever with thrombocytopenia syndrome in South Korea, 2013-2015. PLoS Negl Trop Dis 10. https://doi.org/10.1371/journal.pntd.0005264
Wada Y, Miyamoto S, Iida S, Sano K, Sato Y, Ainai A, Saito K, Katano H, Hasegawa H, Suzuki T (2022) Propagation of activated B cells by in vitro severe fever with thrombocytopenia syndrome virus infection of human peripheral blood mononuclear cells. J Infect Dis 225:269–281. https://doi.org/10.1093/infdis/jiab343
Takahashi T, Sano K, Suzuki T, Matsumura T, Sakai K, Tominaga T, Sato Y, Katano H, Hasegawa H (2020) Virus-infected peripheral blood plasmablasts in a patient with severe fever with thrombocytopenia syndrome. Int J Hematol5. https://doi.org/10.1007/s12185-020-03040-3
Liu Q, He B, Huang S-Y, Wei F, Zhu X-Q (2014) Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 14:763–772. https://doi.org/10.1016/s1473-3099(14)70718-2
Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W (2012) Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol 53:48–53. https://doi.org/10.1016/j.jcv.2011.09.031
Kang CK, Choi SJ, Koh J, Jeon YK, Kim KH, Chung J, Choe PG, Kim N-J, Park WB, Oh M-d (2018) 18F-FDG PET and histopathologic findings in a patient with severe fever with thrombocytopenia syndrome. Ticks Tick Borne Dis 9:972–975. https://doi.org/10.1016/j.ttbdis.2018.03.030
Kim AR, Kim T, Shin D-H, Lee S, Lim S (2021) Severe fever with thrombocytopenia syndrome with necrotizing lymphadenitis in a patient who underwent 18F-FDG PET/CT: a case report. Int J Environ Res Public Health 18:10488. https://doi.org/10.3390/ijerph181910488
Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T (2013) The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209:816–827. https://doi.org/10.1093/infdis/jit603
Takada A, Shimizu K, Nakazato Y, Ohikata K, Tsuchida S, Iijima M, Kojima M (2017) Infectious mononucleosis lymphadenitis resembling Kikuchi’s disease: cytological, histopathological, and immunohistological findings. J Clin Exp Hematop 56:176–178. https://doi.org/10.3960/jslrt.56.176
Louissaint A, Ferry JA, Soupir CP, Hasserjian RP, Harris NL, Zukerberg LR (2012) Infectious mononucleosis mimicking lymphoma: distinguishing morphological and immunophenotypic features. Mod Pathol 25:1149–1159. https://doi.org/10.1038/modpathol.2012.70
Hutchinson CB, Wang E (2010) Kikuchi-Fujimoto disease. Arch Pathol Lab Med 134:289–293. https://doi.org/10.5858/134.2.289
Asano S, Mori K, Yamazaki K, Sata T, Kurata A, Sato Y, Odajima H, Akaike Y, Wakasa H, Kojima M (2014) Necrotizing lymphadenitis (NEL) is a systemic disease characterized by blastic transformation of CD8+ cells and apoptosis of CD4+ cells. Virchows Arch 464:95–103. https://doi.org/10.1007/s00428-013-1516-z
Perry AM, Choi SM (2018) Kikuchi-Fujimoto disease: a review. Arch Pathol Lab Med 142:1341–1346. https://doi.org/10.5858/arpa.2018-0219-RA
Irie M, Miyoshi T, Hiramoto A, Hirata M, Takanosu M, Park E-S, Maeda K (2022) Diagnosis of severe fever with thrombocytopenia syndrome (SFTS) in a cat with clinical findings resembling lymphoma. J Vet Med Sci 84:21–0519. https://doi.org/10.1292/jvms.21-0519
Sakai Y, Kuwabara Y, Ishijima K, Kagimoto S, Mura S, Tatemoto K, Kuwata R, Yonemitsu K, Minami S, Kuroda Y (2021) Histopathological characterization of cases of spontaneous fatal feline severe fever with thrombocytopenia syndrome. Japan Emerg Infect Dis 27:1068. https://doi.org/10.3201/eid2704.204148
Finke D, Baribaud F, Diggelmann H, Acha-Orbea H (2001) Extrafollicular plasmablast B cells play a key role in carrying retroviral infection to peripheral organs. J Immunol 166:6266–6275. https://doi.org/10.4049/jimmunol.166.10.6266
Sajadi MM, Farshidpour M, Brown EP, Ouyang X, Seaman MS, Pazgier M, Ackerman ME, Robinson H, Tomaras G, Parsons MS (2016) λ light chain bias associated with enhanced binding and function of anti-HIV env glycoprotein antibodies. J Infect Dis 213:156–164. https://doi.org/10.1093/infdis/jiv448
Du M-Q, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin N, Meignin V, Oksenhendler E, Boshoff C, Isaacson PG (2001) Kaposi sarcoma–associated herpesvirus infects monotypic (IgMλ) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 97:2130–2136. https://doi.org/10.1182/blood.V97.7.2130
Baumgarth N (2021) The shaping of a B cell pool maximally responsive to infections. Annu Rev Immunol 39:103–129. https://doi.org/10.1146/annurev-immunol-042718-041238
Brighenti A, Andrulis M, Geissinger E, Roth S, Müller-Hermelink HK, Rüdiger T (2005) Extrafollicular proliferation of B cells in the absence of follicular hyperplasia: a distinct reaction pattern in lymph nodes correlated with primary or recall type responses. Histopathology 47:90–100. https://doi.org/10.1111/j.1365-2559.2005.02173.x
Lam JH, Smith FL, Baumgarth N (2020) B cell activation and response regulation during viral infections. Viral immunol 33:294–306. https://doi.org/10.1089/vim.2019.0207
Fink K (2012) Origin and function of circulating plasmablasts during acute viral infections. Front Immunol 3:78. https://doi.org/10.3389/fimmu.2012.00078
Schwartz JC, Murtaugh MP (2018) Viral infection skews immunoglobulin light chain repertoire diversity. bioRxiv398529. https://doi.org/10.1101/398529
Kirchenbaum GA, Allen JD, Layman TS, Sautto GA, Ross TM (2017) Infection of ferrets with influenza virus elicits a light chain–biased antibody response against hemagglutinin. J Immunol 199:3798–3807. https://doi.org/10.4049/jimmunol.1701174
Takahashi T, Suzuki T, Hiroshige S, Nouno S, Matsumura T, Tominaga T, Yujiri T, Katano H, Sato Y, Hasegawa H (2019) Transient appearance of plasmablasts in the peripheral blood of japanese patients with severe fever with thrombocytopenia syndrome. J Infect Dis 220:23–27. https://doi.org/10.1093/infdis/jiz054
McCarron MJ, Park PW, Fooksman DR (2017) CD138 mediates selection of mature plasma cells by regulating their survival. Blood 129:2749–2759. https://doi.org/10.1182/blood-2017-01-761643
Uehara N, Yano T, Ishihara A, Saijou M, Suzuki T (2016) Fatal severe fever with thrombocytopenia syndrome: an autopsy case report. Intern Med 55:831–838. https://doi.org/10.2169/internalmedicine.55.5262
Kaneko M, Shikata H, Matsukage S, Maruta M, Shinomiya H, Suzuki T, Hasegawa H, Shimojima M, Saijo M (2018) A patient with severe fever with thrombocytopenia syndrome and hemophagocytic lymphohistiocytosis-associated involvement of the central nervous system. J Infect Chemother 24:292–297. https://doi.org/10.1016/j.jiac.2017.10.016
Iwao K, Kawaguchi T, Kimura M, Iwao C, Rikitake M, Aizawa A, Kariya Y, Matsuda M, Miyauchi S, Takajo I (2021) Severe fever with thrombocytopenia syndrome accompanied by invasive pulmonary aspergillosis: an autopsy case. Viruses 13:1086. https://doi.org/10.3390/v13061086
Tsuru M, Suzuki T, Murakami T, Matsui K, Maeda Y, Yoshikawa T, Kurosu T, Shimojima M, Shimada T, Hasegawa H (2021) Pathological characteristics of a patient with severe fever with thrombocytopenia syndrome (SFTS) infected with SFTS virus through a sick cat’s bite. Viruses 13:204. https://doi.org/10.3390/v13020204
Acknowledgements
This work was supported by the Inha University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
The need for informed consent was waived by the IRB of Inha University Hospital. For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, Y., Prome, S.A., Kim, L. et al. Florid lambda-monotypic B-cell proliferation in fatal severe fever with thrombocytopenia syndrome virus infection-associated necrotizing lymphadenitis: a potential diagnostic pitfall. J Hematopathol 15, 221–228 (2022). https://doi.org/10.1007/s12308-022-00520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-022-00520-9