Abstract
Mixed phenotype acute leukemia (MPAL) is an uncommon disease characterized by currently only limited knowledge concerning biology, clinical presentation, and treatment outcome. We here describe a most unusual case of simultaneous occurrence of T-lymphoblastic lymphoma in cervical and mediastinal lymph nodes and acute myeloid leukemia in the bone marrow (BM) successfully treated with allogeneic stem cell transplantation (SCT). Although the blasts in both locations showed additional aberrant expression of other lineage markers (even B-cell markers), diagnostic criteria of MPAL were not fulfilled either in the LN or in the BM. We performed next generation sequencing (NGS) with the objective to look for common genetic aberrations in both tissues. Histology, immunohistochemistry, flow cytometry, AML-associated genetic alterations (FLT3, NPM1, KIT D816V, CEPBA), and clonal T-cell receptor β and γ gene rearrangements were performed according to routine diagnostic workflows. Next generation sequencing and Sanger sequencing were additionally performed in BM and LN. Somatic mutation in the EZH2 gene (p.(Arg684Cys)) was detected in the BM by NGS, and the same mutation was found in the LN. Since an identical genetic aberration (EZH2 mutation) was detected in both locations, a common progenitor with regional dependent differentiation may be involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early T-precursor lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) is a high-risk subtype of T-ALL/LBL newly recognized in the 2016 update to the WHO classification. It derives from cells from a very early T-cell maturation stage, specifically from early T-cell precursors (ETPs) that migrate from the bone marrow (BM) to the thymus where they retain their capability of multilineage differentiation [1]. Diagnostic criteria of ETP-ALL/LBL include aberrant expression of myeloid and hematopoietic stem cell markers, weak (< 75% cells positive) or no CD5 expression, and absence of T-lineage cell surface markers CD1a and CD8. Cases with higher CD5 expression (> 75% CD5 + blasts) and otherwise identical immunophenotype are called “near” ETP-ALL/LBL. Both entities are early/immature T-cell neoplasms and share a similar transcriptional and mutational profile [2, 3]. ETP-ALL is a rare neoplasm, accounting for 10–13% of cases of pediatric T-ALL and 5–10% of cases of adult ALL. The genetic landscape of ETP-ALL is different to conventional T-ALL cases and shows some overlapping features with acute myeloid leukemia (AML). A lineage switch of ETP-ALL to AML or mixed phenotype acute leukemia (MPAL-T/myeloid subtype) after therapy or as relapse has been reported in sporadic cases [4,5,6].
Here we present a unique case of an adult patient with co-occurrence of near ETP-LBL in LN and AML in BM. EZH2 mutation in both entities may point to a clonal relationship.
Clinical history
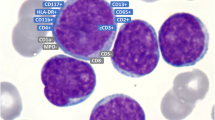
In November 2016, a 26-year-old male from Bangladesh was admitted to our hospital due to rapid cervical LN enlargement during the preceding 6 weeks. He had no other medical history. Peripheral blood (PB) cell counts were within normal ranges, LDH was not elevated, and clonal lymphocytic cells or blasts were not detectable in PB. A PET-CT showed cervical, axillary, mediastinal, para-aortal, and hilar LN involvement. Histologic examination of the cervical LN biopsy revealed a diffuse infiltration with medium-sized monomorphic blasts having round, slightly irregular nuclei with dispersed chromatin (Fig. 1). Immunohistochemistry showed strong expression of CD3, CD7, CD33, CD34, and terminal deoxynucleotidyl transferase (TdT) and weak expression of CD5, CD10, CD19, CD79a, and CD56. A few scattered blasts were found to be CD2, PAX5, and CD117 positive. All blasts were negative for T-cell receptor βF1, CD4, CD8, CD20, CD1a, and MPO (Figs. 1–3 left column). The diagnosis of immature T-LBL/ALL was made, due to CD5 expression in > 75% of blasts subclassified as near ETP-ALL/LBL. Surprisingly, staging BM biopsy showed a dense infiltration (70%) with mainly myeloid-differentiated blasts (Figs. 1, 2 and 3 right column) with fine granulated chromatin and agranular cytoplasm without Auer rods. Flow cytometry revealed positivity for CD34, TdT, HLA-DR, myeloid antigens (CD117, CD11b, CD33, CD13, and cytMPO), and some B-cell markers (cytCD79a, weak expression of CD19). Blasts were also positive for CD7, CD56, and CD4 and negative for CD2, CD3, cytCD3, CD5, CD10, CD20, and CD22. Immunohistochemistry of the biopsy displayed—besides the above mentioned immunophenotype—PAX5 positivity in one-third of the blasts. BM diagnosis of AML with minimal differentiation and aberrant co-expression of CD19, CD79a, CD7, and CD56 was made; MPAL criteria were not fulfilled. The lumbar puncture showed no blasts in the cerebrospinal fluid.
No AML-associated genetic alterations (FLT3, NPM1, KIT D816V, CEPBA) were found in BM, and interphase fluorescence in situ hybridization (FISH) with commercially available probes on a BM sample showed one signal of 13q14 in 30% of all cells, and BM karyogram showed a heterozygote deletion of 13q. PCR demonstrated clonal T-cell receptor β and γ gene rearrangements both in PB and BM. Next generation sequencing of BM (Ion Torrent, Thermo Fisher Scientific, Oncomine Comprehensive Assay v3) revealed a somatic mutation in the EZH2 gene (p.(Arg684Cys)) with a variant allele frequency of 25.8% (estimated tumor cell content in the analyzed tissue: 40%). The same EZH2 mutation was detected by Sanger sequencing of the LN sample.
An intensive induction therapy employing daunorubicin (45 mg/m2, days 1–3), cytarabine (100 mg/m2, days 1–7), and etoposide (100 mg/m2, days 1–5) was initiated. Re-staging revealed partial remission with 10% blasts in the BM as detected by flow cytometry, and PET-MRI showed regression of LN involvement. A second induction with high-dose cytarabine (1000 mg/m2, days 1–4) and ebetrexate (10 mg/m2, days 2–5) resulted in complete hematologic, molecular, and radiologic remission, and the patient received one cycle of high-dose cytarabine (3000 mg/m2 twice on days 1, 3, 5) as consolidation therapy. Due to the high risk of disease relapse, he underwent allogeneic stem cell transplantation (SCT) from an unrelated donor in April 2017 after consolidation with cyclophosphamide (60 mg/kg), anti-thymocyte globulin (20 mg/kg), and radiation (13.2 Gy). In July 2018, a control PET-CT detected cervical lymphadenopathy and a suspicious glucose uptake (SUV 20) mass in the right tonsil. Histologic examination of the tonsil revealed EBV-positive polymorphic posttransplant lymphoproliferative disease (P-PTLD) with CD30 expression. NGS did not reveal any mutation in the PTLD. After 4 cycles of rituximab monotherapy without any response, he received 4 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone), which resulted in partial remission with demonstrable regression of the tonsillar mass and cervical lymphadenopathy in the PET-CT. To achieve complete remission, treatment was changed to brentuximab vedotin. After two cycles, a repeat PET-CT confirmed CR which has been sustained since then.
Materials and methods
Histology and immunochemistry
Lymph node and EDTA-decalcified BM were embedded in paraffin after formalin fixation and sectioned according to routine methods. Hematoxylin/eosin staining and immunohistochemical staining with anti-CD2, CD3, CD4, CD5, CD7, CD8, CD1a, T-cell receptor βF1, CD33, CD117, MPO, CD19, CD20, CD79a, PAX-5, CD56, CD30, TdT, CD10, and CD34 antibodies were performed according to standardized automated operating protocols.
Flow cytometry
Mononuclear cells from heparinized BM aspirates were prepared by Ficoll density gradient centrifugation, followed by red blood cell lysis, staining for 4–5 color immunophenotyping with a broad panel of fluorochrome-conjugated antigen-specific monoclonal antibodies, and analysis on a FACS Canto II flow cytometer equipped with FACS Diva™ software (Becton Dickinson).
The antibody panels used for cell surface staining were as follows:
-
CD65-FITC/CD15-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD33-APC/CD45-V500
-
CD16-FITC/CD13-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD11b-APC/CD45-V500
-
HLA-DR-FITC/CD10-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD19-APC/CD45-V500
-
CD2-FITC/CD7-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD5-APC/CD45-V500
-
CD4-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD56-APC/CD14-APC-Cy7/CD45-V500
-
CD3-FITC/CD1a-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD4-APC/CD8-APC-Cy7/CD45-V500
-
CD20-FITC/CD22-PE/CD34-PerCP-cy5/CD19-PE-Cy7/CD10-APC/CD24-APC-Cy7/CD45-V500
-
Kappa-FITC/Lambda-PE/CD34-PerCP-cy5/CD19-PE-Cy7/CD23-APC/IgM-V450/CD45-V500
The antibody panels used for intracytoplasmatic staining were as follows:
-
MPO-FITC/Lactoferrin-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD14-APC/CD3-V450/CD45-V500
-
TdT-FITC/CD22-PE/CD34-PerCP-cy5/CD117-PE-Cy7/CD79a-APC/CD45-V500
PCR clonal T-cell receptor β and γ gene rearrangements
DNA was prepared from white blood cells (WBC) from BM aspirate and PB using the QIAsymphony® DSP DNA Midi Kit (Qiagen, Hilden, Germany).
PCR-based detection of clonal T-cell receptor β chain and γ chain gene rearrangements was performed using the IdentiClone® TCRB + TCRG T-Cell Clonality Assay–Gel Detection Kit (Invivoscribe, San Diego, CA, USA) according to the manufacturer’s instructions.
NGS and Sanger sequencing
DNA was extracted from paraffin embedded tissue blocks with a QIAamp Tissue Kit™ (Qiagen, Hilden, Germany). DNA library from the BM and the tonsil sample was generated by multiplex polymerase chain reaction with the DNA Oncomine™ Comprehensive Panel v3 (Ion Torrent, Thermo Fisher Scientific, Waltham, MA) covering 161 genes. Sequencing was performed with an Ion S5™ Sequencer (Thermo Fisher Scientific). Sequencing data were analyzed using Variant Caller™ and Ion Reporter™ (both Thermo Fisher Scientific). The presence of EZH2 mutation in the lymph node tissue was investigated by capillary sequencing using PCR primers flanking the DNA mutation. Sequence analysis was performed with the SeqScape Version 2.7 software (Thermo Fisher Scientific).
Discussion
We here present an interesting, complex case of co-occurrence of two immature blast cell diseases with different phenotypes—mainly T-lymphoblastic in the LN and mainly myeloid in the BM. Although an aberrant co-expression of various different lineage (intriguingly even B-cell) markers was present in both biopsies, the WHO-established MPAL criteria were not fulfilled either in the LN or in the BM.
ETP-ALL/LBL was first described by Coustan-Smith et al. in 2009 [2] and represents a subtype of T-ALL/LBL with a higher risk of induction failure or relapse as compared to conventional T-ALL. Near ETP-ALL represents a transcriptional and genetic similar subset that only differs by the virtue of CD5 expression [2, 3]. The unique immunophenotype of blasts indicates an early T-cell differentiation. It is supposed that blasts derive from a subset of thymocytes that retain the potential for myeloid/dendritic cell differentiation. T-cell lineage commitment is promoted by the intrathymic microenvironment where thymic epithelial-, endothelial-, and mesenchymal-stromal cells communicate with precursor T-cells via Eph receptors and their ligands. For the diagnosis of T-LBL, blasts have to be MPO-negative, thus precluding its diagnosis in BM in our case, where additionally there was no evidence of T-cell differentiation at all, thus qualifying for a distinct diagnosis. However, the fact that some markers (CD34, TdT, CD33, CD7, CD79a) occurred in both location hints toward a common progenitor for tissue-dependent local differentiation with thymus and LN-promoting lymphocyte proliferation and the BM-regulating myeloid differentiation. We performed NGS to gain insight into the underlying molecular alteration and to determine any clonal relationship. Unfortunately, the LN specimen was only a small biopsy, and only sparse material was left for molecular analysis, thus not allowing for broad-range PCR required for NGS. However, Sanger sequencing could be performed and detected the EZH2 mutation which had initially been found in the BM by NGS, thus supporting a clonal relationship. The detection of clonal T-cell rearrangement in the BM is another indicator of a shared derivation with multilineage diversity.
ETP-ALL shows greater genomic instability [7], a stem-cell like gene expression program [8], frequent mutations in genes regulating cytokine receptor and RAS signaling (NRAS, KRAS, NFLT3, IL7R, JAK3, JAK1, SH2B3, BRAF), genes encoding key transcription factors responsible for hematopoiesis (GATA3, ETV6, RUNX1, IKZF1, EP300), and genes encoding histone modifiers (EZH2, EED, SUZ12, SETD2) [8]. The methyltransferase Enhancer of Zeste2 (EZH2) is a subunit of the developmental regulator polycomb repressive complex 2 (PRC2) whose components are frequently altered in hematologic malignancies, including T-ALL, especially ETP-ALL where inactivating EZH2 mutations have been linked to inferior outcome [8]. The role of EZH2 was studied in a murine model recapitulating ETP-ALL [9]. EZH2 inactivation was shown to accelerate leukemia onset and to contribute to silencing of the stem-cell and early progenitor cell associated transcriptional program. Furthermore, a link between EZH2 inactivation and JAK/STAT signaling was demonstrated via accentuated phosphorylation on tyrosine 705 and hypersensitive STAT3 phosphorylation in response to IL6 [9]. EZH2 mutations have been reported in different hematologic malignancies including lymphomas and acute leukemias [10,11,12,13]. EZH2 has also been described as a potential therapeutic target; however, future research is still necessary [10, 11].
Gibson et al. described EZH2 mutation to cause Weaver syndrome [14], and there are few case reports in the literature of patients with Weaver syndrome who developed acute myeloid [15] or lymphoblastic [16] leukemias suggesting an important role of EZH2 mutations in leukemogenesis. ETP-ALL with EZH2 and RUNX1 inactivating mutations showed dismal prognosis with co-expression of myeloid/lymphoid genes if additional FLT3-ITD mutation occurs [17]. Our patient developed a rare and complex hematological immature blastoid disease with different phenotypes in BM and LN but with the identical EZH2 mutation in both entities. So, we can speculate that epigenetic instability might have led to simultaneous leukemogenesis with microenvironment dependent lineage differentiation.
Reports of lineage switches exist, but these switches are usually described to occur at the time of relapse [4,5,6, 18, 19]. Ortin et al. presented a case of childhood T-ALL which relapsed as a minimal differentiated AML [4]. A very similar report was published by Paganin et al. where a 4-year-old boy diagnosed with T-ALL relapsed with AML [5]. Here, T-cell receptor gene rearrangement analyses, array comparative genomic hybridization, and NGS revealed a clonal relationship between T-ALL and AML. The phenomenon of lineage switch from T-cell to myeloid differentiation at relapse has also been reported in adolescence [6] and adulthood [18, 19]. The same cytogenetic alterations verified clonality in the latter age group. These cases share some similarities with our report; in particular the very immature T-cell phenotype was present in most instances. However, the concurrence of T-LBL and AML is an exceptional finding and definitely rules out secondary AML induced by high-dose chemotherapy, which was taken into consideration to some extent in the previous case studies.
The therapeutic approach of such a complex and rare neoplasia proves most difficult due to a low incidence rate and a lack of treatment guidelines. ETP-ALL/LBL is per se a high-risk subtype of ALL in both childhood [2] and adulthood [20]. Disadvantages in outcome may probably be offset by an intensification of therapy. The clinical manifestation of near ETP-ALL is less aggressive; Morita et al. reported a 5-year overall survival (OS) rate of 56% compared to 63% in the non-ETP-ALL group [3]. The significantly worse 10-year OS (19% vs 84%) and event-free survival (EFS) (22% vs 69%) reported for childhood ETP-ALL/LBL vs non ETP-T-ALL/LBL patients in the St. Jude Children’s Research Hospital Group [2] could not be confirmed in the Children’s Oncology Group, where MRD-positive cases received intensified treatment at the end of induction [21]. Another reason for the better outcome in this study group could be the inclusion of cases with either unknown or positive CD5 expression, the latter most likely representing near ETP-ALL. EZH2 mutation and additional co-occurrence of myeloid blasts in the BM prompted us to focus our assessment on an aggressive systemic immature disease assumed to be biologically comparable with MPAL and involving an additional risk factor, viz., residual disease morphologically detectable after induction therapy, thus arguing in support of allogeneic SCT. This case report presents the successful treatment in a complex case that poses a number of therapeutic and diagnostic challenges, emphasizing the importance of detailed phenotypic diagnostic work up of every effected organ system in a patient presenting with lymphadenopathy and AML. The rapid development of diagnostic techniques and the rapidly growing amount on new insights in molecular biology require precise and elaborate diagnostic work-up to find the optimal therapeutic approach for each patient.
References
Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y et al (2008) Adult T-cell progenitors retain myeloid potential. Nature 452(7188):768–772
Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D et al (2009) Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 10(2):147–156
Morita K, Jain N, Kantarjian H, Takahashi K, Fang H, Konopleva M et al (2021) Outcome of T-cell acute lymphoblastic leukemia/lymphoma: focus on near-ETP phenotype and differential impact of nelarabine. Am J Hematol 96(5):589–598
Ortin X, Escoda L, Nomdedeu J, Llorente A, Cabezudo E, Boixadera J et al (2003) Childhood T-acute lymphoblastic leukemia relapsed as minimally differentiated acute myeloid leukemia (AML-M0). Leuk Lymphoma 44(12):2159–2161
Paganin M, Buldini B, Germano G, Seganfreddo E, Meglio A, Magrin E et al (2016) A Case of T-cell acute lymphoblastic leukemia relapsed as myeloid acute leukemia. Pediatr Blood Cancer 63(9):1660–1663
Mantadakis E, Danilatou V, Stiakaki E, Paterakis G, Papadhimitriou S, Kalmanti M (2007) T-cell acute lymphoblastic leukemia relapsing as acute myelogenous leukemia. Pediatr Blood Cancer 48(3):354–357
Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al (2007) Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446(7137):758–764
Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D et al (2012) The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481(7380):157–163
Danis E, Yamauchi T, Echanique K, Zhang X, Haladyna JN, Riedel SS et al (2016) Ezh2 controls an early hematopoietic program and growth and survival signaling in early T cell precursor acute lymphoblastic leukemia. Cell Rep 14(8):1953–1965
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492(7427):108–112
Lue JK, Amengual JE (2018) Emerging EZH2 inhibitors and their application in lymphoma. Curr Hematol Malig Rep 13(5):369–382
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ et al (2018) Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378(15):1396–1407
Rinke J, Chase A, Cross NCP, Hochhaus A, Ernst T (2020) EZH2 in myeloid malignancies. Cells 9(7)
Gibson WT, Hood RL, Zhan SH, Bulman DE, Fejes AP, Moore R et al (2012) Mutations in EZH2 cause Weaver syndrome. Am J Hum Genet 90(1):110–118
Basel-Vanagaite L (2010) Acute lymphoblastic leukemia in Weaver syndrome. Am J Med Genet A 152a(2):383–6
Usemann J, Ernst T, Schäfer V, Lehmberg K, Seeger K (2016) EZH2 mutation in an adolescent with Weaver syndrome developing acute myeloid leukemia and secondary hemophagocytic lymphohistiocytosis. Am J Med Gen A 170a(5):1274–7
Booth CAG, Barkas N, Neo WH, Boukarabila H, Soilleux EJ, Giotopoulos G et al (2018) Ezh2 and Runx1 mutations collaborate to initiate lympho-myeloid leukemia in early thymic progenitors. Cancer Cell 33(2):274–91.e8
Rossi M, Camera A, Barone M, Castagnola C, Del Vecchio L, Rotoli B (2001) CD7(+) acute leukemia switching from a lymphoid to a myeloid phenotype. Haematologica 86(8):877
Kawakami K, Miyanishi S, Amakawa R, Hayashi T, Kurata M, Nakamura F et al (1999) A case of T-lineage lymphoblastic lymphoma/leukemia with t(4;11)(q21;p15) that switched to myelomonocytic leukemia at relapse. Int J Hematol 69(3):196–199
Jain N, Lamb AV, O’Brien S, Ravandi F, Konopleva M, Jabbour E et al (2016) Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 127(15):1863–1869
Patrick K, Wade R, Goulden N, Mitchell C, Moorman AV, Rowntree C et al (2014) Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 166(3):421–424
Acknowledgements
We thank Gertrude Krainz for proofreading.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
All authors have been involved in the care of the patient.
E.P.: Treated the patient and wrote the manuscript.
W.R.S.: Treated the patient.
R.T.: Provided flow cytometry and molecular diagnostics.
G.MH.: Provided PCR.
I.S-K.: Provided pathological diagnostics.
L.M.: NGS
A-I.S.: Provided pathological diagnostics and histological images and wrote the manuscript.
Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porpaczy, E., Sperr, W.R., Thalhammer, R. et al. Co-occurrence of immature T-lymphoblastic lymphoma and acute myeloid leukemia—microenvironment-dependent lineage differentiation derived from a common progenitor?. J Hematopathol 14, 325–332 (2021). https://doi.org/10.1007/s12308-021-00466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-021-00466-4