Abstract
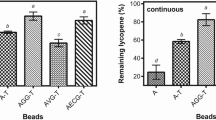
Characteristics of naringinase nano-encapsulated forms on different carrier materials (chitosan and alginate polymers) were investigated in this study. Screening of twelve fungal isolates for naringinase production indicated that Trichoderma longibrachiatum was the most promising. Grapefruit rind was used as a substrate containing naringin for naringinase production. TEM micrographs showed that chitosan nano-capsules were applied for the production of morphologically homogeneous enzymatic nano-particles with high enzyme encapsulation efficiency, small asymmetric sizes (from 15.09 to 27.07 nm with the mean of 21.8 nm) and rough surfaces compared to nano-encapsulated naringinase in alginate which showed nano-particle size (from 33.37 to 51.01 nm with the mean of 43.03 nm). It was revealed that the highest naringinase activity was found in case of chitosan nano-capsule naringinase compared to alginate nano-capsule one. Thermogram analysis (TGA) showed that the free enzyme loses about 92% of its weight at approximately 110°C, while the nano-encapsulated ones show more stability at higher temperatures. Conclusively, the nano-capsulation process improves the kinetics and operational stability so could be useful as a debittering agent for various thermal processing applications in citrus juices industries which makes the fruit juice more acceptable and cost-effective to the consumer.
Similar content being viewed by others
References
Aponso, M.M.W., Marapana, R., and Manawaduge, R. 2017. Physicochemical analysis of grape juice from Israel blue (Vitisvini fera L.) grape cultivar under different processing conditions and a comparison with Red Globe and Michele Palieri grape varieties. J. Pharmacogn. Phytochem. 6, 381–385.
Awad, G.E.A, El Aty, A.A.A., Shehata, A.N., Hassan, M.E., and Elnashar, M.M. 2016. Covalent immobilization of microbial naringinase using novel thermally stable biopolymer for hydrolysis of naringin. 3 Biotech. 6, 14.
Beever, R.E. and Bollard, E.G. 1970. The nature of the stimulation of fungal growth by potato extract. J. Gen. Microbiol. 60, 273–279.
Cao, L. 2005. Carrier-bound immobilized enzymes: principles, application and design. John Wiley & Sons.
Cipolatti, E.P., Valerio, A., Henriques, R.A., Moritz, D.E., Ninow, J.L., Freire, D.M.G., Manoel, E.A., Fernandez-Lafuente, R., and de Oliveira, D. 2016. Nanomaterials for biocatalyst immobilization — State of the art and future trends. RSC Adv. 6, 104675–104692.
Costa, S.A., Tzanova, T., Paarb, A., Gudeljb, M., Gübitzb, G.M., and Cavaco-Paulo, A. 2001. Immobilization of catalases from Bacillus SF on alumina for the treatment of textile bleaching effluents. Enzyme Microbiol. Technol. 28, 815–819.
De Silva, G.O., Raug, M., and Ranmalie, M. 2017. Effect of naringinase enzymatic treatment on the bitter compound naringin in fresh juice of “Bibila” sweet oranges. J. Pharmacogn. Phytochem. 6, 174–178.
Deene, M. and Lingappa, K. 2013. Microwave assisted rapid biobased synthesis of gold nanorods using pigment produced by Streptomyces coelicolor klmp33. Acta Metall. Sin. 26, 613–617.
Donsi, F.M., Annunziata, M., Sessa, M., and Ferrari, G. 2011. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 44, 1908–1914.
Elnashar, M.M., Danial, E.N., and Awad, G.E. 2009. Novel carrier of grafted alginate for covalent immobilization of inulinase. Ind. Eng Chem. Res. 48, 9781–9785.
Elnashar, M.M., Mohamed, E.H., and Ghada, E.A. 2013. Grafted carrageenan gel disks and beads with polyethylenimine and glutaraldehyde for covalent immobilization of penicillin G acylase. J. Colloid Sci. Biotechnol. 2, 1–7.
Ferreira, L., Cristina, A., Helder, V., António, A., and Maria, H.L. 2008. Evaluation of the effect of high pressure on naringin hydrolysis in grapefruit juice with naringinase immobilized in calcium alginate beads. Food Technol. Biotechnol. 46, 146–150.
Ge, L., Chen, A., Pei, J.J., Zhao, L., Fang, X., Ding, G., Wang, Z., Xiao, W., and Tang, F. 2017. Enhancing the thermostability of α-L-rhamnosidase from Aspergillus terreus and the enzymatic conversion of rutin to isoquercitrin by adding sorbitol. BMC Biotechnol. 17, 21.
Hector, L.R., Ana, I.B., Juan, U., and Maria, A. 2013. Immobilization of pectinase by adsorption on an alginate-coated chitin support. Biotecnol. Apl. 30, 101–104.
Homaei, A.A., Sariri, R., Vianello, F., and Stevanato, R. 2013. Enzyme immobilization: An update. J. Chem. Biol. 6, 185–205.
Horwitz, W. and Latimer, G.W. 2005. Official methods of analysis of AOAC international. 18th ed, AOAC International, Gaithersburg, MD, USA.
Hwang, E.T. and Gu, M.B. 2013. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 13, 49–61.
Jagjiwan, D. 2001. M. Sc. Thesis. Physicochemical debittering and processing of kinnow juice with by product recovery. Thapar Institute of Engineering and Technology, Patiala, Punjab, India.
Joo, A.R., Jeya, M., Lee, K.M., Lee, K.M., Moon, H.J., Kim, Y.S., and Lee, J.K. 2010. Production and characterization of β-1,4-glucosidase from a strain of Penicillium pinophilum. Process Biochem. 45, 851–858.
Kaur, K. 2002. M. Sc. Thesis. Application of novel juice extraction methods and bacterial utilization of limonin for control of bitterness in kinnowjuice. Thapar Institute of Engineering and Technology, Patiala, India.
Khaled, F.M., Heba, I.A., and Manal, M.H. 2018. Micro- and nanocapsulated fungal pectinase with outstanding capabilities of eliminating turbidity in freshly produced juice. Food Sci. Technol. Int. 24, 330–340.
Kim, J., Grate, J.W., and Wang, P. 2006. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 61, 1017–1026.
Kurita, K. 2001. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 26, 1921–1971.
Lowry, O.H., Rosebroug, N.J., Farr, A.L., and Randall, R.J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428.
Mostafa, F.A. and Abd El Aty, A.A. 2013. Enzyme activities of the marine-derived fungus Alternaria alternate cultivated on selected agricultural wastes. JABS 7, 39–46.
Navaratnam, P., Ketheeswary, N., Senthuran, A., and Arasaratnam, V. 2003. Preliminary studies on the isolation of naringinase producing fungus. Jaffna Sci. Assoc. 11, 10.
Norouzian, D., Hosseinzadeh, A., Inanlou, D.N., and Moazami, N. 2000. Production and partial purification of naringinase by Penicillium decumbens PTCC 5248. World J. Microbiol. Biotechnol. 16, 471–473.
Nunes, M.A.P., Pedro, C.B.F., and Maria, H.L.R. 2010. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 160, 2129–2147.
Palmer, T. 1991. Extraction and purification of enzymes, pp. 301–317. In Understanding enzymes, Ellis Horwood, Ltd., England.
Param, P.S. and Nayjot, K. 2015. Characterization of enzyme naringinase and the production of debittered low alcoholic kinnow (Citrus raticulatablanco) beverage. Int. J. Adv. Res. 3, 1220–1233.
Plummer, D.T. 1978. The practice of column chromatography, pp. 61–66. In An introduction of practical biochemistry. McGraw-Hill Book Company Ltd., UK.
Pruthi, J.S., Mann, J.K., and Teotia, M.S. 1984. Studies on utilization of kinnow and malta oranges. J. Food Sci. Technol. 21, 123–127.
Puri, M. 2012. Updates on naringinase: structural and biotechnological aspects. Appl. Microbiol. Biotechnol. 93, 49–60.
Puri, M., Banerjee, A., and Banerjee, U.C. 2005. Optimization of process parameters for the production of naringinase by Aspergillus niger MTCC 1344. Process Biochem. 40, 195–201.
Puri, M. and Kalra, S. 2005. Purification and characterization of naringinase from a newly isolated strain of Aspergillus niger 1344 for the transformation of flavonoids. World J. Microbiol. Biotechnol. 21, 753–758.
Puri, M., Marwaha, S.S., Kothari, R.M., and Kennedy, J.F. 1996. Biochemical basis of bitterness in citrus fruit juices and biotech approaches for debittering. Crit. Rev. Biotechnol. 16, 145–155.
Ramani, G., Meera, B., Vanitha, C., Rao, M., and Gunasekaran, P. 2012. Production, purification and characterization of a β-glucosidase of Penicillium funiculosum NCL1. Appl. Biochem. Biotechnol. 167, 959–972.
Rampino, A., Borgogna, M., Blasi, P., Bellich, B., and Cesàro, A. 2013. Chitosan nanoparticles: preparation, size evolution and stability. Int. J. Pharm. 455, 219–228.
Rangana, S. 1991. Handbook of analysis and quality and control for fruits and vegetables products. Tata McGraw Hill Publishing Co., New Delhi, India.
Ribeiro, F.B., Lanna, E.A.T., Bomfim, M.A.D., Donzele, J.L., Quadros, M., and Cunha, S.L. 2011. True and apparent digestibility of protein and amino acids of feed in Nile tilapia. R. Bras. Zootec. 40, 939–946.
Roitner, M., Schalkhammer, T., and Pittner, F. 1984. Preparation of prunin with the help of immobilized naringinase pretreated with alkaline buffer. Appl. Biochem. Biotechnol. 9, 483–488.
Sadighi, A. and Faramarzi, M.A. 2013. Congo red decolorization by immobilized laccase through chitosan nanoparticles on the glass beads. J. Taiwan Inst. Chem. Eng. 44, 156–162.
Sadler, G.D. and Murphy, P.A. 1998. pH and titratable acidity, pp. 99–117. In Nielsen, S.S. (ed.), Food analysis. 2nd ed. Aspen Publishers, Gaithersburg, MD, USA.
Sahota, P.P. and Kaur, N. 2015. Characterization of enzyme naringinase and the production of debittered low alcoholic kinnow (Citrus raticulatablanco) beverage. Int. J. Adv. Res. 3, 1220–1233.
Saloko, S., Darmadji, P., Setiaji, B., Pranoto, Y., and Anal, A.K. 2013. Encapsulation of coconut shell liquid smoke in chitosan-maltodextrin based nanoparticles. Int. Food Res. J. 20, 1269–1276.
Segel, I.W. 1993. Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady-state enzyme systems. John Wiley & Sons, New York, NY, USA.
Shehata, A.N. and Abd El Aty, A.A. 2014. Optimization of process parameters by statistical experimental designs for the production of naringinase enzyme by marine fungi. Int. J. Chem. Eng. 1, 1–10.
Thammawat, K., Pongtanya, P., Juntharasri, V., and Wongvithoonyaporn, P. 2008. Isolation, preliminary enzyme characterization and optimization of culture parameters for production of naringinase isolated from Aspergillus niger BCC 25166. Kasetsart J. Nat. Sci. 42, 61–72.
Williams, P. and Besler, S. 1993. Thermogravimetric analysis of the components of biomass, pp. 771–783. In Advances in thermochemical biomass conversion, Springer, Dordrecht, The Netherlands.
Xu, Y.M. and Du, Y.M. 2003. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 250, 215–226.
Xu, W., Jin, W., Zhang, C., Li, Z., Lin, L., Huang, Q., Ye, S., and Li, B. 2014. Curcumin loaded and protective system based on complex of K-carrageenan and lysozyme. Food Res. Int. 59, 61–66.
Zhu, Y., Huiyong, J., Menglu, X., Jinlong, L., Li, Y., and Xiuting, L. 2017. Characterization of a naringinase from Aspergillus oryzae 11250 and its application in the debitterization of orange juice. Proc. Biochem. 62, 114–121.
Zhu, Y., Jia, H., Xi, M., Xu, L., Wu, S., and Li, X. 2016. Purification and characterization of a naringinase from a newly isolated strain of Bacillus amyloliquefaciens 11568 suitable for the transformation of flavonoids. Food Chem. 14, 39–46.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Housseiny, M.M., Aboelmagd, H.I. Nano-encapsulation of naringinase produced by Trichoderma longibrachiatum ATCC18648 on thermally stable biopolymers for citrus juice debittering. J Microbiol. 57, 521–531 (2019). https://doi.org/10.1007/s12275-019-8528-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-019-8528-6