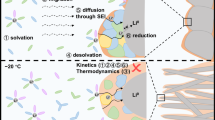
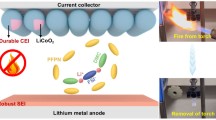
Abstract
Along with the keeping growing demand for high-energy-density energy storage system, high-voltage Li-metal batteries (LMBs) have attracted many attentions. In view of many defects of the commercial electrolytes, such as flammability, limited operation temperature range, and severe Li dendrite growth, non-flammable phosphate-based localized highly concentrated electrolytes (LHCE) have been explored as one of the safe electrolytes for LMBs. But until now there is rare report on wide-temperature range LMBs using phosphate-based electrolytes. Here, we prepare a wide-temperature LHCE, which is composed of lithium difluoro(oxalato)borate (LiDFOB), triethyl phosphate (TEP), and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (HFE), and explore the applicability in wide-temperature LMBs from −40 to 70 °C. In the LHCE, both TEP and HFE are non-flammable, and Li+ is highly coordinated with TEP and DFOB−, which can effectively inhibit the TEP decomposition on anode, and facilitate the preferential reduction of DFOB−, thus obtain a robust solid electrolyte interphase (SEI) to suppress Li dendrite growth and side reactions. Therefore, this LHCE can not only endow Li/Cu and Li/Li cells with high Coulombic efficiency (CE) and long cycling lifespan, but also be applied to LiFePO4 (LFP)/Li and LiNi0.5Co0.2Mn0.3O2 (NCM523)/Li LMBs. Most importantly, the NCM523/Li LMBs with LHCE can deliver stable cycling performance at 4.5 V high-voltage and high-temperature (70 °C), as well as excellent low-temperature capacity retention even though both charging and discharging process were carried out at −40 °C.

Similar content being viewed by others
References
Wu, F. X.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614.
Zou, J.; Yuan, K. G.; Zhao, J.; Wang, B. J.; Chen, S. Y.; Huang, J. Y.; Li, H.; Niu, X. B.; Wang, L. P. Delithiation-driven topotactic reaction endows superior cycling performances for high-energy-density FeSx (1 ≤ x ≤ 1.14) cathodes. Energy Storage Mater. 2021, 43, 579–584.
Whittingham, M. S. History, evolution, and future status of energy storage. Proc. IEEE 2012, 100, 1518–1534.
Cheng, X. B.; Zhang, R.; Zhao, C. Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473.
Xu, J. J.; Hu, Y. Y.; Liu, T.; Wu, X. D. Improvement of cycle stability for high-voltage lithium-ion batteries by in-situ growth of SEI film on cathode. Nano Energy 2014, 5, 67–73.
Li, T.; Zhang, X. Q.; Shi, P.; Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 2019, 3, 2647–2661.
Wang, J. H.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29.
Xiao, J. How lithium dendrites form in liquid batteries. Science 2019, 366, 426–427.
Wang, Z. C.; Sun, Y. Y.; Mao, Y. Y.; Zhang, F. R.; Zheng, L.; Fu, D. S.; Shen, Y. B.; Hu, J. C.; Dong, H. L.; Xu, J. J. et al. Highly concentrated dual-anion electrolyte for non-flammable high-voltage Li-metal batteries. Energy Storage Mater. 2020, 30, 228–237.
Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212.
Li, Y. C.; Veith, G. M.; Browning, K. L.; Chen, J. H.; Hensley, D. K.; Paranthaman, M. P.; Dai, S.; Sun, X. G. Lithium malonatoborate additives enabled stable cycling of 5 V lithium metal and lithium-ion batteries. Nano Energy 2017, 40, 9–19.
Landesfeind, J.; Gasteiger, H. A. Temperature and concentration dependence of the ionic transport properties of lithium-ion battery electrolytes. J. Electrochem. Soc. 2019, 166, A3079–A3097.
Fan, X. L.; Ji, X.; Chen, L.; Chen, J.; Deng, T.; Han, F. D.; Yue, J.; Piao, N.; Wang, R. X.; Zhou, X. Q. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 2019, 4, 882–890.
Zhang, S. S.; Xu, K.; Allen, J. L.; Jow, T. R. Effect of propylene carbonate on the low temperature performance of Li-ion cells. J. Power Sources 2002, 110, 216–221.
Guo, F.; Kang, T.; Liu, Z. J.; Tong, B.; Guo, L. M.; Wang, Y.; Liu, C. H.; Chen, X.; Zhao, Y. F.; Shen, Y. B. et al. An advanced lithium metal-carbon nanotube composite anode for high-performance lithium-oxygen batteries. Nano Lett. 2019, 19, 6377–6384.
Park, S. J.; Hwang, J. Y.; Yoon, C. S.; Jung, H. G.; Sun, Y. K. Stabilization of lithium-metal batteries based on in-situ formation of stable solid electrolyte interphase layer. ACS Appl. Mater. Interfaces 2018, 10, 17985–17993.
Wan, G. J.; Guo, F. H.; Li, H.; Cao, Y L.; Ai, X. P.; Qian, J. F.; Li, Y. X.; Yang, H. X. Suppression of dendritic lithium growth by in-situ formation of a chemically stable and mechanically strong solid electrolyte interphase. ACS Appl. Mater. Interfaces 2018, 10, 593–601.
Chen, L.; Sun, W. L.; Xu, K.; Dong, Q. Y.; Zheng, L.; Wang, J.; Lu, D. R.; Shen, Y. B.; Zhang, J. Y.; Fu, F. et al. How Prussian blue analogues can be stable in concentrated aqueous electrolytes. ACS Energy Lett. 2022, 7, 1672–1678.
Huang, Y. F.; Sun, W. L.; Xu, K.; Zhang, J. S.; Zhang, H.; Li, J. L.; He, L. W.; Cai, L. F.; Fu, F.; Qin, J. Q. et al. Robust interphase on both anode and cathode enables stable aqueous lithium-ion battery with coulombic efficiency exceeding 99%. Energy Storage Mater. 2022, 46, 577–582.
Zhou, D.; Liu, R. L.; He, Y. B.; Li, F. Y.; Liu, M.; Li, B. H.; Yang, Q. H.; Cai, Q.; Kang, F. Y. SiO2 hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life. Adv. Energy Mater. 2016, 6, 1502214.
Guo, Q. P.; Han, Y.; Wang, H.; Hong, X. B.; Zheng, C. M.; Liu, S. K.; Xie, K. Safer lithium metal battery based on advanced ionic liquid gel polymer nonflammable electrolytes. RSC Adv. 2016, 6, 101638–101644.
Basile, A.; Bhatt, A. I.; O’Mullane, A. P. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 2016, 7, ncomms11794.
Wang, Z. C.; Zhang, F. R.; Sun, Y. Y.; Zheng, L.; Shen, Y. B.; Fu, D. S.; Li, W. F.; Pan, A. R.; Wang, L.; Xu, J. J. et al. Intrinsically nonflammable ionic liquid-based localized highly concentrated electrolytes enable high-performance Li-metal batteries. Adv. Energy Mater. 2021, 11, 2003752.
Xiao, L. F.; Zeng, Z. Q.; Liu, X. W.; Fang, Y. J.; Jiang, X. Y.; Shao, Y. Y.; Zhuang, L.; Ai, X. P.; Yang, H. X.; Cao, Y. L. et al. Stable Li metal anode with “ion-solvent-coordinated” nonflammable electrolyte for safe Li metal batteries. ACS Energy Lett. 2019, 4, 483–488.
Zeng, Z. Q.; Murugesan, V.; Han, K. S.; Jiang, X. Y.; Cao, Y. L.; Xiao, L. F.; Ai, X. P.; Yang, H. X.; Zhang, J. G.; Sushko, M. L. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681.
Chen, S. R.; Zheng, J. M.; Yu, L.; Ren, X. D.; Engelhard, M. H.; Niu, C. J.; Lee, H.; Xu, W.; Xiao, J.; Liu, J. et al. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2018, 2, 1548–1558.
Yang, H. J.; Guo, C.; Chen, J. H.; Naveed, A.; Yang, J.; Nuli, Y. N.; Wang, J. L. An intrinsic flame-retardant organic electrolyte for safe lithium-sulfur batteries. Angew. Chem., Int. Ed. 2019, 58, 791–795.
Schedlbauer, T.; Krüger, S.; Schmitz, R.; Schmitz, R. W.; Schreiner, C.; Gores, H. J.; Passerini, S.; Winter, M. Lithium difluoro(oxalato)borate: A promising salt for lithium metal based secondary batteries? Electrochim. Acta 2013, 92, 102–107.
Allen, J. L.; Han, S. D.; Boyle, P. D.; Henderson, W. A. Crystal structure and physical properties of lithium difluoro(oxalato)borate (LiDFOB or LiBF2Ox). J. Power Sources 2011, 196, 9737–9742.
Zhou, H. M.; Liu, F. R.; Li, J. Preparation, thermal stability and electrochemical properties of LiODFB. J. Mater. Sci. Technol. 2012, 28, 723–727.
Zhang, F. R.; Sun, Y. Y.; Wang, Z. C.; Fu, D. S.; Li, J.; Hu, J. C.; Xu, J. J.; Wu, X. D. Highly conductive polymeric ionic liquid electrolytes for ambient-temperature solid-state lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 23774–23780.
Evans, J.; Vincent, C. A.; Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328.
Gholizadeh, R.; Wang, Y. J. Molecular dynamics simulation of the aggregation phenomenon in the late stages of silica materials preparation. Chem. Eng. Sci. 2018, 184, 62–71.
Wang, J. H.; Yamada, Y.; Sodeyama, K.; Chiang, C. H.; Tateyama, Y.; Yamada, A. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 2016, 7, 12032.
Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046.
Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517.
Fan, X. L.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J. J.; Xu, K. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 2018, 4, 174–185.
Li, X.; Zheng, J. M.; Engelhard, M. H.; Mei, D. H.; Li, Q. Y.; Jiao, S. H.; Liu, N.; Zhao, W. G.; Zhang, J. G.; Xu, W. Effects of imideorthoborate dual-salt mixtures in organic carbonate electrolytes on the stability of lithium metal batteries. ACS Appl. Mater. Interfaces 2018, 10, 2469–2479.
Zhou, H. M.; Xiao, K. W.; Li, J.; Xiao, D. M.; Jiang, Y. X. Synthesis of lithium difluoro(oxalate)borate (LiODFB), phase diagram and ions coordination of LiODFB in dimethyl carbonate. J. Cent. South Univ. 2018, 25, 550–560.
Polu, A. R.; Rhee, H. W. Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int. J. Hydrog. Energy 2017, 42, 7212–7219.
Septiani, N. L. W.; Kaneti, Y. V.; Fathoni, K. B.; Kani, K.; Allah, A. E.; Yuliarto, B.; Nugraha; Dipojono, H. K.; Alothman, Z. A.; Golberg, D. et al. Self-assembly of two-dimensional bimetallic nickel-cobalt phosphate nanoplates into one-dimensional porous chainlike architecture for efficient oxygen evolution reaction. Chem. Mater. 2020, 32, 7005–7018.
Wang, Z. C.; Zhang, H. Y.; Xu, J. J.; Pan, A. R.; Zhang, F. R.; Wang, L.; Han, R.; Hu, J. C.; Liu, M. N.; Wu, X. D. Advanced ultralow-concentration electrolyte for wide-temperature and high-voltage Li-metal batteries. Adv. Funct. Mater. 2022, 32, 2112598.
Takada, K.; Yamada, Y.; Yamada, A. Optimized nonflammable concentrated electrolytes by introducing a low-dielectric diluent. ACS Appl. Mater. Interfaces 2019, 11, 35770–35776.
Dong, Y.; Zhang, N.; Li, C. X.; Zhang, Y. F.; Jia, M.; Wang, Y. Y.; Zhao, Y. R.; Jiao, L. F.; Cheng, F. Y.; Xu, J. Z. Fire-retardant phosphate-based electrolytes for high-performance lithium metal batteries. ACS Appl. Energy Mater. 2019, 2, 2708–2716.
Shen, X.; Zhang, R.; Chen, X.; Cheng, X. B.; Li, X. Y.; Zhang, Q. The failure of solid electrolyte interphase on Li metal anode: Structural uniformity or mechanical strength? Adv. Energy Mater. 2020, 10, 1903645.
Zhou, H. M.; Yang, Z. H.; Xiao, D. M.; Xiao, K. W.; Li, J. An electrolyte to improve the deep charge-discharge performance of LiNi0.8Co0.15Al0.05O2 cathode. J. Mater. Sci. Mater. Electron. 2018, 29, 6648–6659.
Feng, D. J.; Chen, S. M.; Wang, R. M.; Chen, T. H.; Gu, S. J.; Su, J. L.; Dong, T.; Liu, Y. W. Mixed lithium salts electrolyte improves the high-temperature performance of nickel-rich based lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 110544.
Zhou, H. M.; Xiao, K. W.; Li, J. Lithium difluoro(oxalate)borate and LiBF4 blend salts electrolyte for LiNi0.5Mn1.5O4 cathode material. J. Power Sources 2016, 302, 274–282.
Du, K.; Wang, C.; Balaya, P.; Gajjela, S. R.; Law, M. A fire-retarding electrolyte using triethyl phosphate as a solvent for sodium-ion batteries. Chem. Commun. 2022, 58, 533–536.
Yang, H. J.; Li, Q. Y.; Guo, C.; Naveed, A.; Yang, J.; Nuli, T.; Wang, J. L. Safer lithium-sulfur battery based on nonflammable electrolyte with sulfur composite cathode. Chem. Commun. 2018, 54, 4132–4135.
Weber, I.; Wang, B.; Bodirsky, C.; Chakraborty, M.; Wachtler, M.; Diemant, T.; Schnaidt, J.; Behm, R. J. Model studies on solid electrolyte interphase formation on graphite electrodes in ethylene carbonate and dimethyl carbonate II: Graphite powder electrodes. ChemElectroChem 2020, 7, 4794–4809.
Holoubek, J.; Liu, H. D.; Wu, Z. H.; Yin, Y. J.; Xing, X.; Cai, G. R.; Yu, S. C.; Zhou, H. Y.; Pascal, T. A.; Chen, Z. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 2021, 6, 303–313.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos 22179142 and 22075314). The XPS characterization is supported by Nano-X (Vacuum Interconnected Nanotech Workstation, Chinese Academy of Sciences, Suzhou 215123, China).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pan, A., Wang, Z., Zhang, F. et al. Wide-temperature range and high safety electrolytes for high-voltage Li-metal batteries. Nano Res. 16, 8260–8268 (2023). https://doi.org/10.1007/s12274-022-4655-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4655-1