Abstract
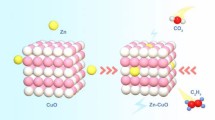
Cu-based catalysts have attracted widespread attention for its capability in electrocatalytically reducing CO2 to a variety of products. Surface modification of Cu has become an interesting method for tuning the catalytic performance. Here, we use Zr-based metal-organic layers (MOLs) as the additive of the Cu surface, which enhanced the Faradaic efficiency of CH4 by two times as compared to the untreated polycrystalline Cu foil. Unexpectedly, the MOLs were found to induce in situ nano-structuring of the Cu foil surface within seconds in the electrolysis, as revealed by a combination of scanning electron microscopy (SEM), grazing incidence X-ray diffractometry (GIXRD), and linear sweep voltammetry (LSV) measurements. These surface changes are responsible for the shift of product selectivity. Control experiments suggest that negatively charged μ3-O− on the Zr-cluster in the MOL might interact with CO-covered Cu surface and induce roughing and nano-structuring. This work reveals a potential role of additive on Cu to induce surface nano-structuring that tunes catalytic activity and selectivity.

Similar content being viewed by others
References
Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X. Y.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672.
Kuhl, K. P.; Cave, E. R.; Abram, D. N.; Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059.
Chen, X. Y.; Chen, J. F.; Alghoraibi, N. M.; Henckel, D. A.; Zhang, R. X.; Nwabara, U. O.; Madsen, K. E.; Kenis, P. J. A.; Zimmerman, S. C.; Gewirth, A. A. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 2021, 4, 20–27.
Xu, H. P.; Rebollar, D.; He, H. Y.; Chong, L. N.; Liu, Y. Z.; Liu, C.; Sun, C. J.; Li, T.; Muntean, J. V.; Winans, R. E. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632.
Dinh, C. T.; Burdyny, T.; Kibria, M. G.; Seifitokaldani, A.; Gabardo, C. M.; García de Arquer, F. P.; Kiani, A.; Edwards, J. P.; De Luna, P.; Bushuyev, O. S. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787.
Guo, Y.; He, X. R.; Su, Y. M.; Dai, Y. H.; Xie, M. C.; Yang, S. L.; Chen, J. W.; Wang, K.; Zhou, D.; Wang, C. Machine-learning-guided discovery and optimization of additives in preparing Cu catalysts for CO2 reduction. J. Am. Chem. Soc. 2021, 143, 5755–5762.
Li, F. W.; Li, Y. C.; Wang, Z. Y.; Li, J.; Nam, D. H.; Lum, Y.; Luo, M. C.; Wang, X.; Ozden, A.; Hung, S. F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule-metal catalyst interfaces. Nat. Catal. 2020, 3, 75–82.
Han, Z. J.; Kortlever, R.; Chen, H. Y.; Peters, J. C.; Agapie, T. CO2 reduction selective for C ≥ 2 products on polycrystalline copper with N-substituted pyridinium additives. ACS Cent. Sci. 2017, 3, 853–859.
Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227.
Li, F. W.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z. Y.; Li, Y. L.; Gabardo, C. M.; Ozden, A.; Dinh, C. T.; Li, J.; Wang, Y. H. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577, 509–513.
Liang, H. Q.; Zhao, S. Q.; Hu, X. M.; Ceccato, M.; Skrydstrup, T.; Daasbjerg, K. Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water. ACS Catal. 2021, 11, 958–966.
Wei, X.; Yin, Z. L.; Lyu, K.; Li, Z.; Gong, J.; Wang, G. W.; Xiao, L.; Lu, J. T.; Zhuang, L. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 2020, 10, 4103–4111.
Cao, L. Y.; Lin, Z. K.; Peng, F.; Wang, W. W.; Huang, R. Y.; Wang, C.; Yan, J. W.; Liang, J.; Zhang, Z. M.; Zhang, T. et al. Self-supporting metal-organic layers as single-site solid catalysts. Angew. Chem., Int. Ed. 2016, 55, 4962–4966.
Guo, Y.; Shi, W. J.; Yang, H. J.; He, Q. F.; Zeng, Z. M.; Ye, J. Y.; He, X. R.; Huang, R. Y.; Wang, C.; Lin, W. B. Cooperative stabilization of the [pyridinium-CO2-Co] adduct on a metal-organic layer enhances electrocatalytic CO2 reduction. J. Am. Chem. Soc. 2019, 141, 17875–17883.
Ji, P. F.; Solomon, J. B.; Lin, Z. K.; M. Wilders, A.; Jordan, R. F.; Lin, W. B. Transformation of metal-organic framework secondary building units into hexanuclear Zr-alkyl catalysts for ethylene polymerization. J. Am. Chem. Soc. 2017, 139, 11325–11328.
DeStefano, M. R.; Islamoglu, T.; Garibay, S. J.; Hupp, J. T.; Farha, O. K. Room-temperature synthesis of UiO-66 and thermal modulation of densities of defect sites. Chem. Mater. 2017, 29, 1357–1361.
Kickelbick, G.; Wiede, P.; Schubert, U. Variations in capping the Zr6O4(OH)4 cluster core: X-ray structure analyses of [Zr6(OH)4O4(OOC-CH=CH2)10]2(μ-OOC-CH=CH2)4 and Zr6(OH)4O4(OOCR)12(PrOH) (R = Ph, CMe=CH2). Inorg. Chim. Acta 1999, 284, 1–7.
Dunwell, M.; Lu, Q.; Heyes, J. M.; Rosen, J.; Chen, J. G.; Yan, Y. S.; Jiao, F.; Xu, B. J. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 2017, 139, 3774–3783.
Heyes, J.; Dunwell, M.; Xu, B. J. CO2 reduction on Cu at low overpotentials with surface-enhanced in situ spectroscopy. J. Phys. Chem. C 2016, 120, 17334–17341.
Malkani, A. S.; Dunwell, M.; Xu, B. J. Operando spectroscopic investigations of copper and oxide-derived copper catalysts for electrochemical CO reduction. ACS Catal. 2019, 9, 474–478.
Malkani, A. S.; Li, J.; Anibal, J.; Lu, Q.; Xu, B. J. Impact of forced convection on spectroscopic observations of the electrochemical CO reduction reaction. ACS Catal. 2020, 10, 941–946.
Ma, M.; Djanashvili, K.; Smith, W. A. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays. Angew. Chem., Int. Ed. 2016, 55, 6680–6684.
Handoko, A. D.; Ong, C. W.; Huang, Y.; Lee, Z. G.; Lin, L. Y.; Panetti, G. B.; Yeo, B. S. Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C 2016, 120, 20058–20067.
Lee, S. H.; Lin, J. C.; Farmand, M.; Landers, A. T.; Feaster, J. T.; Avilés Acosta, J. E.; Beeman, J. W.; Ye, Y. F.; Yano, J.; Mehta, A. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 2021, 143, 588–592.
Zhu, C. Y.; Zhang, Z. B.; Zhong, L. X.; Hsu, C. S.; Xu, X. Z.; Li, Y. Z.; Zhao, S. W.; Chen, S. H.; Yu, J. Y.; Chen, S. L. et al. Product-specific active site motifs of Cu for electrochemical CO2 reduction. Chem 2021, 7, 406–420.
Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surf. Sci. 1995, 335, 258–263.
Iijima, G.; Yamaguchi, H.; Inomata, T.; Yoto, H.; Ito, M.; Masuda, H. Methanethiol SAMs induce reconstruction and formation of Cu+ on a Cu catalyst under electrochemical CO2 reduction. ACS Catal. 2020, 10, 15238–15249.
Raciti, D.; Cao, L.; Livi, K. J. T.; Rottmann, P. F.; Tang, X.; Li, C. Y.; Hicks, Z.; Bowen, K. H.; Hemker, K. J.; Mueller, T. et al. Low-overpotential electroreduction of carbon monoxide using copper nanowires. ACS Catal. 2017, 7, 4467–4472.
Xiong, L. K.; Zhang, X.; Chen, L.; Deng, Z.; Han, S.; Chen, Y. F.; Zhong, J.; Sun, H.; Lian, Y. B.; Yang, B. Y. et al. Geometric modulation of local CO flux in Ag@Cu2O nanoreactors for steering the CO2RR pathway toward high-efficacy methane production. Adv. Mater. 2021, 33, 2101741.
Hollins, P. The influence of surface defects on the infrared spectra of adsorbed species. Surf. Sci. Rep. 1992, 16, 51–94.
Roth, J. D.; Weaver, M. J. Role of double-layer cation on the potential-dependent stretching frequencies and binding geometries of carbon monoxide at platinum-nonaqueous interfaces. Langmuir 1992, 8, 1451–1458.
Zhang, L.; Li, X. X.; Lang, Z. L.; Liu, Y.; Liu, J.; Yuan, L.; Lu, W. Y.; Xia, Y. S.; Dong, L. Z.; Yuan, D. Q. et al. Enhanced cuprophilic interactions in crystalline catalysts facilitate the highly selective electroreduction of CO2 to CH4. J. Am. Chem. Soc. 2021, 143, 3808–3816.
Wang, T. T.; Zeng, Z. M.; Cao, L. Y.; Li, Z.; Hu, X. F.; An, B.; Wang, C.; Lin, W. B. A Dynamically stabilized single-nickel electrocatalyst for selective reduction of oxygen to hydrogen peroxide. Chem.—Eur. J. 2018, 24, 17011–17018.
Klet, R. C.; Liu, Y. Y.; Wang, T. C.; Hupp, J. T.; Farha, O. K. Evaluation of Brønsted acidity and proton topology in Zr- and Hf-based metal-organic frameworks using potentiometric acid-base titration. J. Mater. Chem. A 2016, 4, 1479–1485.
Eren, B.; Zherebetskyy, D.; Patera, L. L.; Wu, C. H.; Bluhm, H.; Africh, C.; Wang, L. W.; Somorjai, G. A.; Salmeron, M. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 2016, 351, 475–478.
Acknowledgements
We acknowledge funding support from the National Natural Science Foundation of China (Nos. 22125502, 22071207, 22121001, and 21721001) and NFFTBS (No. J1310024)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
He, X., Chen, J., Xu, Y. et al. Metal-organic layers induce in situ nano-structuring of Cu surface in electrocatalytic CO2 reduction. Nano Res. 16, 4554–4561 (2023). https://doi.org/10.1007/s12274-022-4461-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4461-9