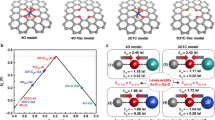
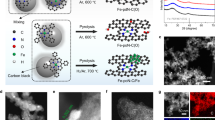
Abstract
Unraveling the substrate adsorption structure-performance relationship is pivotal for heterogeneous carbon supported metal single-atom catalysts (M1/C SACs). However, due to the complexity of the functional groups on carbon material surface, it is still a great challenge. Herein, inspired by structure of enzymes, we used activated carbon (AC), which has adjustable surface oxygen functional groups (OFGs), supported atomically dispersed Fe-N4 sites as heme-like catalyst. And based on a combination of scanning transmission electron microscopy (STEM), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), Mössbauer spectroscopy, Fourier transform infrared (FT-IR) characterizations, kinetics experiments and density functional theory (DFT) calculations, we revealed the effect of substrate adsorption behavior on AC support surface, that is, with the increase of carboxyl group in OFGs, the adsorbed 3,3′,5,5′-tetramethylbenzidine (TMB) molecular increased, and consequently the substrate enriched on AC surface. Such carboxyl group as well as Fe-N4 active sites synergistically realized high-efficiency peroxidase-like activity, just like the heme. This work suggests that simultaneously constructing metal single-atom active sites and specific functional groups on carbon support surface may open an avenue for engineering metal-support synergistic catalysis in M1/C SACs, which can further improve catalytic performance.

Similar content being viewed by others
References
Fei, H. L.; Dong, J. C.; Chen, D. L.; Hu, T. D.; Duan, X. D.; Shakir, I.; Huang, Y.; Duan, X. F. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241.
He, Y. H.; Liu, S. W.; Priest, C.; Shi, Q. R.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524.
Wang, Y.; Mao, J.; Meng, X. G.; Yu, L.; Deng, D. H.; Bao, X. H. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications. Chem. Rev. 2019, 119, 1806–1854.
Zhang, H. B.; Lu, X. F.; Wu, Z. P.; Lou, X. W. D. Emerging multifunctional single-atom catalysts/nanozymes. ACS Cent. Sci. 2020, 6, 1288–1301.
Zhang, L. L.; Zhou, M. X.; Wang, A. Q.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733.
Chen, Y. J.; Ji, S. F.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264.
Ji, S. F.; Jiang, B.; Hao, H. G.; Chen, Y. J.; Dong, J. C.; Mao, Y.; Zhang, Z. D.; Gao, R.; Chen, W. X.; Zhang, R. F. et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 2021, 4, 407–417.
Huang, L.; Chen, J. X.; Gan, L. F.; Wang, J., Dong, S. J. Single-atom nanozymes. Sci. Adv. 2019, 5, eaav5490.
Kaiser, S. K.; Fako, E.; Manzocchi, G.; Krumeich, F.; Hauert, R.; Clark, A. H.; Safonova, O. V.; López, N.; Pérez-Ramírez, J. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production. Nat. Catal. 2020, 3, 376–385.
Qi, H. F.; Yang, J.; Liu, F.; Zhang, L. L.; Yang, J. Y.; Liu, X. Y.; Li, L.; Su, Y.; Liu, Y. F.; Hao, R. et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones. Nat. Commun. 2021, 12, 3295.
Xiang, H. J.; Feng, W.; Chen, Y. Single-atom catalysts in catalytic biomedicine. Adv. Mater. 2020, 32, 1905994.
Zhang, Z.; Liu, W. G.; Zhang, Y. Y.; Bai, J. W.; Liu, J. Bioinspired atomic manganese site accelerates oxo-dehydrogenation of N-heterocycles over a conjugated tri-s-triazine framework. ACS Catal. 2020, 11, 313–322.
Feng, S. Q.; Song, X. G.; Liu, Y.; Lin, X. S.; Yan, L.; Liu, S. Y.; Dong, W. R.; Yang, X. M.; Jiang, Z.; Ding, Y. J. In situ formation of mononuclear complexes by reaction-induced atomic dispersion of supported noble metal nanoparticles. Nat. Commun. 2011, 10, 5281.
Huang, F.; Deng, Y. C.; Chen, Y. L.; Cai, X. B.; Peng, M.; Jia, Z. M.; Ren, P. J.; Xiao, D. Q.; Wen, X. D.; Wang, N. et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 2018, 140, 13142–13146.
Li, X. Y.; Rong, H. P.; Zhang, J. T.; Wang, D. S.; Li, Y. D. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855.
Sun, T.; Mitchell, S.; Li, J.; Lyu, P.; Wu, X. B.; Pérez-Ramírez, J.; Lu, J. Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions. Adv. Mater. 2021, 33, 2003075.
Liu, P. X.; Zheng, N. F. Coordination chemistry of atomically dispersed catalysts. Natl. Sci. Rev. 2018, 5, 636–638.
Liu, W. G.; Zhang, L. L.; Liu, X.; Liu, X. Y.; Yang, X. F.; Miao, S.; Wang, W. T.; Wang, A. Q.; Zhang, T. Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C-H bond. J. Am. Chem. Soc. 2017, 139, 10790–10798.
Jiang, R.; Li, L.; Sheng, T.; Hu, G. F.; Chen, Y. G.; Wang, L. Y. Edge-site engineering of atomically dispersed Fe-N4 by selective C-N bond cleavage for enhanced oxygen reduction reaction activities. J. Am. Chem. Soc. 2018, 140, 11594–11598.
Yin, X. P.; Wang, H. J.; Tang, S. F.; Lu, X. L.; Shu, M.; Si, R.; Lu, T. B. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew. Chem., Int. Ed. 2018, 57, 9382–9386.
Wang, Y.; Jia, G. R.; Cui, X. Q.; Zhao, X.; Zhang, Q. H.; Gu, L.; Zheng, L. R.; Li, L. H.; Wu, Q.; Singh, D. J. et al. Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specificity. Chem 2021, 7, 436–449.
Zhou, D.; Zhang, L. L.; Liu, X. Y.; Qi, H. F.; Liu, Q. G.; Yang, J.; Su, Y.; Ma, J. Y.; Yin, J. Z.; Wang, A. Q. Tuning the coordination environment of single-atom catalyst M-N-C towards selective hydrogenation of functionalized nitroarenes. Nano Res. 2022, 15, 519–527.
Wang, L. L.; Zhu, C. W.; Xu, M. Q.; Zhao, C. L.; Gu, J.; Cao, L. N.; Zhang, X. H.; Sun, Z. H.; Wei, S. Q.; Zhou, W. et al. Boosting activity and stability of metal single-atom catalysts via regulation of coordination number and local composition. J. Am. Chem. Soc. 2021, 143, 18854–18858.
Xu, W. Q.; Song, W. Y.; Kang, Y. K.; Jiao, L.; Wu, Y.; Chen, Y. F.; Cai, X. L.; Zheng, L. R.; Gu, W. L.; Zhu, C. Z. Axial ligand-engineered single-atom catalysts with boosted enzyme-like activity for sensitive immunoassay. Anal. Chem. 2021, 93, 12758–12766.
Jiao, L.; Xu, W. Q.; Zhang, Y.; Wu, Y.; Gu, W. L.; Ge, X. X.; Chen, B. B.; Zhu, C. Z.; Guo, S. J. Boron-doped Fe-N-C single-atom nanozymes specifically boost peroxidase-like activity. Nano Today 2020, 35, 100971.
Zhang, J.; Wang, L.; Shao, Y.; Wang, Y. Q.; Gates, B. C.; Xiao, F. S. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew. Chem. 2017, 129, 9879–9883.
Zhao, X.; Wang, F. L.; Kong, X. P.; Fang, R. Q.; Li, Y. W. Dual-metal hetero-single-atoms with different coordination for efficient synergistic catalysis. J. Am. Chem. Soc. 2021, 143, 16068–16077.
Zhang, Y. F.; Ge, J.; Liu, Z. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal. 2015, 5, 4503–4513.
Zhou, T. J.; Mo, Y. R.; Liu, A. M.; Zhou, Z. H.; Tsai, K. R. Enzymatic mechanism of Fe-only hydrogenase: Density functional study on H-H making/breaking at the diiron cluster with concerted proton and electron transfers. Inorg. Chem. 2004, 43, 923–930.
Anderson, J. S.; Rittle, J.; Peters, J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 2013, 501, 84–87.
Seefeldt, L. C.; Yang, Z. Y.; Lukoyanov, D. A.; Harris, D. F.; Dean, D. R.; Raugei, S.; Hoffman, B. M. Reduction of substrates by nitrogenases. Chem. Rev. 2020, 120, 5082–5106.
Xue, T.; Jiang, S.; Qu, Y. Q.; Su, Q.; Cheng, R.; Dubin, S.; Chiu, C. Y.; Kaner, R.; Huang, Y.; Duan, X. F. Graphene-supported hemin as a highly active biomimetic oxidation catalyst. Angew. Chem., Int. Ed. 2012, 51, 3822–3825.
Gao, L. Z.; Gao, X. F.; Yan, X. Y. Kinetics and mechanisms for nanozymes. In Nonozomology: Cnnnenting biolygy and nanotechnology. Yan, X. Y., Ed.; Springer: Singapore, 2020; pp 17–39.
Ma, N. N.; Chen, Z. F.; Chen, J.; Chen, J. F.; Wang, C.; Zhou, H. F.; Yao, L. S.; Shoji, O.; Watanabe, Y.; Cong, Z. Q. Dual-functional small molecules for generating an efficient cytochrome P450BM3 peroxygenase. Angew. Chem., Int. Ed. 2018, 57, 7628–7633.
Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J. J.; Wu, H.; Luo, G. Q.; Xu, M. H.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8.
Han, G. F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J. P.; Kim, S. W.; Kim, S. J.; Bu, Y. F.; Fu, Z. P.; Lu, Y. L. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209.
Chen, C. M.; Zhang, Q.; Yang, M. G.; Huang, C. H.; Yang, Y. G.; Wang, M. Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584.
Seoudi, R.; El-Bahy, G. S.; El Sayed, Z. A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126.
Westre, T. E.; Kennepohl, P.; DeWitt, J. G.; Hedman, B.; Hodgson, K. O.; Solomon, E. I. A multiplet analysis of Fe K-edge 1s→3d pre-edge features of iron complexes. J. Am. Chem. Soc. 1997, 119, 6297–6314.
Wu, Z. Y.; Ouvrard, G.; Gressier, P.; Natoli, C. R. Ti and O K edges for titanium oxides by multiple scattering calculations: Comparison to XAS and EELS spectra. Phys. Rev. B 1997, 55, 10381–10391.
Yamamoto, T. Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: Electric dipole or quadrupole? X-Ray Spectrom. 2008, 37, 572–584.
Maruyama, J.; Abe, I. Fuel cell cathode catalyst with heme-like structure formed from nitrogen of glycine and iron. J. Electrochem. Soc. 2007, 154, B297–B304.
Kramm, U. I.; Herranz, J.; Larouche, N.; Arruda, T. M.; Lefèvre, M.; Jaouen, F.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Mukerjee, S. et al. Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 2012, 14, 11673–11688.
Ren, Y. J.; Wei, H. S.; Yin, G. Z.; Zhang, L. L.; Wang, A. Q.; Zhang, T. Oxygen surface groups of activated carbon steer the chemoselective hydrogenation of substituted nitroarenes over nickel nanoparticles. Chem. Commun. 2017, 53, 1969–1972.
Chuang, C. H.; Ray, S. C.; Mazumder, D.; Sharma, S.; Ganguly, A.; Papakonstantinou, P; Chiou, J. W.; Tsai, H. M.; Shiu, H. W.; Chen, C. H. et al. Chemical modification of graphene oxide by nitrogenation: An X-ray absorption and emission spectroscopy study. Sci. Rep. 2017, 7, 42235.
Qi, W.; Liu, W.; Zhang, B. S.; Gu, X. M.; Guo, X. L.; Su, D. S. Oxidative dehydrogenation on nanocarbon: Identification and quantification of active sites by chemical titration. Angew. Chem., Int. Ed. 2013, 52, 14224–14228.
Lu, Z. Y.; Chen, G. X.; Siahrostami, S.; Chen, Z. H.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D. C.; Liu, Y. Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162.
Kim, H. W.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J. H.; Yang, P. D.; McCloskey, B. D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282–290.
Yang, S.; Cheng, Q. Q.; Mao, J. N.; Xu, Q.; Zhang, Y. J.; Guo, Y.; Tan, T. Y.; Luo, W.; Yang, H.; Jiang, Z. Rational design of edges of covalent organic networks for catalyzing hydrogen peroxide production. Appl. Catal. B 2021, 298, 120605.
Jeong, H. K.; Noh, H. J.; Kim, J. Y.; Jin, M. H.; Park, C. Y.; Lee, Y. H. X-ray absorption spectroscopy of graphite oxide. Eur. Lett. 2008, 82, 67004.
Xu, B. L.; Wang, H.; Wang, W. W.; Gao, L. Z.; Li, S. S.; Pan, X. T.; Wang, H. Y.; Yang, H. L.; Meng, X. Q.; Wu, Q. W. et al. A singleatom nanozyme for wound disinfection applications. Angew. Chem., Int. Ed. 2019, 58, 4911–4916.
Ota, N.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Performance, Structure, and mechanism of ReOt-Pd/CeO2 catalyst for simultaneous removal of vicinal OH groups with H2. ACS Catal. 2016, 6, 3213–3226.
Thornton, D. A. Metal complexes of aniline: Infrared and Raman spectra. J. Coord. Chem. 1991, 24, 261–289.
Tamura, M.; Yuasa, N.; Nakagawa, Y.; Tomishige, K. Selective hydrogenation of nitroarenes to aminoarenes using a MoOx-modified Ru/SiO2 catalyst under mild conditions. Chem. Commun. 2017, 53, 3377–3380.
Acknowledgements
The authors wish to acknowledge the support of National Natural Science Foundation of China (NSFC, Nos. 21802094, 22002118, 22172119, and 22102167), Postdoctoral Research Foundation of China (Nos. 2020TQ0245 and 2021M693060) and Natural Science Basic Research Plan in Shaanxi Province of China (No. 2021JM-047). We also thank the BL 14W beamline at the SSRF and MCD-A beamline at NSRL. The calculations were performed on the Supercomputing Center of the University of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Duan, J., Zhou, Y., Ren, Y. et al. Insights into the effect of substrate adsorption behavior over heme-like Fe1/AC single-atom catalyst. Nano Res. 15, 5970–5976 (2022). https://doi.org/10.1007/s12274-022-4274-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4274-x