Abstract
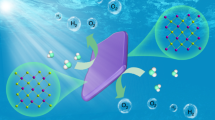
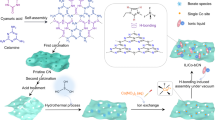
The utilization of perovskites as photocatalysts to convert CO2 into fuels and chemicals has received wide attention recently. However, their instability in water hinders their long-term application for overall photocatalytic CO2 reduction. Herein, we integrate the water-stable perovskite-like organolead iodide crystalline material [Pb8I8(H2O)3]8+[−O2C(CH2)4CO2−]4 (TJU-16) with Au co-catalyst for photocatalytic CO2 reduction in aqueous solution without sacrificial reagent. Under the AM 1.5 G simulated illumination, the TJU-16 with 0.19 wt.‰ Au co-catalyst steadily generated electrons for CO2 reduction reaction, which was 2.2 times of pure TJU-16. The Au0.19/TJU-16 catalyzed CO2 reduction at a rate of 84.2 µmol·g−1·h−1, and achieved a solar-to-fuel (STF) conversion efficiency of 0.034%. Our work will motivate the rational design of water-stable perovskite-like materials for photocatalytic applications.

Similar content being viewed by others
References
Chen, J.; Dong, C. W.; Idriss, H.; Mohammed, O. F.; Bakr, O. M. Metal halide perovskites for solar-to-chemical fuel conversion. Adv. Energy Mater. 2020, 10, 1902433.
Shyamal, S.; Pradhan, N. Halide perovskite nanocrystal photocatalysts for CO2 reduction: Successes and challenges. J. Phys. Chem. Lett. 2020, 11, 6921–6934.
Ahmed, G. H.; Yin, J.; Bakr, O. M.; Mohammed, O. F. Successes and challenges of core/shell lead halide perovskite nanocrystals. ACS Energy Lett. 2021, 6, 1340–1357.
Spanopoulos, I.; Hadar, I.; Ke, W. J.; Guo, P. J.; Sidhik, S.; Kepenekian, M.; Even, J.; Mohite, A. D.; Schaller, R. D.; Kanatzidis, M. G. Water-stable 1D hybrid tin(II) iodide emits broad light with 36% photoluminescence quantum efficiency. J. Am. Chem. Soc. 2020, 142, 9028–9038.
Cho, H.; Jeong, S. H.; Park, M. H.; Kim, Y. H.; Wolf, C.; Lee, C. L.; Heo, J. H.; Sadhanala, A.; Myoung, N.; Yoo, S. et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225.
Guan, W. H.; Li, Y.; Zhong, Q. X.; Liu, H. Y.; Chen, J. N.; Hu, H. C.; Lv, K. X.; Gong, J.; Xu, Y.; Kang, Z. H. et al. Fabricating MAPbI3/MoS2 composites for improved photocatalytic performance. Nano Lett. 2021, 21, 597–604.
Zhou, P.; Chen, H.; Chao, Y. G.; Zhang, Q. H.; Zhang, W. Y.; Lv, F.; Gu, L.; Zhao, Q.; Wang, N.; Wang, J. S.; Guo, S. J. Single-atom Pt-I3 sites on all-inorganic Cs2SnI6 perovskite for efficient photocatalytic hydrogen production. Nat. Commun. 2021, 12, 4412.
Pornrungroj, C.; Andrei, V.; Rahaman, M.; Uswachoke, C.; Joyce, H. J.; Wright, D. S.; Reisner, E. Bifunctional perovskite-BiVO4 tandem devices for uninterrupted solar and electrocatalytic water splitting cycles. Adv. Funct. Mater. 2021, 31, 2008182.
Wang, H.; Zhang, H. F.; Wang, J. H.; Gao, Y. Y.; Fan, F. T.; Wu, K. F.; Zong, X.; Li, C. Mechanistic understanding of efficient photocatalytic H2 evolution on two-dimensional layered lead iodide hybrid perovskites. Angew. Chem., Int. Ed. 2021, 60, 7376–7381.
Chen, Y. H.; Ye, J. K.; Chang, Y. J., Liu, T. W.; Chuang, Y. H.; Liu, W. R.; Liu, S. H.; Pu, Y. C. Mechanisms behind photocatalytic CO2 reduction by CsPbBr3 perovskite-graphene-based nanoheterostructures. Appl. Catal. B:Environ. 2021, 284, 119751.
Mu, Y. F.; Zhang, C.; Zhang, M. R.; Zhang, W.; Zhang, M.; Lu, T. B. Direct Z-scheme heterojunction of ligand-free FAPbBr3/α-Fe2O3 for boosting photocatalysis of CO2 reduction coupled with water oxidation. ACS Appl. Mater. Interfaces 2021, 13, 22314–22322.
Xu, Y.; Zhang, W.; Su, K.; Feng, Y. X.; Mu, Y. F.; Zhang, M.; Lu, T. B. Glycine-functionalized CsPbBr3 nanocrystals for efficient visible-light photocatalysis of CO2 reduction. Chem.—Eur. J. 2021, 27, 2305–2309.
Xu, F. Y.; Meng, K.; Cheng, B.; Wang, S. Y.; Xu, J. S.; Yu, J. G. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613.
Mu, Y. F.; Zhang, W.; Dong, G. X.; Su, K.; Zhang, M.; Lu, T. B. Ultrathin and small-size graphene oxide as an electron mediator for perovskite-based Z-scheme system to significantly enhance photocatalytic CO2 reduction. Small 2020, 16, 2002140.
Cheng, R. L.; Jin, H. D.; Roeffaers, M. B. J.; Hofkens, J.; Debroye, E. Incorporation of cesium lead halide perovskites into g-C3N4 for photocatalytic CO2 reduction. ACS Omega 2020, 5, 24495–24503.
Wang, Y. Y.; Huang, H. L.; Zhang, Z. Z.; Wang, C.; Yang, Y. Y.; Li, Q.; Xu, D. S. Lead-free perovskite Cs2AgBiBr6@g-C3N4 Z-scheme system for improving CH4 production in photocatalytic CO2 reduction. Appl. Catal. B:Environ. 2021, 282, 119570.
Chen, Z. J.; Hu, Y. G.; Wang, J.; Shen, Q.; Zhang, Y. H.; Ding, C.; Bai, Y.; Jiang, G. C.; Li, Z. Q.; Gaponik N. Boosting photocatalytic CO2 reduction on CsPbBr3 perovskite nanocrystals by immobilizing metal complexes. Chem. Mater. 2020, 32, 1517–1525.
Wang, Q.; Wang, J.; Wang, J. C.; Hu, X.; Bai, Y.; Zhong, X. H.; Li, Z. Q. Coupling CsPbBr3 quantum dots with covalent triazine frameworks for visible-light-driven CO2 reduction. ChemSusChem 2021, 14, 1131–1139.
Bera, S.; Shyamal, S.; Pradhan, N. Chemically spiraling CsPbBr3 perovskite nanorods. J. Am. Chem. Soc. 2021, 143, 14895–14906.
Xu, Y. F.; Yang, M. Z.; Chen, B. X.; Wang, X. D.; Chen, H. Y.; Kuang, D. B.; Su, C. Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663.
Xu, Y. F.; Yang, M. Z.; Chen, H. Y.; Liao, J. F.; Wang, X. D.; Kuang, D. B. Enhanced solar-driven gaseous CO2 conversion by CsPbBr3 nanocrystal/Pd nanosheet schottky-junction photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089.
Que, M. D.; Zhao, Y.; Yang, Y. W.; Pan, L. K.; Lei, W. Y.; Cai, W. H.; Yuan, H. D.; Chen, J.; Zhu, G. Q. Anchoring of formamidinium lead bromide quantum dots on Ti3C2 nanosheets for efficient photocatalytic reduction of CO2. ACS Appl. Mater. Interfaces 2021, 13, 6180–6187.
Kong, Z. C.; Liao, J. F.; Dong, Y. J.; Xu, Y. F.; Chen, H. Y.; Kuang, D. B.; Su, C. Y. Core@shell CsPbBr3@zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett. 2018, 3, 2656–2662.
Wu, L. Y.; Mu, Y. F.; Guo, X. X.; Zhang, W.; Zhang, Z. M.; Zhang, M.; Lu, T. B. Encapsulating perovskite quantum dots in iron-based metal—organic frameworks (MOFs) for efficient photocatalytic CO2 reduction. Angew. Chem., Int. Ed. 2019, 58, 9491–9495.
Ou, M.; Tu, W. G.; Yin, S. M.; Xing, W. N.; Wu, S. Y.; Wang, H. J.; Wan, S. P.; Zhong, Q.; Xu, R. Amino-assisted anchoring of CsPbBr3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction. Angew. Chem. 2018, 130, 13758–13762.
Su, K.; Dong, G. X.; Zhang, W.; Liu, Z. L.; Zhang, M.; Lu, T. B. In situ coating CsPbBr3 nanocrystals with graphdiyne to boost the activity and stability of photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2020, 12, 50464–50471.
Song, X. L.; Wei, G. F.; Sun, J.; Peng, C. D.; Yin, J. L.; Zhang, X.; Jiang, Y. L.; Fei, H. H. Overall photocatalytic water splitting by an organolead iodide crystalline material. Nat. Catal. 2020, 3, 1027–1033.
Li, R. G.; Han, H. X.; Zhang, F. X.; Wang, D. E.; Li, C. Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4. Energy Environ. Sci. 2014, 7, 1369–1376.
Lin, F.; Wang, D. E.; Jiang, Z. X.; Ma, Y.; Li, J.; Li, R. G.; Li, C. Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalysts Pt and RuO2 under visible light irradiation using molecular oxygen as oxidant. Energy Environ. Sci. 2012, 5, 6400–6406.
Wang, X. D.; Huang, Y. H.; Liao, J. F.; Jiang, Y.; Zhou, L.; Zhang, X. Y.; Chen, H. Y.; Kuang, D. B. In situ construction of a Cs2SnI6 perovskite nanocrystal/SnS2 nanosheet heterojunction with boosted interfacial charge transfer. J. Am. Chem. Soc. 2019, 141, 13434–13441.
Acknowledgements
J. S. L. acknowledges the funding support from the National Key Research and Development Program of China (No. 2019YFE0123400), the Excellent Young Scholar Fund from the National Natural Science Foundation of China (No. 22122903), and the Tianjin Distinguished Young Scholars Fund (No. 20JCJQJC00260).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Chen, R., Gao, G. & Luo, J. A water-stable organolead iodide material for overall photocatalytic CO2 reduction. Nano Res. 15, 10084–10089 (2022). https://doi.org/10.1007/s12274-022-4216-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4216-7