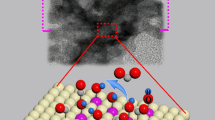
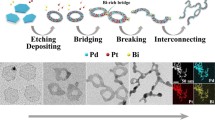
Abstract
Highly active and durable Pd-based electrocatalysts for ethanol oxidation reaction (EOR) play a crucial role in the commercialization of direct ethanol fuel cells (DEFCs). However, the poisonous intermediates (especially adsorbed CO species (COad)) formed during the EOR process can easily adsorb and block the active sites on Pd electrodes, which in turn limits the catalytic efficiency. Hence, we present a series of Pd-based composites with a strong coupling interface consisting of Pd nanosheets and amorphous Bi(OH)3 species. The incorporation of Bi(OH)3 can induce an electron-rich state adjacent to the Pd sites and effectively separate the Pd ensemble, leading to excellent CO tolerance. The optimal Pd-Bi(OH)3 NSs catalyst manifests a mass activity of 2.2 A·mgPd−1, which is 5.7 and 2.0 times higher than that of Pd NSs and commercial Pd/C catalyst, respectively. Further CO-stripping experiments and CO-DRIFTS tests confirm the excellent CO tolerance on Pd-Bi(OH)3 NSs electrode, leading to the enhanced EOR durability.

Similar content being viewed by others
References
Bianchini, C.; Shen, P. K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206.
Bai, J.; Liu, D. Y.; Yang, J.; Chen, Y. Nanocatalysts for electrocatalytic oxidation of ethanol. ChemSusCeem 2019, 12, 2117–2132.
Bai, S. X.; Xu, Y.; Cao, K. L.; Huang, X. Q. Selective ethanol oxidation reaction at the Rh-SnO2 irrtrfacee. Adv. Mater. 2021, 33, 2005767.
Mao, J. J.; Chen, W. X.; He, D. S.; Wan, J. W.; Pei, J. J.; Dong, J. C.; Wang, Y.; An, P. F.; Jin, Z.; Xing, W. et al. Design of ultrathin Pt-Mo-Ni nanowire catalysts for ethanol electrooxidation. Sci. Adv. 2017, 3, e1603068.
Feng, Q. C.; Zhao, S.; He, D. S.; Tian, S. B.; Gu, L.; Wen, X. D.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga. J. Am. Chem. Soc. 2018, 140, 2773–2776.
Wang, H.; Jusys, Z.; Behm, R. J. Ethanol electrooxidation on a carbon-supported Pt catalyst: Reaction kinetics and product yields. J. Phyu. Chem. B 2004, 108, 19413–19424.
Monyoncho, E. A.; Steinmann, S. N.; Michel, C.; Baranova, E. A.; Woo, T. K.; Sautet, P. Ethanol electro-oxidation on palladium revisited using polarization modulation infrared reflection absorption spectroscopy (PM-IRRAS) and density functional theory (DFT): Why is it difficult to break the C-C bond? ACS Catal. 2016, 6, 4894–4906.
Rizo, R.; Aran-Ais, R. M.; Padgett, E.; Muller, D. A.; Lázaro, M. J.; Solla-Gullón, J.; Feliu, J. M.; Pastor, E.; Abruña, H. D. Pt-richcore/Sn-richsubsurface/Ptskin nanocubes as highly active and stable electrocatalysts for the ethanol oxidation reaction. J. Am. Chem. Soc. 2018, 140, 3791–3797.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 31, 2003300.
Han, S. H.; Liu, H. M.; Chen, P.; Jiang, J. X.; Chen, Y. Porous trimetallic PtRhCu cubic nanoboxes for ethanol electrooxidation. Adv. Energy Mater. 2018, 8, 1801326.
Kowal, A.; Li, M.; Shao, M.; Sasaki, K.; Vukmirovic, M. B.; Zhang, J.; Marinkovic, N. S.; Liu, P.; Frenkel, A. I.; Adzic, R. R. Ternary Pt/Rh/SnO2 electrocatalysts for oxidizing ethanol to CO2. Nat. Mater. 2009, 8, 325–330.
Zhang, L. L.; Chang, Q. W.; Chen, H. M.; Shao, M. H. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219.
Yang, Y. Y.; Ren, J.; Li, Q. X.; Zhou, Z. Y.; Sun, S. G.; Cai, W. B. Electrocatalysis of ethanol on a Pd electrode in alkaline media: An in uitu attenuated total reflection surface-enhanced infrared absorption spectroscopy study. ACS Catal. 2014, 4, 798–803.
Zhang, Y.; Yuan, X. L.; Lyu, F. L.; Wang, X. C.; Jiang, X. J.; Cao, M. H.; Zhang, Q. Facile one-step synthesis of PdPb nanochains for high-performance electrocatalytic ethanol oxidation. Rare Met. 2020, 39, 792–799.
Kang, Y. Q.; Xue, Q.; Zhao, Y.; Li, X. F.; Jin, P. J.; Chen, Y. Selective etching induced synthesis of hollow Rh nanospheres electrocatalyst for alcohol oxidation reactions. Small 2018, 14, 1801239.
Zhang, J. W.; Ye, J. Y.; Fan, Q. Y.; Jiang, Y. T.; Zhu, Y. F.; Li, H. Q.; Cao, Z. M.; Kuang, Q.; Cheng, J.; Zheng, J. et al. Cyclic penta-twinned rhodium nanobranches as superior catalysts for ethanol electro-oxidation. J. Am. Chem. Soc. 2018, 140, 11232–11240.
Yuan, Q.; Zhou, Z. Y.; Zhuang, J.; Wang, X. Seed displacement, epitaxial synthesis of Rh/Pt bimetallic ultrathin nanowires for highly selective oxidizing ethanol to CO2. Chem. Mater. 2010, 22, 2395–2402.
Antolini, E.; Gonzalez, E. R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450.
Ren, L.; Yang, L. F.; Yu, P.; Wang, Y. X.; Mao, L. Q. Electrochemical post-treatment of infinite coordination polymers: An effective route to preparation of Pd nanoparticles supported onto carbon nanotubes with enhanced electrocatalytic activity toward ethanol oxidation. ACS Appl. Mater. Interfaces 2013, 5, 11471–11478.
Zhou, Z. Y.; Wang, Q.; Lin, J. L.; Tian, N.; Sun, S. G. In situ FTIR spectroscopic studies of electrooxidation of ethanol on Pd electrode in alkaline media. Electrochimi. Acta 2010, 55, 7995–7999.
Wu, H. X.; Li, H. J.; Zhai, Y. J.; Xu, X. L.; Jin, Y. D. Facile synthesis of free-standing Pd-based nanomembranes with enhanced catalytic performance for methanol/ethanol oxidation. Adv. Mater. 2012, 14, 1594–1597.
Tiwari, J. N.; Dang, N. K.; Park, H. J.; Sultan, S.; Kim, M. G.; Haiyan, J.; Lee, Z.; Kim, K. S. Remarkably enhanced catalytic activity by the synergistic effect of palladium single atoms and palladium-cobalt phosphide nanoparticles. Nano Energy 2020, 78, 105166.
Peng, C.; Hu, Y. L.; Liu, M. R.; Zheng, Y. X. Hollow raspberry-like PdAg alloy nanospheres: High electrocatalytic activity for ethanol oxidation in alkaline media. J. Power Sources 2005, 278, 69–75.
Li, C. Z.; Yuan, Q.; Ni, B.; He, T.; Zhang, S. M.; Long, Y.; Gu, L.; Wang, X. Dendritic defect-rich palladium-copper-cobalt nanoalloys as robust multifunctional non-platinum electrocatalysts for fuel cells. Nat. Commun. 2018, 9, 3702.
Chen, J. Y.; Li, Y.; Lu, N. L.; Tian, C. H.; Han, Z. D.; Zhang, L.; Fang, Y.; Qian, B.; Jiang, X. F.; Cui, R. J. Nanoporous PdCe bimetallic nanocubes with high catalytic activity towards ethanol electro-oxidation and the oxygen reduction reaction in alkaline media. J. Mater. Chem. A 2018, 6, 23560–23568.
Kim, D.; Resasco, J.; Yu, Y.; Asiri, A. M.; Yang, P. D. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948.
Liu, P.; Nerskov, J. K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818.
Loffreda, D.; Simon, D.; Sautet, P. Dependence of stretching frequency on surface coverage and adsorbate-adsorbate interactions: A density-functional theory approach of CO on Pd (111). Surf. Sci. 1999, 425, 68–80.
Chen, Y.; Fan, Z. X.; Luo, Z. M.; Liu, X. Z.; Lai, Z. C.; Li, B.; Zong, Y.; Gu, L.; Zhang, H. High-yield synthesis of crystal-phase-heterostructured 4H/fcc Au@Pd core-shell nanorods for electrocatalytic ethanol oxidation. Adv. Mater. 2017, 29, 1701331.
Yang, M.; Lao, X. Z.; Sun, J.; Ma, N.; Wang, S. Q.; Ye, W. N.; Guo, P. Z. Assembly of bimetallic PdAg nanosheets and their enhanced electrocatalytic activity toward ethanol oxidation. Langmuir 2020, 36, 11094–11101.
De, A.; Datta, J. Synergistic combination of Pd and Co catalyst nanoparticles over self-designed MnO2 structure: Green synthetic approach and unprecedented electrode kinetics in direct ethanol fuel cell. ACS Sustainable Chem. Eng. 2018, 6, 13706–13718.
Jiang, K. Z.; Wang, P. T.; Guo, S. J.; Zhang, X.; Shen, X.; Lu, G.; Su, D.; Huang, X. Q. Ordered PdCu-based nanoparticles as bifunctional oxygen-reduction and ethanol-oxidation electrocatalysts. Angew. Chem., Int. Ed. 2016, 55, 9030–9035.
Chen, L.; Lu, L. L.; Zhu, H. L.; Chen, Y. G.; Huang, Y.; Li, Y. D.; Wang, L. Y. Improved ethanol electrooxidation performance by shortening Pd-Ni active site distance in Pd-Ni-P nanocatalysts. Nat. Commun. 2017, 8, 14136.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Norskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Chen, X. M.; Wu, G. H.; Chen, J. M.; Chen, X.; Xie, Z. X.; Wang, X. R. Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695.
Antolini, E. Palladium in fuelcell catalysis. Energy Environ. Sci. 2009, 2, 915–931.
Xu, C. W.; Tian, Z. Q.; Shen, P. K.; Jiang, S. P. Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochimi. Acta 2008, 53, 2610–2618.
Abdel Hameed, R. M. Facile preparation of Pd-metal oxide/C electrocatalysts and their application in the electrocatalytic oxidation of ethanol. Appl. Surf. Sci. 2017, 411, 91–104.
Gao, Q.; Mou, T. Y.; Liu, S. K.; Johnson, G.; Han, X.; Yan, Z. H.; Ji, M. X.; He, Q.; Zhang, S.; Xin, H. L. et al. Monodisperse PdSn/SnO, core/shell nanoparticles with superior electrocatalytic ethanol oxidation performance. J. Mater. Chem. A 2020, 8, 20931–20938.
Zhang, B. W.; Jiang, Y. X.; Ren, J.; Qu, X. M.; Xu, G. L.; Sun, S. G. PtBi intermetallic and PtBi intermetallic with the Bi-rich surface supported on porous graphitic carbon towards HCOOH electro-oxidation. Electrochimi. Acta 2015, 162, 254–262.
Yuan, X. L.; Jiang, B.; Cao, M. H.; Zhang, C. Y.; Liu, X. Z.; Zhang, Q. H.; Lyu, F. L.; Gu, L.; Zhang, Q. Porous Pt nanoframes decorated with Bi(OH)3 as highly efficient and stable electrocatalyst for ethanol oxidation reaction. Nano Res. 2020, 13, 265–272.
Huang, W. J.; Ma, X. Y.; Wang, H.; Feng, R. F.; Zhou, J. G.; Duchesne, P. N.; Zhang, P.; Chen, F. J.; Han, N.; Zhao, F. P. et al. Promoting effect of Ni(OH)2 on palladium nanocrystals leads to greatly improved operation durability for electrocatalytic ethanol oxidation in alkaline solution. Adv. Mater. 2017, 29, 1703057.
Huang, X. Q.; Tang, S. H.; Mu, X. L.; Dai, Y.; Chen, G. X.; Zhou, Z. Y.; Ruan, F. X.; Yang, Z. L.; Zheng, N. F. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nanotechnol. 2011, 6, 28–32.
Ding, Y.; Fan, F. R.; Tian, Z. Q.; Wang, Z. L. Atomic structure of Au-Pd bimetallic alloyed nanoparticles. J. Am. Chem. Soc. 2010, 132, 12480–12486.
Chen, Z. L.; Zhang, J. F.; Zhang, Y.; Liu, Y. W.; Han, X. P.; Zhong, C.; Hu, W. B.; Deng, Y. D. NiO-induced synthesis of PdNi bimetallic hollow nanocrystals with enhanced electrocatalytic activities toward ethanol and formic acid oxidation. Nano Energy 2017, 42, 353–362.
Yuan, X. L.; Zhang, Y.; Cao, M. H.; Zhou, T.; Jiang, X. J.; Chen, J. X.; Lyu, F. L.; Xu, Y.; Luo, J.; Zhang, Q. et al. Bi(OH)3/PdBi composite nanochains as highly active and durable electrocatalysts for ethanol oxidation. Nano Lett. 2019, 19, 4752–4759.
Zalineeva, A.; Serov, A.; Padilla, M.; Martinez, U.; Artyushkova, K.; Baranton, S.; Coutanceau, C.; Atanassov, P. B. Self-supported PdxBi catalysts for the electrooxidation of glycerol in alkaline media. J. Am. Chem. Soc. 2014, 136, 3937–3945.
Casella, I. G.; Contursi, M. Characterization of bismuth adatom-modified palladium electrodes: The electrocatalytic oxidation of aliphatic aldehydes in alkaline solutions. Electrochim. Acta 2006, 52, 649–657.
Wang, X. C.; Xie, M.; Lyu, F. L.; Yiu, Y. M.; Wang, Z. Q.; Chen, J. T.; Chang, L. Y.; Xia, Y. J.; Zhong, Q. X.; Chu, M. Y. et al. Bismuth oxyhydroxide-Pt inverse interface for enhanced methanol electrooxidation performance. Nano Lett. 2020, 20, 7751–7759.
Su, Y. Z.; Xiao, K.; Li, N.; Liu, Z. Q.; Qiao, S. Z. Amorphous Ni(OH)2 @ three-dimensional Ni core-shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 13845–13853.
Lou, B. H.; Kang, H. Q.; Yuan, W. T.; Ma, L.; Huang, W. X.; Wang, Y.; Jiang, Z.; Du, Y. H.; Zou, S. H.; Fan, J. Highly selective acetylene semihydrogenation catalyst with an operation window exceeding 150 °C. ACS Catal. 2021, 11, 6073–6080.
Bistoni, G.; Rampino, S.; Scafuri, N.; Ciancaleoni, G.; Zuccaccia, D.; Belpassi, L.; Tarantelli, F. How n back-donation quantitatively controls the CO stretching response in classical and non-classical metal carbonyl complexes. Chem. Sci. 2016, 7, 1174–1184.
Zou, S. H.; Lou, B. H.; Yang, K. R.; Yuan, W. T.; Zhu, C. Z.; Zhu, Y. H.; Du, Y. H.; Lu, L. F.; Liu, J. J.; Huang, W. X. et al. Grafting nanometer metal/oxide interface towards enhanced low-temperature acetylene semi-hydrogenation. Nat. Commun. 2021, 12, 5770.
Liu, Z. L.; Ling, X. Y.; Su, X. D.; Lee, J. Y. Carbon-supported Pt and PtRu nanoparticles as catalysts for a direct methanol fuel cell. J. Phys. Chem. B 2004, 108, 8234–8240.
Du, W. X.; Wang, Q.; Saxner, D.; Deskins, N. A.; Su, D.; Krzanowski, J. E.; Frenkel, A. I.; Teng, X. W. Highly active iridium/iridium-tin/tin oxide heterogeneous nanoparticles as alternative electrocatalysts for the ethanol oxidation reaction. J. Am. Chem. Soc. 2011, 133, 15172–15183.
Hong, J. W.; Kim, Y.; Wi, D. H.; Lee, S.; Lee, S. U.; Lee, Y. W.; Choi, S. I.; Han, S. W. Ultrathin free-standing ternary-alloy nanosheets. Angew. Chem., Int. Ed. 2016, 55, 2753–2758.
Huang, L.; Zhang, X. P.; Wang, Q. Q.; Han, Y. J.; Fang, Y. X.; Dong, S. J. Shape-control of Pt-Ru nanocrystals: Tuning surface structure for enhanced electrocatalytic methanol oxidation. J. Am. Chem. Soc. 2018, 140, 1142–1147.
Chung, D. Y.; Lee, K. J.; Sung, Y. E. Methanol electro-oxidation on the Pt surface: Revisiting the cyclic voltammetry interpretation. J. Phys. Chem. C 2016, 120, 9028–9035.
Zhang, B. W.; Sheng, T.; Wang, Y. X.; Qu, X. M.; Zhang, J. M.; Zhang, Z. C.; Liao, H. G.; Zhu, F. C.; Dou, S. X.; Jiang, Y. X. et al. Platinum-cobalt bimetallic nanoparticles with Pt skin for electro-oxidation of ethanol. ACS Catal. 2016, 7, 892–895.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 51922073 and 21902109), the Natural Science Foundation of Jiangsu Province (Nos. BK20200960 and BK20180097), the Natural Science Foundation of Higher Education in Jiangsu Province (No. 20KJB150041), and the Natural Science Foundation of Nantong University for High-Level Talent (No. 03083033). We also acknowledge the financial support from the 111 Project, Collaborative Innovation Center of Suzhou Nano Science, Technology (NANO-CIC) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_4049_MOESM1_ESM.pdf
Synergistic combination of Pd nanosheets and porous Bi(OH)3 boosts activity and durability for ethanol oxidation reaction
Rights and permissions
About this article
Cite this article
Chu, M., Huang, J., Gong, J. et al. Synergistic combination of Pd nanosheets and porous Bi(OH)3 boosts activity and durability for ethanol oxidation reaction. Nano Res. 15, 3920–3926 (2022). https://doi.org/10.1007/s12274-021-4049-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-4049-9