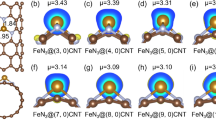
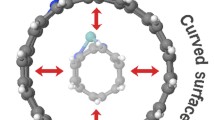
Abstract
Elucidating the reaction mechanism of hydrazine oxidation reaction (HzOR) over carbon-based catalysts is highly propitious for the rational design of novel electrocatalysts for HzOR. In present work, isolated first-row transition metal atoms have been coordinated with N atoms on the graphite layers of carbon nanotubes via a M-N4-C configuration (MSA/CNT, M=Fe, Co and Ni). The HzOR over the three single atom catalysts follows a predominant 4-electron reaction pathway to emit N2 and a negligible 1-electron pathway to emit trace of NH3, while their electrocatalytic activity for HzOR is dominated by the absorption energy of N2H4 on them. Furthermore, FeSA/CNT reverses the passivation effect on Fe/C and shows superior performance than CoSA/CNT and NiSA/CNT with a recorded high mass activity for HzOR due to the higher electronic charge of Fe over Co and Ni in the M-N4-C configuration and the lowest absorption energy of N2H4 on FeSA/CNT among the three MSA/CNT catalysts.

Similar content being viewed by others
References
Zhao, Y.; Setzler, B. P.; Wang, J. H.; Nash, J.; Wang, T.; Xu, B. J.; Yan, Y. S. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule 2019, 3, 2472–2484.
Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9.
Feng, G.; Kuang, Y.; Li, Y. J.; Sun, X. M. Three-dimensional porous superaerophobic nickel nanoflower electrodes for high-performance hydrazine oxidation. Nano Res. 2015, 8, 3365–3371.
Du, X. Q.; Liu, C.; Du, C.; Cai, P.; Cheng, G. Z.; Luo, W. Nitrogen-doped graphene hydrogel-supported NiPt-CeOx nanocomposites and their superior catalysis for hydrogen generation from hydrazine at room temperature. Nano Res. 2017, 10, 2856–2865.
Xia, B. Q.; Chen, K.; Luo, W.; Cheng, G. Z. NiRh nanoparticles supported on nitrogen-doped porous carbon as highly efficient catalysts for dehydrogenation of hydrazine in alkaline solution. Nano Res. 2015, 8, 3472–3479.
Burshtein, T. Y.; Farber, E. M.; Ojha, K.; Eisenberg, D. Revealing structure-activity links in hydrazine oxidation: Doping and nanostructure in carbide-carbon electrocatalysts. J. Mater. Chem. A 2019, 7, 23854–23861.
Zhang, T.; Asefa, T. Heteroatom-doped carbon materials for hydrazine oxidation. Adv. Mater. 2019, 31, 1804394.
Cazetta, A. L.; Zhang, T.; Silva, T. L.; Almeida, V. C.; Asefa, T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal. B Environ. 2018, 225, 30–39.
Jeong, J.; Choun, M.; Lee, J. Tree-bark-shaped n-doped porous carbon anode for hydrazine fuel cells. Angew. Chem., Int. Ed. 2017, 56, 13513–13516.
Meng, Y. Y.; Zou, X. X.; Huang, X. X.; Goswami, A.; Liu, Z. W.; Asefa, T. Polypyrrole-derived nitrogen and oxygen Co-doped mesoporous carbons as efficient metal-free electrocatalyst for hydrazine oxidation. Adv. Mater. 2014, 26, 6510–6516.
Qiao, B. T.; Wang, A. Q.; Yang, X. F.; Allard, L. F.; Jiang, Z.; Cui, Y. T.; Liu, J. Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641.
Cheng, Y.; Zhao, S. Y.; Li, H. B.; He, S.; Veder, J. P.; Johannessen, B.; Xiao, J. P.; Lu, S. F.; Pan, J.; Chisholm, M. F. et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2. Appl. Catal. B Environ. 2019, 243, 294–303.
Cheng, Y.; Zhao, S. Y.; Johannessen, B.; Veder, J. P.; Saunders, M.; Rowles, M. R.; Cheng, M.; Liu, C.; Chisholm, M. F.; De Marco, R. et al. Atomically dispersed transition metals on carbon nanotubes with ultrahigh loading for selective electrochemical carbon dioxide reduction. Adv. Mater. 2018, 30, 1706287.
Wang, Y. C.; Liu, Y.; Liu, W.; Wu, J.; Li, Q.; Feng, Q. G.; Chen, Z. Y.; Xiong, X.; Wang, D. S.; Lei, Y. P. Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy Environ. Sci. 2020, 13, 4609–4624.
Chen, Y. J.; Ji, S. F.; Sun, W. M.; Lei, Y. P.; Wang, Q. C.; Li, A.; Chen, W. X.; Zhou, G.; Zhang, Z. D.; Wang, Y. et al. Engineering the atomic interface with single platinum atoms for enhanced photocatalytic hydrogen production. Angew. Chem., Int. Ed. 2020, 59, 1295–1301.
Li, H.; Zhang, H. X.; Yan, X. L.; Xu, B. S.; Guo, J. J. Carbon-supported metal single atom catalysts. New Carbon Mater. 2018, 33, 1–11.
Cheng, Y.; He, S.; Lu, S. F.; Veder, J. P.; Johannessen, B.; Thomsen, L.; Saunders, M.; Becker, T.; De Marco, R.; Li, Q. F. et al. Iron single atoms on graphene as nonprecious metal catalysts for high-temperature polymer electrolyte membrane fuel cells. Adv. Sci. 2019, 6, 1802066.
Zhang, N. Q.; Ye, C. L.; Yan, H.; Li, L. C.; He, H.; Wang, D. S.; Li, Y. D. Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13, 3165–3182.
Ou, H. H.; Wang, D. S.; Li, Y. D. How to select effective electrocatalysts: Nano or single atom? Nano Sel., in press. DOI: https://doi.org/10.1002/nano.202000239.
Xiong, Y.; Dong, J. C.; Huang, Z. Q.; Xin, P. Y.; Chen, W. X.; Wang, Y.; Li, Z.; Jin, Z.; Xing, W.; Zhuang, Z. B. et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 2020, 15, 390–397.
Cui, L. T.; Cui, L. R.; Li, Z. J.; Zhang, J.; Wang, H. N.; Lu, S. F.; Xiang, Y. A copper single-atom catalyst towards efficient and durable oxygen reduction for fuel cells. J. Mater. Chem. A 2019, 7, 16690–16695.
Wang, J.; Liu, W.; Luo, G.; Li, Z. J.; Zhao, C.; Zhang, H. R.; Zhu, M. Z.; Xu, Q.; Wang, X. Q.; Zhao, C. M. et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379.
Qu, Y. T.; Li, Z. J.; Chen, W. X.; Lin, Y.; Yuan, T. W.; Yang, Z. K.; Zhao, C. M.; Wang, J.; Zhao, C.; Wang, X. et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 2018, 1, 781–786.
Cheng, Y.; He, S.; Veder, J. P.; De Marco, R.; Yang, S. Z.; Jiang, S. P. Atomically dispersed bimetallic feni catalysts as highly efficient bifunctional catalysts for reversible oxygen evolution and oxygen reduction reactions. ChemElectroChem 2019, 6, 3478–3487.
Sun, T. T.; Xu, L. B.; Wang, D. S.; Li, Y. D. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 2019, 12, 2067–2080.
Wang, T. Z.; Wang, Q.; Wang, Y. C.; Da, Y. L.; Zhou, W.; Shao, Y.; Li, D. B.; Zhan, S. H.; Yuan, J. Y.; Wang, H. Atomically dispersed semimetallic selenium on porous carbon membrane as an electrode for hydrazine fuel cells. Angew. Chem., Int. Ed. 2019, 58, 13466–13471.
Ojha, K.; Farber, E. M.; Burshtein, T. Y.; Eisenberg, D. A multi-doped electrocatalyst for efficient hydrazine oxidation. Angew. Chem., Int. Ed. 2018, 57, 17168–17172.
Wang, J.; Huang, Z. Q.; Liu, W.; Chang, C. R.; Tang, H. L.; Li, Z. J.; Chen, W. X.; Jia, C. J.; Yao, T.; Wei, S. Q. et al. Design of n-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284.
Li, Z. J.; Wang, D. H.; Wu, Y. E.; Li, Y. D. Recent advances in the precise control of isolated single-site catalysts by chemical methods. Natl. Sci. Rev. 2018, 5, 673–689.
Asazawa, K.; Sakamoto, T.; Yamaguchi, S.; Yamada, K.; Fujikawa, H.; Tanaka, H.; Oguro, K. Study of anode catalysts and fuel concentration on direct hydrazine alkaline anion-exchange membrane fuel cells. J. Electrochem. Soc. 2009, 156, B509–B512.
Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561.
Cui, L. T.; Li, Z. J.; Wang, H. N.; Cui, L. R.; Zhang, J.; Lu, S. F.; Xiang, Y. Atomically dispersed Cu-N-C as a promising support for low-Pt loading cathode catalysts of fuel cells. ACS Appl. Energy Mater. 2020, 3, 3807–3814.
Guo, M.; Wang, H. N.; Cui, L. T.; Zhang, J.; Xiang, Y.; Lu, S. F. Nickel promoted palladium nanoparticles for electrocatalysis of carbohydrazide oxidation reaction. Small 2019, 15, 1900929.
Xu, H. X.; Cheng, D. J.; Cao, D. P.; Zeng, X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Peng, H. Q.; Li, Q. H.; Hu, M. X.; Xiao, L.; Lu, J. T.; Zhuang, L. Alkaline polymer electrolyte fuel cells stably working at 80 °C. J. Power Sources 2018, 390, 165–167.
Xiong, Y.; Sun, W. M.; Han, Y. H.; Xin, P. Y.; Zheng, X. S.; Yan, W. S.; Dong, J. C.; Zhang, J.; Wang, D. S.; Li, Y. D. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res., in press, DOI: https://doi.org/10.1007/s12274-020-3244-4.
Fu, Y.; Yu, H. Y.; Jiang, C.; Zhang, T. H.; Zhan, R.; Li, X. W.; Li, J. F.; Tian, J. H.; Yang, R. Z. Nico alloy nanoparticles decorated on N-doped carbon nanofibers as highly active and durable oxygen electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705094.
Li, X. Y.; Rong, H. P.; Zhang, J. T.; Wang, D. S.; Li, Y. D. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855.
Zhang, J.; Zheng, C. Y.; Zhang, M. L.; Qiu, Y. J.; Xu, Q.; Cheong, W. C.; Chen, W. X.; Zheng, L. R.; Gu, L.; Hu, Z. P. et al. Controlling N-doping type in carbon to boost single-atom site Cu catalyzed transfer hydrogenation of quinoline. Nano Res. 2020, 13, 3082–3087.
Asazawa, K.; Yamada, K.; Tanaka, H.; Taniguchi, M.; Oguro, K. Electrochemical oxidation of hydrazine and its derivatives on the surface of metal electrodes in alkaline media. J. Power Sources 2009, 191, 362–365.
Jeon, T. Y.; Watanabe, M.; Miyatake, K. Carbon segregation-induced highly metallic Ni nanoparticles for electrocatalytic oxidation of hydrazine in alkaline media. ACS Appl. Mater. Interfaces 2014, 6, 18445–18449.
Fragal, V. H.; Fragal, E. H.; Zhang, T.; Huang, X. X.; Cellet, T. S. P.; Pereira, G. M.; Jitianu, A.; Rubira, A. F.; Silva, R.; Asefa, T. Deriving efficient porous heteroatom-doped carbon electrocatalysts for hydrazine oxidation from transition metal ions-coordinated casein. Adv. Funct. Mater. 2019, 29, 1808486.
Liu, C. B.; Zhang, H.; Tang, Y. H.; Luo, S. L. Controllable growth of graphene/Cu composite and its nanoarchitecture-dependent electrocatalytic activity to hydrazine oxidation. J. Mater. Chem. A 2014, 2, 4580–4587.
Sanabria-Chinchilla, J.; Asazawa, K.; Sakamoto, T.; Yamada, K.; Tanaka, H.; Strasser, P. Noble metal-free hydrazine fuel cell catalysts: EPOC effect in competing chemical and electrochemical reaction pathways. J. Am. Chem. Soc. 2011, 133, 5425–5431.
Wang, H.; Ma, Y. J.; Wang, R. F.; Key, J.; Linkov, V.; Ji, S. Liquid-liquid interface-mediated room-temperature synthesis of amorphous NiCo pompoms from ultrathin nanosheets with high catalytic activity for hydrazine oxidation. Chem. Commun. 2015, 51, 3570–3573.
Wen, X. P.; Dai, H. B.; Wu, L. S.; Wang, P. Electroless plating of Ni-B film as a binder-free highly efficient electrocatalyst for hydrazine oxidation. Appl. Surf. Sci. 2017, 409, 132–139.
Wang, Y. H.; Liu, X. Y.; Tan, T.; Ren, Z. L.; Lei, Z. Q.; Wang, W. A phosphatized pseudo-core-shell Fe@Cu-P/C electrocatalyst for efficient hydrazine oxidation reaction. J. Alloys Compd. 2019, 787, 104–111.
Wen, H.; Gan, L. Y.; Dai, H. B.; Wen, X. P.; Wu, L. S.; Wu, H.; Wang, P. In situ grown Ni phosphide nanowire array on Ni foam as a high-performance catalyst for hydrazine electrooxidation. Appl. Catal. B Environ. 2019, 241, 292–298.
Chen, Y. J.; Ji, S. F.; Wang, Y. G.; Dong, J. C.; Chen, W. X.; Li, Z.; Shen, R. A.; Zheng, L. R.; Zhuang, Z. B.; Wang, D. S. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2017, 56, 6937–6941.
Yin, W. X.; Li, Z. P.; Zhu, J. K.; Qin, H. Y. Effects of NaOH addition on performance of the direct hydrazine fuel cell. J. Power Sources 2008, 182, 520–523.
Yamada, K.; Yasuda, K.; Tanaka, H.; Miyazaki, Y.; Kobayashi, T. Effect of anode electrocatalyst for direct hydrazine fuel cell using proton exchange membrane. J. Power Sources 2003, 122, 132–137.
Kodera, T.; Honda, M.; Kita, H. Electrochemical behaviour of hydrazine on platinum in alkaline solution. Electrochim. Acta. 1985, 30, 669–675.
Yamada, K.; Asazawa, K.; Yasuda, K.; Ioroi, T.; Tanaka, H.; Miyazaki, Y.; Kobayashi, T. Investigation of PEM type direct hydrazine fuel cell. J. Power Sources 2003, 115, 236–242.
Agusta, M. K.; Diño, W. A.; David, M.; Nakanishi, H.; Kasai, H. Theoretical study of hydrazine adsorption on Pt(111): Anti or cis? Surf. Sci. 2011, 605, 1347–1353.
Feng, G.; An, L.; Li, B.; Zuo, Y. X.; Song, J.; Ning, F. H.; Jiang, N.; Cheng, X. P.; Zhang, Y. F.; Xia, D. G. Atomically ordered non-precious Co3Ta intermetallic nanoparticles as high-performance catalysts for hydrazine electrooxidation. Nat. Commun. 2019, 10, 4514.
Zhang, J.; Cao, X. Y.; Guo, M.; Wang, H. N.; Saunders, M.; Xiang, Y.; Jiang, S. P.; Lu, S. F. Unique Ni crystalline core/Ni phosphide amorphous shell heterostructured electrocatalyst for hydrazine oxidation reaction of fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 19048–19055.
Rosca, V.; Koper, M. T. M. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim. Acta. 2008, 53, 5199–5205.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300.
Chen, Y. J.; Gao, R.; Ji, S. F.; Li, H. J.; Tang, K.; Jiang, P.; Hu, H. B.; Zhang, Z. D.; Hao, H. G.; Qu, Q. Y. et al. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: Enhanced oxygen reduction performance. Angew. Chem., Int. Ed. 2021, 60, 3212–3221.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Single-atom materials: Small structures determine macroproperties. Small Struct. 2021, 2, 2170006.
Martinez, U.; Rojas-Carbonell, S.; Halevi, B.; Artyushkova, K.; Kiefer, B.; Sakamoto, T.; Asazawa, K.; Tanaka, H.; Datye, A.; Atanassov, P. Ni-La electrocatalysts for direct hydrazine alkaline anion-exchange membrane fuel cells. J. Electrochem. Soc. 2014, 161, H3106–H3112.
Lu, Z. Y.; Sun, M.; Xu, T. H.; Li, Y. J.; Xu, W. W.; Chang, Z.; Ding, Y.; Sun, X. M.; Jiang, L. Superaerophobic electrodes for direct hydrazine fuel cells. Adv. Mater. 2015, 27, 2361–2366.
Acknowledgements
Project supported by Beijing Natural Science Foundation (No. 2194076), the National Natural Science Foundation of China (Nos. 21908001, 21872003, and U19A2017), and the Fundamental Research Funds for the Central Universities. XAS measurements were performed on the soft X-ray and XAS beamlines of the Australian Synchrotron, Victoria, Australia. The electron microscopy carried out at ORNL was supported by the U.S. Department of Energy, Office of Basic Energy Sciences and through a user proposal supported by ORNL’s Center for Nanophase Materials Sciences. This research was also supported by the high performance computing (HPC) resources at Beihang University.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3397_MOESM1_ESM.pdf
Elucidating the electro-catalytic oxidation of hydrazine over carbon nanotube-based transition metal single atom catalysts
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, Y., Yang, C. et al. Elucidating the electro-catalytic oxidation of hydrazine over carbon nanotube-based transition metal single atom catalysts. Nano Res. 14, 4650–4657 (2021). https://doi.org/10.1007/s12274-021-3397-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3397-9