Abstract
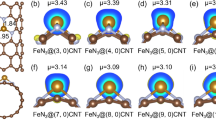
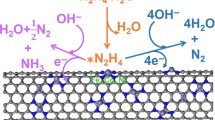
Developing efficient and stable catalysts for the electrocatalytic N2 reduction reaction (NRR) shows promise in nitrogen fixation. Here, we proposed active and stable single-atom catalysts (SACs) toward NRR, where transition metals are anchored on nitrogenated carbon nanotubes (NCNTs). Among the screened nine common transition metals (Ti, V, Cr, Mn, Fe, Mo, Ru, Rh, and Ag) on (4, 4) NCNTs, we found Mo-NCNT possesses the most excellent NRR catalytic activity and selectivity with a low overpotential of 0.29 V. Then, the NRR performance of Mo-NCNT was further engineered by controlling the nanotube diameter, where the lowest overpotential is 0.18 V at a diameter of 9.6 Å. In addition, we found a linear scaling relation between *NNH and *NH2 on the studied catalysts with the exception of (2, 2) and (3, 3) Mo-NCNTs, owing to their extremely unstable structures. We attribute the outstanding NRR performance of Mo-NCNT to the moderate adsorption of N2 due to the slightly low d-band center of Mo, and the charge donating and accepting capacity of NCNTs. This work has provided a deeper insight into designing high-efficiency and stable NRR SACs supported by NCNTs.

Similar content being viewed by others
References
Gruber, N.; Galloway, J. N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296.
Licht, S.; Cui, B. C.; Wang, B. H.; Li, F. F.; Lau, J.; Liu, S. Z. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 2014, 345, 637–640.
Rosca, V.; Duca, M.; De Groot, M. T.; Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 2009, 109, 2209–2244.
Chae, H. K.; Siberio-Pérez, D. Y.; Kim, J.; Go, Y. B.; Eddaoudi, M.; Matzger, A. J.; O’Keeffe, M.; Yaghi, O. M.; Materials Design and Discovery Group. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527.
Erisman, J. W.; Sutton, M. A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639.
Deng, J.; Iñiguez, J. A.; Liu, C. Electrocatalytic nitrogen reduction at low temperature. Joule 2018, 2, 846–856.
Xie, K.; Wang, F. T.; Wei, F. F.; Zhao, J.; Lin, S. Revealing the origin of nitrogen electroreduction activity of molybdenum disulfide supported iron atoms. J. Phys. Chem. C 2022, 126, 5180–5188.
Qi, J. M.; Zhou, S. L.; Xie, K.; Lin, S. Catalytic role of assembled Ce Lewis acid sites over ceria for electrocatalytic conversion of dinitrogen to ammonia. J. Energy Chem. 2021, 60, 249–258.
Lin, L. H.; Gao, L. Y.; Xie, K.; Jiang, R.; Lin, S. Ru-polyoxometalate as a single-atom electrocatalyst for N2 reduction to NH3 with high selectivity at applied voltage: A perspective from DFT studies. Phys. Chem. Chem. Phys. 2020, 22, 7234–7240.
Gao, L. Y.; Wang, F. T.; Yu, M. A.; Wei, F. F.; Qi, J. M.; Lin, S.; Xie, D. Q. A novel phosphotungstic acid-supported single metal atom catalyst with high activity and selectivity for the synthesis of NH3 from electrochemical N2 reduction: A DFT prediction. J. Mater. Chem. A 2019, 7, 19838–19845.
Qi, J. M.; Gao, L. Y.; Wei, F. F.; Wan, Q.; Lin, S. Design of a high-performance electrocatalyst for N2 conversion to NH3 by trapping single metal atoms on stepped CeO2. ACS Appl. Mater. Interfaces 2019, 11, 47525–47534.
Li, R. Z.; Wang, D. S. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res. 2022, 15, 6888–6923.
Zheng, X. B.; Li, B. B.; Wang, Q. S.; Wang, D. S.; Li, Y. D. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res., in press, DOI: 10.1007/s12274-022-4429-9.
Zhu, P.; Xiong, X.; Wang, D. S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013.
Gawande, M. B.; Fornasiero, P.; Zbořil, R. Carbon-based single-atom catalysts for advanced applications. ACS Catal. 2020, 10, 2231–2259.
Zhang, T. J.; Walsh, A. G.; Yu, J. H.; Zhang, P. Single-atom alloy catalysts: Structural analysis, electronic properties and catalytic activities. Chem. Soc. Rev. 2021, 50, 569–588.
Singh, B.; Sharma, V.; Gaikwad, R. P.; Fornasiero, P.; Zbořil, R.; Gawande, M. B. Single-atom catalysts: A sustainable pathway for the advanced catalytic applications. Small 2021, 17, 2006473.
Yuan, F.; Sun, R. S.; Fu, L.; Zhao, G. Z. Defect engineering for high-selection-performance of N2 activation over CeO2(111) surface. Chin. Chem. Lett. 2022, 33, 2188–2194.
Shang, Y. N.; Duan, X. G.; Wang, S. B.; Yue, Q. Y.; Gao, B. Y.; Xu, X. Carbon-based single atom catalyst: Synthesis, characterization, DFT calculations. Chin. Chem. Lett. 2022, 33, 663–673.
Bian, W. Y.; Shen, X. L.; Tan, H.; Fan, X.; Liu, Y. X.; Lin, H. P.; Li, Y. Y. The triggering of catalysis via structural engineering at atomic level: Direct propane dehydrogenation on Fe−N3P−C. Chin. Chem. Lett., in press, DOI: 10.1016/j.cclet.2022.03.012.
Yan, Y. B.; Miao, J. W.; Yang, Z. H.; Xiao, F. X.; Yang, H. B.; Liu, B.; Yang, Y. H. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346.
Gong, K. P.; Du, F.; Xia, Z. H.; Durstock, M.; Dai, L. M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764.
Yu, D. S.; Zhang, Q.; Dai, L. M. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129.
Li, Y. H.; Hung, T. H.; Chen, C. W. A first-principles study of nitrogen- and boron-assisted platinum adsorption on carbon nanotubes. Carbon 2009, 47, 850–855.
García-García, F. R.; Álvarez-Rodríguez, J.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. The use of carbon nanotubes with and without nitrogen doping as support for ruthenium catalysts in the ammonia decomposition reaction. Carbon 2010, 48, 267–276.
Lee, D. H.; Lee, W. J.; Lee, W. J.; Kim, S. O.; Kim, Y. H. Theory, synthesis, and oxygen reduction catalysis of fe-porphyrin-like carbon nanotube. Phys. Rev. Lett. 2011, 106, 175502.
Aoyama, S.; Kaiwa, J.; Chantngarm, P.; Tanibayashi, S.; Saito, H.; Hasegawa, M.; Nishidate, K. Oxygen reduction reaction of FeN4 center embedded in graphene and carbon nanotube: Density functional calculations. AIP Adv. 2018, 8, 115113.
Omidvar, A. Dissociation of O2 molecule on Fe/Nx clusters embedded in C60 fullerene, carbon nanotube and graphene. Synth. Met. 2017, 234, 38–46.
Yang, L.; Wu, Y. X.; Wu, F.; Zhao, Y.; Zhuo, Z. W.; Wang, Z. W.; Li, X. Y.; Luo, Y.; Jiang, J. Emerging linear activity trend in the oxygen evolution reaction with dual-active-sites mechanism. J. Mater. Chem. A 2020, 8, 20946–20952.
Niu, J. T.; Qi, W. J.; Li, C.; Mao, M.; Zhang, Z. G.; Chen, Y.; Li, W. L.; Ge, S. S. Mechanisms of oxygen reduction reaction on B doped FeN4-G and FeN4-CNT catalysts for proton-exchange membrane fuel cells. Inter. J. Energy Res. 2021, 45, 8524–8535.
Sun, H.; Wang, M. F.; Du, X. C.; Jiao, Y.; Liu, S. S.; Qian, T.; Yan, Y. C.; Liu, C.; Liao, M.; Zhang, Q. H. et al. Modulating the d-band center of boron doped single-atom sites to boost the oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 20952–20957.
Ma, Y. Y.; Yang, T.; Zou, H. Y.; Zang, W. J.; Kou, Z. K.; Mao, L.; Feng, Y. P.; Shen, L.; Pennycook, S. J.; Duan, L. L. et al. Synergizing Mo single atoms and Mo2C nanoparticles on CNTs synchronizes selectivity and activity of electrocatalytic N2 reduction to ammonia. Adv. Mater. 2020, 32, 2002177.
Wang, Y.; Cui, X. Q.; Zhao, J. X.; Jia, G. R.; Gu, L.; Zhang, Q. H.; Meng, L. K.; Shi, Z.; Zheng, L. R.; Wang, C. Y. et al. Rational design of Fe−N/C hybrid for enhanced nitrogen reduction electrocatalysis under ambient conditions in aqueous solution. ACS Catal. 2019, 9, 336–344.
Ou, P. F.; Zhou, X.; Meng, F. C.; Chen, C.; Chen, Y. Q.; Song, J. Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation. Nanoscale 2019, 11, 13600–13611.
Lv, X. S.; Wei, W.; Huang, B. B.; Dai, Y.; Frauenheim, T. High-throughput screening of synergistic transition metal dual-atom catalysts for efficient nitrogen fixation. Nano Lett. 2021, 21, 1871–1878.
Li, X.; Zhou, Q. Y.; Wang, S. F.; Li, Y.; Liu, Y. F.; Gao, Q.; Wu, Q. Tuning the coordination environment to effect the electrocatalytic behavior of a single-atom catalyst toward the nitrogen reduction reaction. J. Phys. Chem. C 2021, 125, 11963–11974.
Feng, Z.; Tang, Y. N.; Chen, W. G.; Li, Y.; Li, R. Y.; Ma, Y. Q.; Dai, X. Q. Graphdiyne coordinated transition metals as single-atom catalysts for nitrogen fixation. Phys. Chem. Chem. Phys. 2020, 22, 9216–9224.
Chen, Z. W.; Yan, J. M.; Jiang, Q. Single or double: Which is the altar of atomic catalysts for nitrogen reduction reaction? Small Methods 2019, 3, 1800291.
Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T. A.; Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106.
Mathew, K.; Kolluru, V. S. C.; Mula, S.; Steinmann, S. N.; Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 2019, 151, 234101.
Dronskowski, R.; Bloechl, P. E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624.
Bultinck, P. The hirshfeld-I method: Atoms in molecules and chemical bonding perspective. Acta Cryst. 2011, A67, C85–C86.
Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J. K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245.
Zafari, M.; Kumar, D.; Umer, M.; Kim, K. S. Machine learning-based high throughput screening for nitrogen fixation on boron-doped single atom catalysts. J. Mater. Chem. A 2020, 8, 5209–5216.
Zheng, S. S.; Li, S. M.; Mei, Z. W.; Hu, Z. X.; Chu, M. H.; Liu, J. H.; Chen, X.; Pan, F. Electrochemical nitrogen reduction reaction performance of single-boron catalysts tuned by MXene substrates. J. Phys. Chem. Lett. 2019, 10, 6984–6989.
Zhao, W. H.; Zhang, L. F.; Luo, Q. Q.; Hu, Z. P.; Zhang, W. H.; Smith, S.; Yang, J. L. Single Mo1(Cr1) atom on nitrogen-doped graphene enables highly selective electroreduction of nitrogen into ammonia. ACS Catal. 2019, 9, 3419–3425.
Ling, C. Y.; Ouyang, Y. X.; Li, Q.; Bai, X. W.; Mao, X.; Du, A. J.; Wang, J. L. A general two-step strategy-based high-throughput screening of single atom catalysts for nitrogen fixation. Small Methods 2019, 3, 1800376.
Li, M. Y.; Cui, Y.; Zhang, X. L.; Luo, Y.; Dai, Y. X.; Huang, Y. C. Screening a suitable Mo form supported on graphdiyne for effectively electrocatalytic N2 reduction reaction: From atomic catalyst to cluster catalyst. J. Phys. Chem. Lett. 2020, 11, 8128–8137.
Guo, X. Y.; Gu, J. X.; Lin, S. R.; Zhang, S. L.; Chen, Z. F.; Huang, S. P. Tackling the activity and selectivity challenges of electrocatalysts toward the nitrogen reduction reaction via atomically dispersed biatom catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721.
Xu, Z. W.; Song, R. F.; Wang, M. Y.; Zhang, X. Z.; Liu, G. W.; Qiao, G. J. Single atom-doped arsenene as electrocatalyst for reducing nitrogen to ammonia: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 26223–26230.
Li, X. H.; Ren, X.; Liu, X. J.; Zhao, J. X.; Sun, X.; Zhang, Y.; Kuang, X.; Yan, T.; Wei, Q.; Wu, D. A MoS2 nanosheet-reduced graphene oxide hybrid: An efficient electrocatalyst for electrocatalytic N2 reduction to NH3 under ambient conditions. J. Mater. Chem. A 2019, 7, 2524–2528.
Qiu, W. B.; Xie, X. Y.; Qiu, J. D.; Fang, W. H.; Liang, R. B.; Ren, X.; Ji, X. Q.; Cui, G. W.; Asiri, A. M.; Cui, G. L. et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun. 2018, 9, 3485.
Montoya, J. H.; Tsai, C.; Vojvodic, A.; Nørskov, J. K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 2015, 8, 2180–2186.
Lin, I. H.; Lu, Y. H.; Chen, H. T. Nitrogen-doped carbon nanotube as a potential metal-free catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2016, 18, 12093–12100.
Hu, X. B.; Wu, Y. T.; Zhang, Z. B. CO oxidation on metal-free nitrogen-doped carbon nanotubes and the related structure-reactivity relationships. J. Mater. Chem. 2012, 22, 15198–15205.
Spatzal, T.; Aksoyoglu, M.; Zhang, L. M.; Andrade, S. L. A.; Schleicher, E.; Weber, S.; Rees, D. C.; Einsle, O. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 2011, 334, 940.
Wang, A. Q.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81.
Zhao, J. X.; Chen, Z. F. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487.
Han, L. L.; Liu, X. J.; Chen, J. P.; Lin, R. Q.; Liu, H. X.; Lü, F.; Bak, S.; Liang, Z. X.; Zhao, S. Z.; Stavitski, E. et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation. Angew. Chem., Int. Ed. 2019, 131, 2343–2347.
Chen, L.; Wang, Q.; Gong, H. R.; Xue, M. S. Single Mo atom supported on defective BC2N monolayers as promising electrochemical catalysts for nitrogen reduction reaction. Appl. Surf. Sci. 2021, 546, 149131.
Zheng, M.; Xu, H. B.; Li, Y.; Ding, K. N.; Zhang, Y. F.; Sun, C. G.; Chen, W. K.; Lin, W. Electrocatalytic nitrogen reduction by transition metal single-atom catalysts on polymeric carbon nitride. J. Phys. Chem. C 2021, 125, 13880–13888.
Evans, M. G.; Warhurst, E. The activation energy of diene association reactions. Trans. Faraday Soc. 1938, 34, 614–624.
Bronsted, J. N. Acid and basic catalysis. Chem. Rev. 1928, 5, 231–338.
Hammer, B.; Nørskov, J. K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220.
Ruban, A.; Hammer, B.; Stoltze, P.; Skriver, H. L.; Nørskov, J. K. Surface electronic structure and reactivity of transition and noble metals. J. Mol. Catal. A:Chem. 1997, 115, 421–429.
Hammer, B.; Nørskov, J. K. Theoretical surface science and catalysis-calculations and concepts. Adv. Catal. 2000, 45, 71–129.
Vojvodic, A.; Nørskov, J. K.; Abild-Pedersen, F. Electronic structure effects in transition metal surface chemistry. Top. Catal. 2014, 57, 25–32.
Nørskov, J. K.; Bligaard, T.; Rossmeisl, J.; Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 22103059). Y. S. acknowledges the “Young Talent Support Plan” of Xi’an Jiaotong University and the Open Funds of State Key Laboratory of Physical Chemistry of Solid Surfaces (Xiamen University No. 202018). Supercomputing facilities were provided by Hefei Advanced Computing Center.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4803_MOESM1_ESM.pdf
Computational study of transition metal single-atom catalysts supported on nitrogenated carbon nanotubes for electrocatalytic nitrogen reduction
Rights and permissions
About this article
Cite this article
Qin, Y., Li, Y., Zhao, W. et al. Computational study of transition metal single-atom catalysts supported on nitrogenated carbon nanotubes for electrocatalytic nitrogen reduction. Nano Res. 16, 325–333 (2023). https://doi.org/10.1007/s12274-022-4803-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4803-7