Abstract
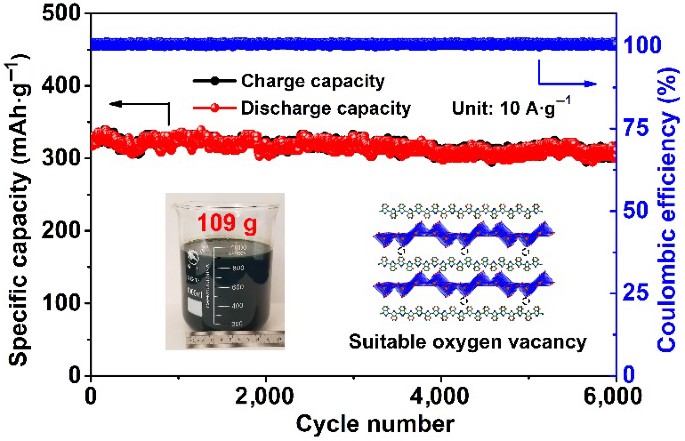
Oxygen vacancy (Vö) is important in the modification of electrode for rechargeable batteries. However, due to the scarcity of suitable preparation strategy with controllable Vö incorporation, the impact of Vö concentration on the electrochemical performances remains unclear. Thus, in this work, Vö-V2O5-PEDOT (VöVP) with tunable Vö concentration is achieved via a spontaneous polymerization strategy, with the capability of mass-production. The introduction of poly(2,3-dihydrothieno-1,4-dioxin) (PEDOT) not only leads to the formation of Vö in V2O5, but it also results in a larger interlayer spacing. The as-prepared Vö-V2O5-PEDOT-20.3% with Vö concentration of 20.3% (denoted as VöVP-20) is able to exhibit high capacity of 449 mAh·g−1 at current density of 0.2 A·g−1, with excellent cyclic performance of 94.3% after 6,000 cycles. It is shown in the theoretical calculations that excessive Vö in V2O5 will lead to an increase in the band gap, which inhibits the electrochemical kinetics and charge conductivity. This is further demonstrated in the experimental results as the electrochemical performance starts to decline when Vö concentration increases beyond 20.3%. Thus, based on this work, scalable fabrication of high-performance electrode with tunable Vö concentration can be achieved with the proposed strategy.
Similar content being viewed by others
References
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Goodenough, J. B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603.
Balogun, M. S.; Qiu, W. T.; Luo, Y.; Meng, H.; Mai, W. J.; Onasanya, A.; Olaniyi, T. K.; Tong, Y. X. A review of the development of full cell lithium-ion batteries: The impact of nanostructured anode materials. Nano Res. 2016, 9, 2823–2851.
Chao, D. L.; Zhou, W. H.; Xie, F. X.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S. Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098.
Xu, W. W.; Wang, Y. Recent progress on zinc-ion rechargeable batteries. Nano-Micro Lett. 2019, 11, 90.
Fang, G. Z; Zhou, J.; Pan, A. Q; Liang, S. Q. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501.
Liu, P. G.; Gao, Y.; Tan, Y. Y.; Liu, W. F.; Huang, Y. P.; Yan, J.; Liu, K. Y. Rational design of nitrogen doped hierarchical porous carbon for optimized zinc-ion hybrid supercapacitors. Nano Res. 2019, 12, 2835–2841.
Zhang, Y. M.; Li, H. N.; Huang, S. Z.; Fan, S.; Sun, L. N.; Tian, B. B.; Chen, F. M.; Wang, Y.; Shi, Y. M.; Yang, H. Y. Rechargeable aqueous zinc-ion batteries in MgSO4/ZnSO4 hybrid electrolytes. Nano-Micro Lett. 2020, 12, 60.
Song, M.; Tan, H.; Chao, D. L.; Fan, H. J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564.
He, P.; Zhang, G. B.; Liao, X. B.; Yan, M. Y.; Xu, X.; An, Q. Y.; Liu, J.; Mai, L. Q. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 2018, 8, 1702463.
Wang, X. Y.; Ma, L. W.; Zhang, P. C.; Wang, H. Y.; Li, S.; Ji, S. J.; Wen, Z. S.; Sun, J. C. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl. Surf. Sci. 2020, 502, 144207.
Jia, D. D.; Zheng, K.; Song, M.; Tan, H.; Zhang, A. T.; Wang, L. H.; Yue, L. J.; Li, D.; Li, C. W.; Liu, J. Q. V2·0.2H2O nanocuboids anchored onto graphene sheets as the cathode material for ultrahigh capacity aqueous zinc ion batteries. Nano Res. 2020, 13, 215–224
Sun, W.; Wang, F.; Hou, S.; Yang, C. Y.; Fan, X. L.; Ma, Z. H.; Gao, T.; Han, F. D.; Hu, R. Z.; Zhu, M. et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778.
Ren, H.; Zhao, J.; Yang, L.; Liang, Q. H.; Madhavi, S.; Yan, Q. Y. Inverse opal manganese dioxide constructed by few-layered ultrathin nanosheets as high-performance cathodes for aqueous zinc-ion batteries. Nano Res. 2019, 12, 1347–1353.
Zhang, L. Y.; Chen, L.; Zhou, X. F.; Liu, Z. P. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015, 5, 1400930.
Liu, Z.; Pulletikurthi, G.; Endres, F. A Prussian blue/zinc secondary battery with a bio-ionic liquid-water mixture as electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164.
Zhao, Q.; Huang, W. W.; Luo, Z. Q.; Liu, L. J.; Lu, Y.; Li, Y. X.; Li, L.; Hu, J. Y.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761.
Kundu, D.; Oberholzer, P.; Glaros, C.; Bouzid, A.; Tervoort, E.; Pasquarello, A.; Niederberger, M. Organic cathode for aqueous Zn-ion batteries: Taming a unique phase evolution toward stable electrochemical cycling. Chem. Mater. 2018, 30, 3874–3881.
Li, G. L.; Yang, Z.; Jiang, Y.; Jin, C. H.; Huang, W.; Ding, X. L.; Huang, Y. H. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217.
Li, G. L.; Yang, Z.; Jiang, Y.; Zhang, W. X.; Huang, Y. H. Hybrid aqueous battery based on Na3V2(PO4)3/C cathode and zinc anode for potential large-scale energy storage. J. Power Sources 2016, 308, 52–57.
Chae, M. S.; Heo, J. W.; Lim, S. C.; Hong, S. T. Electrochemical zinc-ion intercalation properties and crystal structures of ZnMo6S8 and Zn2Mo6S8 Chevrel phases in aqueous electrolytes. Inorg. Chem. 2016, 55, 3294–3301.
Cheng, Y. W.; Luo, L. L.; Zhong, L.; Chen, J. Z.; Li, B.; Wang, W.; Mao, S. X.; Wang, C. M.; Sprenkle, V. L.; Li, G. S. et al. Highly reversible zinc-ion intercalation into Chevrel phase Mo6S8 nanocubes and applications for advanced zinc-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 13673–13677.
Wan, F.; Niu, Z. Q. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2019, 58, 16358–16367.
Wang, X. Y.; Ma, L. W.; Sun, J. C. Vanadium pentoxide nanosheets in-situ spaced with acetylene black as cathodes for high-performance zinc-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 41297–41303.
Tang, B. Y.; Shan, L. T.; Liang, S. Q.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304.
Pang, Q.; He, W.; Zhao, H. N.; Yu, X. Y.; Wei, Y. J.; Tian, Y.; Xing, M. M.; Fu, Y.; Luo, X. X. Hierarchical aluminum vanadate microspheres with structural water: High-performance cathode materials for aqueous rechargeable zinc batteries. ChemPlusChem 2020, 85, 1–8.
Kundu, D.; Adams, B. D.; Duffort, V.; Vajargah, S. H.; Nazar, L. F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119.
Yan, M. Y.; He, P.; Chen, Y.; Wang, S. Y.; Wei, Q. L.; Zhao, K. N.; Xu, X.; An, Q. Y.; Shuang, Y.; Shao, Y. Y. et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725.
Pang, Q.; Sun, C. L.; Yu, Y. H.; Zhao, K. N.; Zhang, Z. Y.; Voyles, P. M.; Chen, G.; Wei, Y. J.; Wang, X. D. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 2018, 8, 1800144.
Liao, M.; Wang, J. W.; Ye, L.; Sun, H.; Wen, Y. Z.; Wang, C.; Sun, X. M.; Wang, B. J.; Peng, H. S. A deep-cycle aqueous zinc-ion battery containing an oxygen-deficient vanadium oxide cathode. Angew. Chem., Int. Ed. 2020, 59, 2273–2278.
Bin, D.; Huo, W. C.; Yuan, Y. B.; Huang, J. H.; Liu, Y.; Zhang, Y. X.; Dong, F.; Wang, Y. G.; Xia, Y. Y. Organic-inorganic-induced polymer intercalation into layered composites for aqueous zinc-ion battery. Chem 2020, 6, 968–984.
Liu, F.; Chen, Z. X.; Fang, G. Z.; Wang, Z. Q.; Cai, Y. S.; Tang, B. Y.; Zhou, J.; Liang, S. Q. V2O5 nanospheres with mixed vanadium valences as high electrochemically active aqueous zinc-ion battery cathode. Nano-Micro Lett. 2019, 11, 25.
Zhao, J.; Ren, H.; Liang, Q. H.; Yuan, D.; Xi, S. B.; Wu, C.; Manalastas, W. Jr.; Ma, J. M.; Fang, W.; Zheng, Y. et al. Highperformance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy 2019, 62, 94–102.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the Projector-Augmented-Wave method. Phys. Rev. B 1999, 59, 1758–1775.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561.
Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Guo, C. X.; Sun, K.; Ouyang, J. Y.; Lu, X. M. Layered V2O5/PEDOT nanowires and ultrathin nanobelts fabricated with a silk reelinglike process. Chem. Mater. 2015, 27, 5813–5819.
Bi, W. C.; Wu, Y. J.; Liu, C. F.; Wang, J. C.; Du, Y. C.; Gao, G. H.; Wu, G. M.; Cao, G. Z. Gradient oxygen vacancies in V2O5/PEDOT nanocables for high-performance supercapacitors. ACS Appl. Energy Mater. 2019, 2, 668–677.
He, P.; Quan, Y. L.; Xu, X.; Yan, M. Y.; Yang, W.; An, Q. Y.; He, L.; Mai, L. Q. High-performance aqueous zinc-ion battery based on layered H2V3O8 nanowire cathode. Small 2017, 13, 1702551.
Alfaruqi, M. H.; Mathew, V.; Song, J. J.; Kim, S.; Islam, S.; Pham, D. T.; Jo, J.; Kim, S.; Baboo, J. P.; Xiu, Z. L. et al. Electrochemical zinc intercalation in lithium vanadium oxide: A high-capacity zinc-ion battery cathode. Chem. Mater. 2017, 29, 1684–1694.
Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M. H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y. K.; Kim, J. K2V6O16·2.7H2O nanorod cathode: An advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 15530–15539.
Guo, X.; Fang, G. Z.; Zhang, W. Y.; Zhou, J.; Shan, L. T.; Wang, L. B.; Wang, C.; Lin, T. Q.; Tang, Y.; Liang, S. Q. Mechanistic insights of Zn2+ storage in sodium vanadates. Adv. Energy Mater. 2018, 8, 1801819.
Soundharrajan, V.; Sambandam, B.; Kim, S.; Alfaruqi, M. H.; Putro, D. Y.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y. K.; Kim, J. Na2V6O16·3H2O barnesite nanorod: An open door to display a stable and high energy for aqueous rechargeable Zn-ion batteries as cathodes. Nano Lett. 2018, 18, 2402–2410.
Chao, D. L.; Zhou, W. H.; Ye, C.; Zhang, Q. H.; Chen, Y. G.; Gu, L.; Davey, K.; Qiao, S. Z. An electrolytic Zn-MnO2 battery for highvoltage and scalable energy storage. Angew. Chem., Int. Ed. 2019, 58, 7823–7828.
Chao, D. L.; Ye, C.; Xie, F. X.; Zhou, W. H.; Zhang, Q. H.; Gu, Q. F.; Davey, K.; Gu, L.; Qiao, S. Z. Atomic engineering catalyzed MnO2 electrolysis kinetics for a hybrid aqueous battery with high power and energy density. Adv. Mater. 2020, 32, 2001894.
Zhang, N.; Jia, M.; Dong, Y.; Wang, Y. Y.; Xu, J. Z.; Liu, Y. C.; Jiao, L. F.; Cheng, F. Y. Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv. Funct. Mater. 2019, 29, 1807331.
Li, Y. K.; Huang, Z. M.; Kalambate, P. K.; Zhong, Y.; Huang, Z. M.; Xie, M. L.; Shen, Y.; Huang, Y. H. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759.
Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y. Y.; Qiu, M. D.; Xu, J. Z.; Liu, Y. C.; Jiao, L. F.; Cheng, F. Y. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372.
Li, R.; Zhang, H. M.; Zheng, Q.; Li, X. F. Porous V2O5 yolk-shell microspheres for zinc ion battery cathodes: Activation responsible for enhanced capacity and rate performance. J. Mater. Chem. A 2020, 8, 5186–5193.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21905037), China Postdoctoral Science Foundation (No. 2020M670719) and the Fundamental Research Funds for the Central Universities (No. 3132019328).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Du, Y., Wang, X. & Sun, J. Tunable oxygen vacancy concentration in vanadium oxide as mass-produced cathode for aqueous zinc-ion batteries. Nano Res. 14, 754–761 (2021). https://doi.org/10.1007/s12274-020-3109-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3109-x




