Abstract
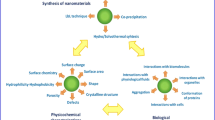
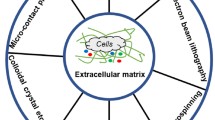
There is a compelling need for delicate nanomaterial design with various intricate functions and applications. Electrohydrodynamics applies electrostatic force to overcome the surface tension of a liquid jet, shrinking the jet through intrinsic jetting instability into submicron fibers or spheres, with versatility from a huge selection of materials, feasibility of extracellular matrix structure mimicry and multi-compartmentalization for tissue engineering and drug delivery. The process typically involves the collection and drying of fibers at a solid substrate, but the introduction of a liquid phase collection by replacing the solid collector with a coagulation bath can introduce a variety of new opportunities for both chemical and physical functionalizations in one single step. The so-called wet electrohydrodynamics is an emerging technique that enables a facile, homogeneous functionalization of the intrinsic large surface area of the submicron fibers/spheres. With a thorough literature sweep, we herein highlight the three main engineering features integrated through the single step wet electrospinning process in terms of creating functional biomaterials: (i) The fabrication of 3D macrostructures, (ii) in situ chemical functionalization, and (iii) tunable nano-topography. Through an emerging technique, wet electrohydrodynamics has demonstrated a great potential in interdisciplinary research for the development of functional 3D interfaces and materials with pertinent applications in all fields where secondary structured, functional surface is desired. Among these, engineered biomaterials bridging materials science with biology have already shown particular potential.

Similar content being viewed by others
References
Luo, C. J.; Stoyanov, S. D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev.2012, 41, 4708–4735.
Huang, Y.; Song, J. N.; Yang, C.; Long, Y. Z.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today2019, 28, 98–113.
Dvir, T.; Timko, B. P.; Kohane, D. S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol.2011, 6, 13–22.
Brown, T. D.; Dalton, P. D.; Hutmacher, D. W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci.2016, 56, 116–166.
Agarwal, S.; Greiner, A.; Wendorff, J. H. Functional materials by electrospinning of polymers. Prog. Polym. Sci.2013, 38, 963–991.
Smit, E.; Biittner, U.; Sanderson, R. D. Continuous yarns from electrospun fibers. Polymer2005, 46, 2419–2423.
Sun, B.; Long, Y. Z.; Zhang, H. D.; Li, M. M.; Duvail, J. L.; Jiang, X. Y.; Yin, H. L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci.2014, 39, 862–890.
Evrova, O.; Hosseini, V.; Milleret, V.; Palazzolo, G.; Zenobi-Wong, M.; Sulser, T.; Buschmann, J.; Eberli, D. Hybrid randomly electrospun poly(lactic-co-glycolic acid): Poly(ethylene oxide) (PLGA: PEO) fibrous scaffolds enhancing myoblast differentiation and alignment. ACSAppl. Mater. Interfaces2016, 8, 31574–31586.
Hwang, P. T. J.; Murdock, K.; Alexander, G. C.; Salaam, A. D.; Ng, J. I.; Lim, D. J.; Dean, D.; Jun, H. W. Poly(s-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. Part A2016, 104, 1017–1029.
Taskin, M. B.; Xu, R. D.; Gregersen, H.; Nygaard, J. V.; Besenbacher, F.; Chen, M. L. Three-dimensional polydopamine functionalized coiled microfibrous scaffolds enhance human mesenchymal stem cells colonization and mild myofibroblastic differentiation. ACS Appl. Mater. Interfaces2016, 8, 15864–15873.
Yokoyama, Y.; Hattori, S.; Yoshikawa, C.; Yasuda, Y.; Koyama, H.; Takato, T.; Kobayashi, H. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater. Lett.2009, 63, 754–756.
Gang, E. H.; Ki, C.S.; Kim, J. W.; Lee, J.; Cha, B. G.; Lee, K. H.; Park, Y. H. Highly porous three-dimensional poly(lactide-co-glycolide) (PLGA) microfibrous scaffold prepared by electrospinning method: A. comparison study with other PLGA type scaffolds on its biological evaluation. Fibers Polym.2012, 13, 685–691.
Hong, S.; Kim, G. H. Fabrication of size-controlled three-dimensional structures consisting of electrohydrodynamically produced poly-caprolactone micro/nanofibers. Appl. Phys. A2011, 103, 1009–1014.
Cai, X. J.; ten Hoopen, S.; Zhang, W. B.; Yi, C.; Yang, W. X.; Yang, F.; Jansen, J. A.; Walboomers, X. R.; Yelick, P. C. Influence of highly porous electrospun PLGA/PCL/nHA fibrous scaffolds on the differentiation of tooth bud cells in vitro. J. Biomed. Mater. Res. Part A2017, 105, 2597–2607.
Dong, X. R.; Zhang, J. Y.; Pang, L.; Chen, J. T.; Qi, M.; You, S. J.; Ren, N. Q. An anisotropic three-dimensional electrospun micro/ nanofibrous hybrid PLA/PCL scaffold. RSC Adv.2019, 9, 9838–9844.
Naseri-Nosar, M.; Salehi, M.; Hojjati-Emami, S. Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int. J. Biol. Macromol.2017,103, 701–708.
Kashiwabuchi, R.; Parikh, K. S.; Omiadze, R.; Zhang, S. M.; Luo, L. X.; Patel, H. V.; Xu, Q. G.; Ensign, L. M.; Mao, H. Q.; Hanes, J. et al. Hojjati-Emami, Development of absorbable, antibiotic-eluting sutures for ophthalmic surgery. Trans. Vis. Sci. Technol.2017, 6, DOI: 10.1167/tvst.6.1.1.
Luo, J. J.; Zhang, H. T.; Zhu, J.; Cui, X. K.; Gao, J. J.; Wang, X.; Xiong, J. Hojjati-Emami, 3-D mineralized silk fibroin/polycaprolactone composite scaffold modified with polyglutamate conjugated with BMP-2 peptide for bone tissue engineering. Colloid. Surf. B. Biointerf2018, 163, 369–378.
Wang, L.; Wu, Y. B.; Guo, B. L.; Ma, P. X. Hojjati-Emami, Nanofiber yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano2015, 9, 9167–9179.
Coburn, J. M.; Gibson, M.; Monagle, S.; Patterson, Z.; Elisseeff, J. H. ACS NanoBioinspired nanofibers support chondrogenesis for articular cartilage repair. Prac. Natl. Acad. Sci. USA2012, 109, 10012–10017.
Wang, H.; Kong, L. Y.; Ziegler, G. R. Aligned wet-electrospun starch fiber mats. Food Hydrocol.2019, 90, 113–117.
Shepherd, L. M.; Frey, M. W.; Joo, Y. L. Immersion electrospinning as a. new method to direct fiber deposition. Macromol. Mater. Eng.2017, 302, 1700148.
Shin, T. J.; Park, S. Y.; Kim, H. J.; Lee, H. J.; Youk, J. H. Development of 3-D poly(trimethylenecarbonate-co-e-caprolactone)-block-poly(p-dioxanone) scaffold for bone regeneration with high porosity using a. wet electrospinning method. Biotechnol. Lett.2010, 32, 877–882.
Lim, J. S.; Ki, C. S.; Kim, J. W.; Lee, K. G.; Kang, S. W.; Kweon, H. Y.; Park, Y. H. Fabrication and evaluation of poly(epsilon-caprolactone)/silk fibroin blend nanofibrous scaffold. Biopolymers2012, 97, 265–275.
Kim, M. S.; Kim, G. H. Highly porous electrospun 3D poly-caprolactone/p-TCP biocomposites for tissue regeneration. Mater. Lett. 2014, 120, 246–250.
Kim, M. S.; Kim, G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohyd. Polym.2014, 114, 213–221.
Yang, W. X.; Yang, F.; Wang, Y. N.; Both, S. K.; Jansen, J. A. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater.2013, 9, 4505–4512.
Kasuga, T.; Obata, A.; Maeda, H.; Ota, Y.; Yao, X. F.; Oribe, K. Siloxane-poly(lactic acid)-vaterite composites with 3D cotton-like structure. J. Mater. Sci. Mater. Med.2012, 23, 2349–2357.
Heo, J.; Nam, H.; Hwang, D.; Cho, S. J.; Jung, S. Y.; Cho, D. W.; Shim, J. H.; Lim, G. Enhanced cellular distribution and infiltration in a. wet electrospun three-dimensional fibrous scaffold using eccentric rotation-based hydrodynamic conditions. Sens. Actuators B-Chem.2016, 226, 357–363.
Shang, S. H.; Yang, F.; Cheng, X. R.; Walboomers, X. F.; Jansen, J. A. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur. Cells Mater.2010, 19, 180–192.
Elliott, M. B.; Ginn, B.; Fukunishi, T.; Bedja, D.; Suresh, A.; Chen, T.; Inoue, T.; Dietz, H. C.; Santhanam, L.; Mao, H. Q. et al. Regenerative and durable small-diameter graft as an arterial conduit. Prac Natl. Acad. Sci. USA2019, 116, 12710–12719.
Yan, L. D.; Si, S. X.; Chen, Y.; Yuan, T.; Fan, H. J.; Yao, Y. Y.; Zhang, Q. Y. Electrospun in-situ hybrid polyurethane/nano-Ti02 as wound dressings. FibersPolym.2011, 12, 207–213.
Zhang, M.; Lin, H.; Wang, Y. L.; Yang, G.; Zhao, H.; Sun, D. H. Fabrication and durable antibacterial properties of 3D porous wet electrospun RCSC/PCL nanofibrous scaffold with silver nanoparticles. Appl. Surf. Sci. 2017, 414, 52–62.
Martrou, G.; Leonetti, M.; Gigmes, D.; Trimaille, T. One-step preparation of surface modified electrospun microfibers as suitable supports for protein immobilization. Polym. Chem. 2017, 8, 1790–1796.
Farzamfar, S.; Naseri-Nosar, M.; Vaez, A.; Esmaeilpour, F.; Ehterami, A.; Sahrapeyma, H.; Samadian, H.; Hamidieh, A. A.; Ghorbani, S.; Goodarzi, A. et al. Neural tissue regeneration by a. gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose2018, 25, 1229–1238.
Barber, P. S.; Griggs, C. S.; Bonner, J. R.; Rogers, R. D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem.2013, 15, 601–607.
Hou, L. J.; Udangawa, W. M. R. N.; Pochiraju, A.; Dong, W. J.; Zheng, Y. Y.; Linhardt, R. J.; Simmons, T. J. Synthesis of heparin-immobilized, magnetically addressable cellulose nanofibers for biomedical applications. ACS Biomater. Sci. Eng.2016, 2, 1905–1913.
Viswanathan, G.; Murugesan, S.; Pushparaj, V.; Nalamasu, O.; Ajayan, P. M.; Linhardt, R. J. Preparation of biopolymer fibers by electro-spinning from room temperature ionic liquids. Biomacromolecules2006, 7,415-418.
Meli, L.; Miao, J. J.; Dordick, J. S.; Linhardt, R. J. Electrospinning from room temperature ionic liquids for biopolymer fiber formation. Green Chem.2010, 12, 1883–1892.
Zheng, Y. Y.; Miao, J. J.; Maeda, N.; Frey, D.; Linhardt, R. J.; Simmons, T. J. Uniform nanoparticle coating of cellulose fibers during wet electrospinning. J. Mater. Chem. A2014, 2, 15029–15034.
Sa, V.; Kornev, K. G. A method for wet spinning of alginate fibers with a. high concentration of single-walled carbon nanotubes. Carbon2011, 49, 1859–1868.
Qin, Y. M. Alginate fibres: An overview of the production processes and applications in wound management. Polym. Int.2008, 57, 171–180.
Miraftab, M.; Qiao, Q.; Kennedy, J. R.; Anand, S. C.; Groocock, M. R. Fibres for wound dressings based on mixed carbohydrate polymer fibres. Carbohydr. Polym.2003, 53, 225–231.
Watthanaphanit, A.; Supaphol, P.; Furuike, T.; Tokura, S.; Tamura, H.; Rujiravanit, R. Novel chitosan-spotted alginate fibers from wet-spinning of alginate solutions containing emulsified chitosan-citrate complex and their characterization. Biomacromolecules2009, 70,320-327.
Cheng, J.; Jun, Y.; Qin, J. H.; Lee, S. H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143.
Majidi, S. S.; Slemming-Adamsen, P.; Hanif, M.; Zhang, Z. Y.; Wang, Z. M.; Chen, M. L. Wet electrospun alginate/gelatin hydrogel nanofibers for 3D cell culture. Int. J. Biol. Macromol.2018, 118, 1648–1654.
Razal, J. M.; Gilmore, K. J.; Wallace, G. G Carbon nanotube biofiber formation in a. polymer-free coagulation bath. Adv. Fund. Mater.2008, 18, 61–66.
Nikoo, A. M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Controlling the morphology and material characteristics of electrospray generated calcium alginate microhydrogels. J. Microencapsulation2016, 33, 605–612.
Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U.; Supaphol, P. Biodegradable alginate microparticles developed by electrohydrodynamic spraying techniques for oral delivery of protein. J. Microencapsulation2009, 26, 563–570.
Suksamran, T.; Ngawhirunpat, T.; Rojanarata, T.; Sajomsang, W.; Pitaksuteepong, T.; Opanasopit, P. Methylated N-(4-N,N-dimethylaminocinnamyl) chitosan-coated electrospray OVA-loaded microparticles for oral vaccination. Int. J. Pharm.2013, 448, 19–27.
Choi, D. H.; Subbiah, R.; Kim, I. H.; Han, D. K.; Park, K. Dual growth factor delivery using biocompatible core-shell microcapsules for angiogenesis. Small2013, 9, 3468–3476.
Lai, W. R.; Susha, A. S.; Rogach, A. L. Multicompartment microgel beads for co-delivery of multiple drugs at individual release rates. ACS Appl. Mater. Interfaces2016, 8, 871–880.
Ward, E.; Chan, E.; Gustafsson, K.; Jayasinghe, S. N. Combining bio-electrospraying with gene therapy: A. novel biotechnique for the delivery of genetic material via living cells. Analyst2010, 135, 1042–1049.
Yao, R.; Zhang, R. J.; Wang, X. H. Design and evaluation of a. cell microencapsulating device for cell assembly technology. J. Bioact. Compat. Polym.2009, 24, 48–62.
Xie, J. W.; Wang, C. H. Electrospray in the dripping mode for cell microencapsulation. J. Colloid Interface Sci. 2007, 312, 247–255.
Nguyen, D. K.; Son, Y. M.; Lee, N. E. Hydrogel encapsulation of cells in core-shell microcapsules for cell delivery. Adv. Healthc. Mater.2015, 4, 1537–1544.
Zhao, S. T.; Agarwal, P.; Rao, W.; Huang, H. S.; Zhang, R. L.; Liu, Z. G.; Yu, J. H.; Weisleder, N.; Zhang, W. J.; He, X. M. Coaxial electrospray of liquid core-hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol.2014, 6, 874–884.
Ma, M. L.; Chiu, A.; Sahay, G.; Doloff, J. C.; Dholakia, N.; Thakrar, R.; Cohen, J.; Vegas, A.; Chen, D. L.; Bratlie, K. M. et al. Core-shell hydrogel microcapsules for improved islets encapsulation. Adv. Healthc. Mater.2013, 2, 667–672.
Kim, P. H.; Yim, H. G.; Choi, Y. J.; Kang, B. J.; Kim, J.; Kwon, S. M.; Kim, B. S.; Hwang, N. S.; Cho, J. Y. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Controlled Release2014, 187, 1–13.
Jayasinghe, S. N. Bio-electrosprays: From bio-analytics to a. generic tool for the health sciences. Analyst2011, 136, 878–890.
Zussman, E. Encapsulation of cells within electrospun fibers. Polym Adv. Technol.2011, 22, 366–371.
Selimovic, S.; Oh, J.; Bae, H.; Dokmeci, M.; Khademhosseini, A. Microscale strategies for generating cell-encapsulating hydrogels. Polymers2012, 4, 1554–1579.
Poncelet, D.; de Vos, P.; Suter, N.; Jayasinghe, S. N. Bio-electrospraying and cell electrospinning: Progress and opportunities for basic biology and clinical sciences. Adv. Healthc. Mater.2012, 7, 27–34.
Lee, S. H.; Park, S. Y.; Choi, J. H. Fiber formation and physical properties of chitosan fiber crosslinked by epichlorohydrin in a. wet spinning system: The effect of the concentration of the crosslinking agent epichlorohydrin. J. Appl. Polym. Sci.2004, 92, 2054–2062.
Lee, S. H.; Park, S. M.; Kim, Y. Effect of the concentration of sodium acetate (SA) on crosslinking of chitosan fiber by epichlorohydrin (ECH) in a. wet spinning system. Carbohydr. Polym. 2007, 70, 53–60.
Denkbas, E. B.; Seyyal, M.; Piskin, E. Implantable 5-fluorouracil loaded chitosan scaffolds prepared by wet spinning. J. Membr. Sci.2000, 172, 33–38.
Wang, X. F.; Min, M. H.; Liu, Z. Y.; Yang, Y.; Zhou, Z.; Zhu, M. R.; Chen, Y. M.; Hsiao, B. S. Poly(ethyleneimine) nanofibrous affinity membrane fabricated via one step wet-electrospinning from poly(vinyl alcohol)-doped poly(ethyleneimine) solution system and its application. J. Membr. Sci.2011, 379, 191–199.
Teng, F. J.; Ding, H. R.; Huang, Y. Q.; Wang, J. W. Fabrication of three-dimensional nanofibrous gelatin scaffolds using one-step crosslink technique. J. Biomater. Sci. Polym. Ed.2018, 29, 1859–1875.
Tsukada, M.; Gotoh, Y.; Nagura, M.; Minoura, N.; Kasai, N.; Freddi, G. Structural changes of silk fibroin membranes induced by immersion in methanol aqueous solutions. J. Polym. Sci. Part B. Polym. Phys.1994, 32, 961–968.
Hofmann, S.; Foo, C. T. W. P.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D. L.; Merkle, H. P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release2006, 111, 219–227.
Baimark, Y.; Srihanam, P. Effect of methanol treatment on regenerated silk fibroin microparticles prepared by the emulsification-diffusion technique. J. Appl. Sci.2009, 9, 3876–3881.
Yu, Q. Z.; Xu, S. L.; Zhang, H.; Gu, L.; Xu, Y. P.; Ko, F. Structure-property relationship of regenerated spider silk protein nano/ microfibrous scaffold fabricated by electrospinning. J. Biomed. Mater. Res. Part A2014, 102, 3828–3837.
Ki, C. S.; Kim, J. W.; Hyun, J. H.; Lee, K. H.; Hattori, M.; Rah, D. K.; Park, Y. H. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. Sci.2007, 106, 3922–3928.
Yang, S. Y.; Hwang, T. H.; Che, L. H.; Oh, J. S.; Ha, Y.; Ryu, W. H. Membrane-reinforced three-dimensional electrospun silk fibroin scaffolds for bone tissue engineering. Biomed. Mater.2015, 10, 035011.
Ding, H. R.; Zhong, J. W.; Xu, R.; Song, F. F.; Yin, M.; Wu, Y. R.; Hu, Q. Y.; Wang, J. W. Establishment of 3D culture and induction of osteogenic differentiation of pre-osteoblasts using wet-collected aligned scaffolds. Mater. Sci. Eng. C2017, 71, 222–230.
Hadisi, Z.; Nourmohammadi, J.; Mohammadi, J. Composite of porous starch-silk fibroin nanofiber-calcium phosphate for bone regeneration. Ceram. Int.2015, 41, 10745–10754.
Akturk, O.; Kismet, K.; Yasti, A. C.; Kuru, S.; Duymus, M. E.; Kaya, R.; Caydere, M.; Hucumenoglu, S.; Keskin, D. Wet electrospun silk fibroin/gold nanoparticle 3D matrices for wound healing applications. RSC Adv.2016, 6, 13234–13250.
Matsuyama, H.; Teramoto, M.; Nakatani, R.; Maki, T. Membrane formation via phase separation induced by penetration of nonsolvent from vapor phase. I. Phase diagram and mass transfer process. J. Appl. Polym. Sci.1999, 74, 159–170.
Megelski, S.; Stephens, J. S.; Chase, D. B.; Rabolt, J. R. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules2002, 35, 8456–8466.
Casper, C. L.; Stephens, J. S.; Tassi, N. G.; Chase, D. B.; Rabolt, J. F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules2004, 37, 573–578.
Dayal, P.; Liu, J.; Kumar, S.; Kyu, T. Experimental and theoretical investigations of porous structure formation in electrospun fibers. Macromolecules2007, 40, 7689–7694.
McCann, J. T.; Marquez, M.; Xia, Y. N. Highly porous fibers by electrospinning into a. cryogenic liquid. J. Am. Chem. Soc.2006, 128, 1436–1437.
Thangaraju, E.; Rajiv, S.; Natarajan, T. S. Comparison of preparation and characterization of water-bath collected porous poly L-lactide microfibers and cellulose/silk fibroin based poly L-lactide nanofibers for biomedical applications. J. Polym. Res.2015, 22, 24.
Katsogiannis, K. A. G.; Vladisavljevic, G. T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J.2015, 69, 284–295.
Li, X. H.; Teng, K. Y.; Shi, J.; Wang, W.; Xu, Z. W.; Deng, H.; Lv, H. M.; Li, F. Y. Electrospun preparation of polylactic acid nanoporous fiber membranes via thermal-nonsolvent induced phase separation. J. Taiwan Inst. Chem. Eng.2016, 60, 636–642.
Nayani, K.; Katepalli, H.; Sharma, C. S.; Sharma, A.; Patil, S.; Venkataraghavan, R. Electrospinning combined with nonsolvent-induced phase separation to fabricate highly porous and hollow submicrometer polymer fibers. Ind. Eng. Chem. Res.2012, 57, 1761–1766.
Wang, Y.; Zhu, L. H.; Chen, A. Z.; Xu, Q.; Hong, Y. J.; Wang, S. B. One-step method to prepare PLLA porous microspheres in a. high-voltage electrostatic anti-solvent process. Materials2016, 9, 368.
Feng, J. T.; Lin, L.; Chen, P. P.; Hua, W. D.; Sun, Q. M.; Ao, Z.; Liu, D. S.; Jiang, L.; Wang, S. T.; Han, D. Topographical binding to mucosa-exposed cancer cells: Pollen-mimetic porous microspheres with tunable pore sizes. ACS Appl. Mater. Interfaces2015, 7, 8961–8967.
Gao, Y.; Bai, Y. T.; Zhao, D.; Chang, M. W.; Ahmad, Z.; Li, J. S. Tuning microparticle porosity during single needle electrospraying synthesis via a. non-solvent-based physicochemical approach. Polymers2015, 7, 2701–2710.
Wu, Y. Q.; Clark, R. L. Controllable porous polymer particles generated by electrospraying. J. Colloid Interface Sci.2007, 310, 529–535.
Taskin, M. B.; Xia, D.; Besenbacher, F.; Dong, M. D.; Chen, M. L. Nanotopography featured polycaprolactone/polyethyleneoxide microfibers modulate endothelial cell response. Nanoscale2017, 9, 9218–9229.
Li, Y. F.; Rubert, M.; Asian, H.; Yu, Y.; Howard, K. A.; Dong, M. D.; Besenbacher, R.; Chen, M. L. Ultraporous interweaving electrospun microfibers from PCL-PEO binary blends and their inflammatory responses. Nanoscale2014, 6, 3392–3402.
Chen, Y. L.; Taskin, M. B.; Zhang, Z. Y.; Su, Y. C.; Han, X. J.; Chen, M. L. Bioadhesive anisotropic nanogrooved microfibers directing three-dimensional neurite extension. Biomater. Sci.2019, 7, 2165–2173.
Muzzarelli, R. A. A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs2011, 9, 1510–1533.
Bazbouz, M. B.; Taylor, M.; Baker, D.; Ries, M. E.; Goswami, P. Dry-jet wet electrospinning of native cellulose microfibers with macroporous structures from ionic liquids. J. Appl. Polym. Sci.2019, 136, 47153.
Acknowledgements
We gratefully acknowledge the funding from Aarhus University Research Foundation (AUFF-E-2015-FLS-7-27).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There are no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Taskin, M.B., Klausen, L.H., Dong, M. et al. Emerging wet electrohydrodynamic approaches for versatile bioactive 3D interfaces. Nano Res. 13, 315–327 (2020). https://doi.org/10.1007/s12274-020-2635-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2635-x