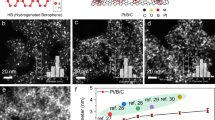
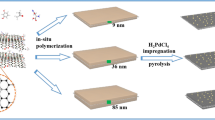
Abstract
Carbon nanospheres (XC-72R) were functionalized by boron-oxygen (B-O) through coannealing with boric acid, to which highly dispersed palladium nanoparticles (Pd NPs) (~ 1.7 nm) were immobilized by a wet chemical reduction for the first time. The resultant Pd/OB-C catalyst exhibits significantly improved activity for the dehydrogenation from formic acid (FA) compared to pristine XC-72R supported Pd NPs (Pd/C). Impressively, by adding melamine precursor, the B-O and nitrogen (N)-functionalized product OB-C-N displays an extremely high B content, ca. 34 times higher than OB-C. The Pd/OB-C-N catalyst with an ultrafine Pd particle size of ~ 1.4 nm shows a superb activity, with a turnover frequency (TOF) as high as 5,354 h−1 at 323 K, owing to the uniform ultrafine Pd NPs and the effect from B-O and N functionalities.

Similar content being viewed by others
References
Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature2001, 414, 353–358.
Hamilton, C. W.; Baker, R. T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogenstorage. Chem. Soc. Rev. 2009, 38, 279–293.
Zhu, Q. L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512.
Yin, H. J.; Tang, H. J.; Wang, D.; Gao, Y.; Tang, Z. Y. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction. ACS Nano2012, 6, 8288–8297.
Yang, X. C.; Sun, J. K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220.
Zhu, Q. L.; Xu, Q. Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chem2016, 1, 220–245.
Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725.
Yadav, M.; Singh, A. K.; Tsumori, N.; Xu, Q. Palladium silica nanosphere-catalyzed decomposition of formic acid for chemical hydrogen storage. J. Mater. Chem. 2012, 22, 19146–19150.
Tan, C. L.; Huang, X.; Zhang, H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today2013, 16, 29–36.
Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material-Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988.
Boddien, A.; Mellmann, D.; Gärtner, F.; Jackstell, R.; Junge, H.; Dyson, P. J.; Laurenczy, G.; Ludwig, R.; Beller, M. Efficient dehydrogenation of formic acid using an iron catalyst. Science2011, 333, 1733–1736.
Hull, J. F.; Himeda, Y.; Wang, W. H.; Hashiguchi, B.; Periana, R.; Szalda, D. J.; Muckerman, J. T.; Fujita, E. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 2012, 4, 383–388.
Fellay, C.; Dyson, P. J.; Laurenczy, G. A viable hydrogen-storage system based on selective formic acid decomposition with a ruthenium catalyst. Angew. Chem., Int. Ed.2008, 47, 3966–3968.
Chen, Y.; Zhu, Q. L.; Tsumori, N.; Xu, Q. Immobilizing highly catalytically active noble metal nanoparticles on reduced graphene oxide: A non-noble metal sacrificial approach. J. Am. Chem. Soc. 2015, 137, 106–109.
Li, Z. P.; Xu, Q. Metal-nanoparticle-catalyzed hydrogen generation from formic acid. Acc. Chem. Res. 2017, 50, 1449–1458.
Sordakis, K.; Tang, C. H.; Vogt, L. K.; Junge, H.; Dyson, P. J.; Beller, M.; Laurenczy, G. Homogeneous catalysis for sustainable hydrogen storage in formic acid and alcohols. Chem. Rev. 2018, 11 8, 372–433.
Song, F. Z.; Zhu, Q. L.; Yang, X. C.; Zhan, W. W.; Pachfule, P.; Tsumori, N.; Xu, Q. Metal-organic framework templated porous carbon-metal oxide/reduced graphene oxide as superior support of bimetallic nanoparticles for efficient hydrogen generation from formic acid. Adv. Energy Mater. 2018, 8, 1701416.
Wang, N.; Sun, Q. M.; Bai, R. S.; Li, X.; Guo, G. Q.; Yu, J. H. In situ confinement of ultrasmall pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484–7487.
Yang, X. C.; Pachfule, P.; Chen, Y.; Tsumori, N.; Xu, Q. Highly efficient hydrogen generation from formic acid using a reduced graphene oxide-supported AuPd nanoparticle catalyst. Chem. Commun. 2016, 52, 4171–4174.
Zhu, Q. L.; Tsumori, N.; Xu, Q. Immobilizing extremely catalytically active palladium nanoparticles to carbon nanospheres: A weakly-capping growth approach. J. Am. Chem. Soc. 2015, 137, 11743–11748.
Li, D. D.; Xu, H. Q.; Jiao, L.; Jiang, H. L. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. EnergyChem2019, 1, 100005.
Song, F. Z.; Zhu, Q. L.; Tsumori, N.; Xu, Q. Diamine-alkalized reduced graphene oxide: Immobilization of sub-2 nm palladium nanoparticles and optimization of catalytic activity for dehydrogenation of formic acid. ACS Catal. 2015, 5, 5141–5144.
Chen, Y. M.; Li, X. Y.; Park, K.; Zhou, L. M.; Huang, H. T.; Mai, Y. W.; Goodenough, J. B. Hollow nanotubes of N-doped carbon on CoS. Angew. Chem., Int. Ed.2016, 55, 15831–15834.
Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A2013, 1, 14–19.
Zhao, X. X.; Yang, H.; Jing, P.; Shi, W.; Yang, G. M.; Cheng, P. A metal- organic framework approach toward highly nitrogen-doped graphitic carbon as a metal-free photocatalyst for hydrogen evolution. Small2017, 13, 1603279.
He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: Catalysis beyond electrochemistry. Angew. Chem., Int. Ed.2016, 55, 12582–12594.
Wang, Q. J.; Tsumori, N.; Kitta, M.; Xu, Q. Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon. ACS Catal. 2018, 8, 12041–12045.
Li, Z. P.; Yang, X. C.; Tsumori, N.; Liu, Z.; Himeda, Y.; Autrey, T.; Xu, Q. Tandem nitrogen functionalization of porous carbon: Toward immobilizing highly active palladium nanoclusters for dehydrogenation of formic acid. ACS Catal. 2017, 7, 2720–2724.
Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S. Z. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem., Int. Ed.2013, 52, 3110–3116.
Wang, X. W.; Sun, G. Z.; Routh, P.; Kim, D. H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098.
Chen, Y. Z.; Cai, G. R.; Wang, Y. M.; Xu, Q.; Yu, S. H.; Jiang, H. L. Palladium nanoparticles stabilized with N-doped porous carbons derived from metal-organic frameworks for selective catalysis in biofuel upgrade: The role of catalyst wettability. Green Chem. 2016, 18, 1212–1217.
Zhu, Q. L.; Tsumori, N.; Xu, Q. Sodium hydroxide-assisted growth of uniform Pd nanoparticles on nanoporous carbon MSC-30 for efficient and complete dehydrogenation of formic acid under ambient conditions. Chem. Sci. 2014, 5, 195–199.
Zhang, Z. Y.; Xi, J. Y.; Zhou, H. P.; Qiu, X. P. KOH etched graphite felt with improved wettability and activity for vanadium flow batteries. Electrochim. Acta2016, 218, 15–23.
Zhao, Y.; Yang, L. J.; Chen, S.; Wang, X. Z.; Ma, Y. W.; Wu, Q.; Jiang, Y. F.; Qian, W. J.; Hu, Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204.
Du, X. Q.; Du, C.; Cai, P.; Luo, W.; Cheng, G. Z. NiPt nanocatalysts supported on boron and nitrogen Co-doped graphene for superior hydrazine dehydrogenation and methanol oxidation. ChemCatChem2016, 8, 1410- 1416.
Zhang, Y. R.; Zhang, H.; Zhao, Y. N.; Han, X. Y.; Wang, H. J.; Gao, Y. J. B/N co-doped carbon derived from the sustainable chitin for C-H bond oxidation. Appl. Surf. Sci. 2018, 457, 439–448.
Chowdhury, S.; Jiang, Y. Q.; Muthukaruppan, S.; Balasubramanian, R. Effect of boron doping level on the photocatalytic activity of graphene aerogels. Carbon2018, 128, 237–248.
Aghili, S.; Panjepour, M.; Meratian, M. Kinetic analysis of formation of boron trioxide from thermal decomposition of boric acid under non-isothermal conditions. J. Therm. Anal. Calorim. 2018, 131, 2443–2455.
Liu, X. X.; Wang, Y. H.; Chen, L. B.; Chen, P. P.; Jia, S. P.; Zhang, Y.; Zhou, S. Y.; Zang, J. B. Co2B and CO nanoparticles immobilized on the N-B-doped carbon derived from nano-B4C for efficient catalysis of oxygen evolution, hydrogen evolution, and oxygen reduction reactions. ACS Appl. Mater. Interfaces2018, 10, 37067–37078.
Goyal, R.; Sarkar, B.; Bag, A.; Lefebvre, F.; Sameer, S.; Pendem, C.; Bordoloi, A. Single-step synthesis of hierarchical BxCN: A metal-free catalyst for low-temperature oxidative dehydrogenation of propane. J. Mater. Chem. A2016, 4, 18559–18569.
Zhao, X. A.; Ong, C. W.; Tsang, Y. C.; Wong, Y. W.; Chan, P. W.; Choy, C. L. Reactive pulsed laser deposition of CNx films. Appl. Phys. Lett. 1995, 66, 2652–2654.
Romanos, J.; Beckner, M.; Stalla, D.; Tekeei, A.; Suppes, G.; Jalisatgi, S.; Lee, M.; Hawthorne, F.; Robertson, J.; Firlej, L. et al. Infrared study of boron-carbon chemical bonds in boron-doped activated carbon. Carbon2013, 54, 208–214.
Shin, S.; Jang, J.; Yoon, S. H.; Mochida, I. A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR. Carbon1997, 35, 1739–1743.
Huang, H. G.; Xiang, C. L.; Ning, Y. S.; Huang, J. Y.; Ang, S. G.; Xu, G. Q. Dry synthesis of triple cumulative double bonds (C=C=C=N) on Si (111)-7× 7 surfaces. J. Phys. Chem. B2005, 109, 19296–19300.
Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nat. Chem. 2016, 8, 718–724.
Wang, D. W.; Li, F.; Chen, Z. G.; Lu, G. Q.; Cheng, H. M. Synthesis and electrochemical property of boron-doped mesoporous carbon in supercapacitor. Chem. Mater. 2008, 20, 7195–7200.
Zhong, S.; Xu, Q. Metal nanoparticle-catalyzed hydrogen generation from liquid chemical hydrides. Bull. Chem. Soc. Jpn. 2018, 91, 1606–1617.
Acknowledgements
The authors are very thankful to Dr. Takeyuki Uchida for TEM measurements, and METI and AIST for financial support. S. Z. is grateful to the Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT) for a PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2019_2539_MOESM1_ESM.pdf
Immobilizing palladium nanoparticles on boron-oxygen-functionalized carbon nanospheres towards efficient hydrogen generation from formic acid
Rights and permissions
About this article
Cite this article
Zhong, S., Tsumori, N., Kitta, M. et al. Immobilizing palladium nanoparticles on boron-oxygen-functionalized carbon nanospheres towards efficient hydrogen generation from formic acid. Nano Res. 12, 2966–2970 (2019). https://doi.org/10.1007/s12274-019-2539-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2539-9