Abstract
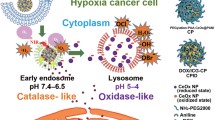
The development of effective nanoplatforms is extremely necessary for cancer therapy. Herein, we prepared polydopamine (PDA) and ammonium bicarbonate (NH4HCO3) coated and doxorubicin (Dox) loaded hollow cerium oxide (CeO2) NPs (PDAC NPs), which showed excellent synergistic effect for photothermal therapy, chemotherapy and chemodynamic therapy. Under near infrared laser irradiation, PDA shell could absorb the incident light and convert it into heat, which could not only kill tumor cells with hyperthermia, but also trigger the decomposition of NH4HCO3 into gaseous carbon dioxide and ammonia, leading to the destroy of PDA shell. The leakage of PDA further accelerated Dox release and exposed CeO2 surface, in which Dox could enter into cell nucleus to induce chemotherapy, and CeO2 could catalyze cellular hydrogen peroxide into hydroxyl radical to present chemodynamic therapy. In fact, PDAC NPs showed an excellent therapeutic efficacy both in vitro and in vivo. This design provides a new strategy for synergistic tumor therapy.

Similar content being viewed by others
References
Yin, C. Y.; Wang, S. H.; Ren, Q. Z.; Shen, X. M.; Chen, X. D.; Liu, Y. J.; Liu, S. J. Radial extracorporeal shock wave promotes the enhanced permeability and retention effect to reinforce cancer nanothermotherapeutics. Sci. Bull.2019, 64, 679–689.
Cheng, Y.; Chang, Y.; Feng, Y. L.; Jian, H.; Wu, X. Q.; Zheng, R. X.; Xu, K. Q.; Zhang, H. Y. Bismuth sulfide nanorods with retractable zinc protoporphyrin molecules for suppressing innate antioxidant defense system and strengthening phototherapeutic effects. Adv. Mater.2019, 31, 1806808.
Wang, Z. Z.; Zhang, Y.; Ju, E. G.; Liu, Z.; Cao, F. F.; Chen, Z. W.; Ren, J. S.; Qu, X. G. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun.2018, 9, 3334.
Feng, W.; Han, X. G.; Wang, R. Y.; Gao, X.; Hu, P.; Yue, W. W.; Chen, Y.; Shi, J. L. Nanocatalysts-augmented and photothermal-enhanced tumorspecific sequential nanocatalytic therapy in both NIR-I and NIR-II biowindows. Adv. Mater.2019, 31, 1805919.
Tang, Z. M.; Zhang, H. L.; Liu, Y. Y.; Ni, D. L.; Zhang, H.; Zhang, J. W.; Yao, Z. W.; He, M. Y.; Shi, J. L.; Bu, W. B. Antiferromagnetic pyrite as the tumor microenvironment-mediated nanoplatform for self-enhanced tumor imaging and therapy. Adv. Mater.2017, 29, 1701683.
Zhang, C.; Liu, W. L.; Bai, X. F.; Cheng, S. X.; Zhong, Z. L.; Zhang, X. Z. A hybrid nanomaterial with NIR-induced heat and associated hydroxyl radical generation for synergistic tumor therapy. Biomaterials2019, 199, 1–9.
Ding, X.; Liu, J. H.; Li, J. Q.; Wang, F.; Wang, Y. H.; Song, S. Y.; Zhang, H. J. Polydopamine coated manganese oxide nanoparticles with ultrahigh relaxivity as nanotheranostic agents for magnetic resonance imaging guided synergetic chemo-/photothermal therapy. Chem. Sci.2016, 7, 6695–6700.
Liu, T.; Liu, W. L.; Zhang, M. K.; Yu, W. Y.; Gao, F.; Li, C. X.; Wang, S. B.; Feng, J.; Zhang, X. Z. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano2018, 12, 12181–12192.
Ma, X. M.; Cheng, Y.; Jian, H.; Feng, Y. L.; Chang, Y.; Zheng, R. X.; Wu, X. Q.; Wang, L.; Li, X.; Zhang, H. Y. Hollow, rough, and nitric oxidereleasing cerium oxide nanoparticles for promoting multiple stages of wound healing. Adv. Healthc. Mater.2019, 8, 1900256.
Cao, F. F.; Zhang, Y.; Sun, Y. H.; Wang, Z. Z.; Zhang, L.; Huang, Y. Y.; Liu, C. Q.; Liu, Z.; Ren, J. S.; Qu, X. G. Ultrasmall nanozymes isolated within porous carbonaceous frameworks for synergistic cancer therapy: Enhanced oxidative damage and reduced energy supply. Chem. Mater.2018, 30, 7831–7839.
Kwon, H. J.; Kim, D.; Seo, K.; Kim, Y. G.; Han, S. I.; Kang, T.; Soh, M.; Hyeon, T. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angew. Chem., Int. Ed.2018, 57, 9408–9412.
Vinothkumar, G.; Lalitha, A. I.; Suresh Babu, K. Cerium phosphate-cerium oxide heterogeneous composite nanozymes with enhanced peroxidase-like biomimetic activity for glucose and hydrogen peroxide sensing. Inorg. Chem.2019, 58, 349–358.
Liu, Q. Y.; Ding, Y. Y.; Yang, Y. T.; Zhang, L. Y.; Sun, L. F.; Chen, P. P.; Gao, C. Enhanced peroxidase-like activity of porphyrin functionalized ceria nanorods for sensitive and selective colorimetric detection of glucose. Mater. Sci. Eng., C2016, 59, 445–453.
Tian, Z. M.; Li, J.; Zhang, Z. Y.; Gao, W.; Zhou, X. M.; Qu, Y. Q. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials2015, 59, 116–124.
Korsvik, C.; Patil, S.; Seal, S.; Self, W. T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun.2007, 1056–1058.
Li, Y. Y.; He, X.; Yin, J. J.; Ma, Y. H.; Zhang, P.; Li, J. Y.; Ding, Y. Y.; Zhang, J.; Zhao, Y. L.; Chai, Z. F. et al, Acquired superoxide-scavenging ability of ceria nanoparticles. Angew. Chem., Int. Ed.2015, 54, 1832–1835.
Yao, C.; Wang, W. X.; Wang, P. Y.; Zhao, M. Y.; Li, X. M.; Zhang, F. Nearinfrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater.2018, 30, 1704833.
Pirmohamed, T.; Dowding, J. M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A. S.; King, J. E. S.; Seal, S.; Self, W. T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun.2010, 46, 2736–2738.
Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J. M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem., Int. Ed.2009, 48, 2308–2312.
Peng, Y. F.; Chen, X. J.; Yi, G. S.; Gao, Z. Q. Mechanism of the oxidation of organic dyes in the presence of nanoceria. Chem. Commun.2011, 47, 2916–2918.
Zhong, X. Y.; Yang, K.; Dong, Z. L.; Yi, X.; Wang, Y.; Ge, C. C.; Zhao, Y. L.; Liu, Z. Polydopamine as a biocompatible multifunctional nanocarrier for combined radioisotope therapy and chemotherapy of cancer. Adv. Funct. Mater.2015, 25, 7327–7336.
Li, W. Q.; Wang, Z. G.; Hao, S. J.; He, H. Z.; Wan, Y.; Zhu, C. D.; Sun, L. P.; Cheng, G.; Zheng, S. Y. Mitochondria-targeting polydopamine nanoparticles to deliver doxorubicin for overcoming drug resistance. ACS Appl. Mater. Interfaces2017, 9, 16793–16802.
Zheng, Q. S.; Lin, T. R.; Wu, H. Y.; Guo, L. Q.; Ye, P. R.; Hao, Y. L.; Guo, Q. Q.; Jiang, J. Z.; Fu, F. F.; Chen, G. N. Mussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Int. J. Pharm.2014, 463, 22–26.
Ding, L.; Zhu, X. B.; Wang, Y. L.; Shi, B. Y.; Ling, X.; Chen, H. J.; Nan, W. H.; Barrett, A.; Guo, Z. L.; Tao, W Intracellular fate of nanoparticles with polydopamine surface engineering and a novel strategy for exocytosisinhibiting, lysosome impairment-based cancer therapy. Nano Lett.2017, 17, 6790–6801.
Lin, L. S.; Cong, Z. X.; Cao, J. B.; Ke, K. M.; Peng, Q. L.; Gao, J. H.; Yang, H. H.; Liu, G.; Chen, X. Y. Multifunctional Fe3O4@polydopamine core-shell nanocomposites for intracellular mrna detection and imagingguided photothermal therapy. ACS Nano2014, 8, 3876–3883.
Li, N.; Li, T. T.; Hu, C.; Lei, X. M.; Zuo, Y. P.; Han, H. Y. Targeted near-infrared fluorescent turn-on nanoprobe for activatable imaging and effective phototherapy of cancer cells. ACS Appl. Mater. Interfaces2016, 8, 15013–15023.
Liu, F. Y.; He, X. X.; Lei, Z.; Liu, L.; Zhang, J. P.; You, H. P.; Zhang, H. M.; Wang, Z. X. Facile preparation of doxorubicin-loaded upconversion@ polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv. Healthc. Mater.2015, 4, 559–568.
Zheng, X. Y.; Zhang, J. X.; Wang, J.; Qi, X. Q.; Rosenholm, J. M.; Cai, K. Y. Polydopamine coatings in confined nanopore space: Toward improved retention and release of hydrophilic cargo. J. Phys. Chem. C2015, 119, 24512–24521.
Chen, J. X.; Lei, S.; Zeng, K.; Wang, M. Z.; Asif, A.; Ge, X. W. Catalaseimprinted Fe3O4/Fe@fibrous SiO2/polydopamine nanoparticles: An integrated nanoplatform of magnetic targeting, magnetic resonance imaging, and dual-mode cancer therapy. Nano Res.2017, 10, 2351–2363.
Ding, X.; Liu, J. H.; Liu, D. P.; Li, J. Q.; Wang, F.; Li, L. J.; Wang, Y. H.; Song, S. Y.; Zhang, H. J. Multifunctional core/satellite polydopamine@Nd3+- sensitized upconversion nanocomposite: A single 808 nm near-infrared light-triggered theranostic platform for in vivo imaging-guided photothermal therapy. Nano Res.2017, 10, 3434–3446.
Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater.2013, 12, 991–1003.
Xia, J. Z.; Feng, G.; Xia, X. R.; Hao, L.; Wang, Z. G. NH4HCO3 gasgenerating liposomal nanoparticle for photoacoustic imaging in breast cancer. Int. J. Nanomed.2017, 12, 1803–1813.
Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci.1968, 26, 62–69.
Wang, Z. H.; Fu, H. F.; Tian, Z. W.; Han, D. M.; Gu, F. B. Strong metal-support interaction in novel core-shell Au-CeO2 nanostructures induced by different pretreatment atmospheres and its influence on CO oxidation. Nanoscale2016, 8, 5865–5872.
Yang, Y.; Wu, Y. K.; Ren, Q. Z.; Zhang, L. G.; Liu, S. J.; Zuo, Y. Y. Biophysical assessment of pulmonary surfactant predicts the lung toxicity of nanomaterials. Small Methods2018, 2, 1700367.
Zhan, Q. C.; Shi, X. Q.; Zhou, J. H.; Zhou, L.; Wei, S. H. Drug-controlled release based on complementary base pairing rules for photodynamicphotothermal synergistic tumor treatment. Small2019, 15, 1803926.
Zhang, D.; Wu, M.; Zeng, Y. Y.; Wu, L. J.; Wang, Q. T.; Han, X.; Liu, X. L.; Liu, J. F. Chlorin e6 conjugated poly(dopamine) nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced cancer therapy. ACS Appl. Mater. Interfaces2015, 7, 8176–8187.
Liu, Y. L.; Ai, K. L.; Liu, J. H.; Deng, M.; He, Y. Y.; Lu, L. H. Dopaminemelanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater.2013, 25, 1353–1359.
Liu, F. Y.; He, X. X.; Zhang, J. P.; Chen, H. D.; Zhang, H. M.; Wang, Z. X. Controllable synthesis of polydopamine nanoparticles in microemulsions with pH-activatable properties for cancer detection and treatment. J. Mat. Chem. B2015, 3, 6731–6739.
Cheng, Y.; Chang, Y.; Feng, Y. L.; Jian, H.; Tang, Z. H.; Zhang, H. Y. Deep-level defect enhanced photothermal performance of bismuth sulfide-gold heterojunction nanorods for photothermal therapy of cancer guided by computed tomography imaging. Angew. Chem., Int. Ed.2018, 57, 246–251.
Cheng, Y.; Chang, Y.; Feng, Y. L.; Liu, N.; Sun, X. J.; Feng, Y. Q.; Li, X.; Zhang, H. Y. Simulated sunlight-mediated photodynamic therapy for melanoma skin cancer by titanium-dioxide-nanoparticle-gold-nanoclustergraphene heterogeneous nanocomposites. Small2017, 13, 1603935.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21703232, 21777152, and 21573216), Hundred Talent Program of Chinese Academy of Sciences, Jilin Provincial Science and Technology Development Program (Nos. 20180520145JH and 20160101304JC).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2019_2532_MOESM1_ESM.pdf
Polydopamine and ammonium bicarbonate coated and doxorubicin loaded hollow cerium oxide nanoparticles for synergistic tumor therapy
Rights and permissions
About this article
Cite this article
Xu, K., Cheng, Y., Yan, J. et al. Polydopamine and ammonium bicarbonate coated and doxorubicin loaded hollow cerium oxide nanoparticles for synergistic tumor therapy. Nano Res. 12, 2947–2953 (2019). https://doi.org/10.1007/s12274-019-2532-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2532-3